The Toxicological Intersection between Allergen and Toxin: A Structural Comparison of the Cat Dander Allergenic Protein Fel d1 and the Slow Loris Brachial Gland Secretion Protein
Abstract
1. Introduction
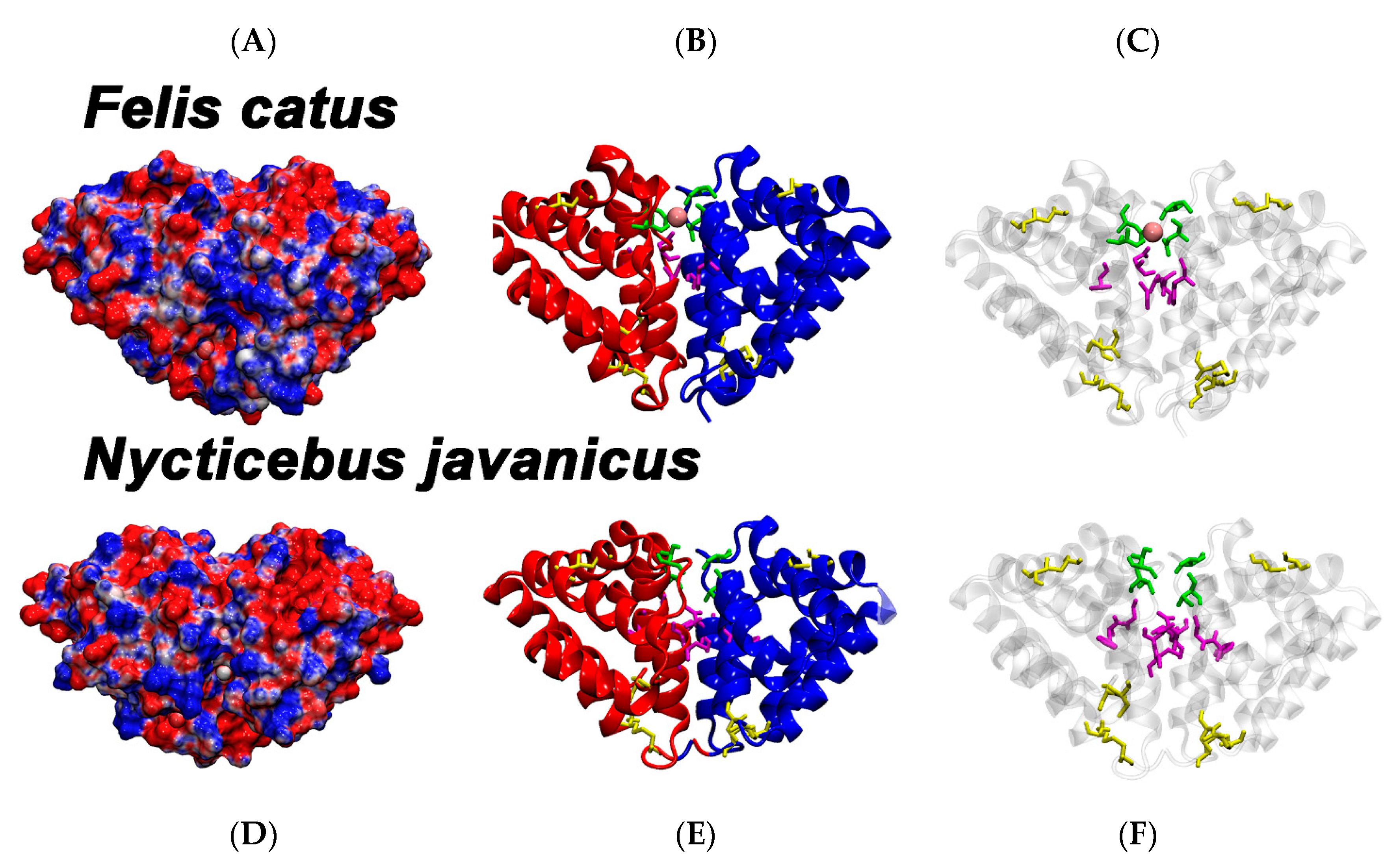
2. Results and Discussion
3. Materials and Methods
3.1. Brachial Gland Secretion
3.2. MS/MS
3.3. LC-MS/MS Analysis
3.4. mRNA
3.5. Modeling
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Petras, D.; Card, D.C.; Suranse, V.; Mychajliw, A.M.; Richards, D.; Koludarov, I.; Albulescu, L.O.; Slagboom, J.; Hempel, B.F.; et al. Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals. Proc. Nat. Acad. Sci. USA 2019, 116, 25745–25755. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wuster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Kakumanu, R.; Hodgson, W.C.; Ravi, R.; Alagon, A.; Harris, R.J.; Brust, A.; Alewood, P.F.; Kemp-Harper, B.K.; Fry, B.G. Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding. Toxins 2019, 11. [Google Scholar] [CrossRef]
- Ligabue-Braun, R.; Verli, H.; Carlini, C.R. Venomous mammals: A review. Toxicon 2012, 59, 680–695. [Google Scholar] [CrossRef]
- Low, D.H.; Sunagar, K.; Undheim, E.A.; Ali, S.A.; Alagon, A.C.; Ruder, T.; Jackson, T.N.; Pineda Gonzalez, S.; King, G.F.; Jones, A.; et al. Dracula’s children: Molecular evolution of vampire bat venom. J. Proteom. 2013, 89, 95–111. [Google Scholar] [CrossRef]
- Rode-Margono, J.E.; Nekaris, K.A. Cabinet of Curiosities: Venom Systems and Their Ecological Function in Mammals, with a Focus on Primates. Toxins 2015, 7, 2639–2658. [Google Scholar] [CrossRef]
- Alterman, L. Toxins and toothcombs: Potential allospecific chemical defenses in Nycticebus and Perodicticus. In Creatures of the Dark: The Nocturnal Prosimians; Alterman, L., Doyle, G.A., Izard, M.K., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 413–424. [Google Scholar]
- Nekaris, K.A.; Moore, R.S.; Rode, E.J.; Fry, B.G. Mad, bad and dangerous to know: The biochemistry, ecology and evolution of slow loris venom. J. Venom. Anim. Toxins Incl. Trop Dis. 2013, 19, 21. [Google Scholar] [CrossRef]
- Fuller, G.; Lukas, K.E.; Kuhar, C.; Dennis, P.M. A retrospective review of mortality in lorises and pottos in North American zoos 1980–2010. Endanger. Species Res. 2014, 23, 205–217. [Google Scholar] [CrossRef]
- Streicher, U. Aspects of Ecology and Conservation of the Pygmy Loris Nycticebus Pygmaeus in Vietnam; Ludwig-Maximilian-Universitat Munchen: Munich, Germany, 2004. [Google Scholar]
- Fung, H.T.; Wong, O.F. Clinical quiz: A potentially toxic primate bite. Hong Kong J. Emerg. Med. 2016, 23, 301–303. [Google Scholar] [CrossRef]
- Gardiner, M.; Weldon, A.; Poindexter, S.A.; Gibson, N.; Nekaris, K.A.I. Survey of practitioners handling slow lorises (Primates: Nycticebus): An assessment of the harmful effects of slow loris bites. J. Venom. Res. 2018, 9, 1–7. [Google Scholar] [PubMed]
- Madani, G.; Nekaris, K.A. Anaphylactic shock following the bite of a wild Kayan slow loris (Nycticebus kayan): Implications for slow loris conservation. J. Venom. Anim. Toxins Incl. Trop Dis. 2014, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Hagey, L.; Fry, B.G.; Snyder, H. Talking Defensively: A Dual Use for the Brachial Gland Exudate of Slow and Pygmy Lorises. In Developments in Primatology; Gursky, N., Ed.; Kluwer: South Holland, The Netherlands, 2007. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.C.; Baer, H.; Ohman, J.L., Jr. A comparative study of the allergens of cat urine, serum, saliva, and pelt. J. Allergy Clin. Immunol. 1985, 76, 563–569. [Google Scholar] [CrossRef]
- Charpin, C.; Mata, P.; Charpin, D.; Lavaut, M.N.; Allasia, C.; Vervloet, D. Fel d I allergen distribution in cat fur and skin. J. Allergy Clin. Immunol. 1991, 88, 77–82. [Google Scholar] [CrossRef]
- Klug, J.; Beier, H.M.; Bernard, A.; Chilton, B.S.; Fleming, T.P.; Lehrer, R.I.; Miele, L.; Pattabiraman, N.; Singh, G. Uteroglobin/Clara cell 10-kDa family of proteins: Nomenclature committee report. Ann. N. Y. Acad. Sci. 2000, 923, 348–354. [Google Scholar] [CrossRef]
- van Milligen, F.J.; Vroom, T.M.; Aalberse, R.C. Presence of Felis domesticus allergen I in the cat’s salivary and lacrimal glands. Int. Arch. Allergy Appl. Immunol. 1990, 92, 375–378. [Google Scholar] [CrossRef]
- Duffort, O.A.; Carreira, J.; Nitti, G.; Polo, F.; Lombardero, M. Studies on the biochemical structure of the major cat allergen Felis domesticus I. Mol. Immunol. 1991, 28, 301–309. [Google Scholar] [CrossRef]
- Kristensen, A.K.; Schou, C.; Roepstorff, P. Determination of isoforms, N-linked glycan structure and disulfide bond linkages of the major cat allergen Fel d1 by a mass spectrometric approach. Biol. Chem. 1997, 378, 899–908. [Google Scholar] [CrossRef]
- Griffith, I.J.; Craig, S.; Pollock, J.; Yu, X.B.; Morgenstern, J.P.; Rogers, B.L. Expression and genomic structure of the genes encoding FdI, the major allergen from the domestic cat. Gene 1992, 113, 263–268. [Google Scholar] [CrossRef]
- Kaiser, L.; Gronlund, H.; Sandalova, T.; Ljunggren, H.G.; van Hage-Hamsten, M.; Achour, A.; Schneider, G. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J. Biol. Chem. 2003, 278, 37730–37735. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Velickovic, T.C.; Badia-Martinez, D.; Adedoyin, J.; Thunberg, S.; Hallen, D.; Berndt, K.; Gronlund, H.; Gafvelin, G.; van Hage, M.; et al. Structural characterization of the tetrameric form of the major cat allergen Fel d 1. J. Mol. Biol. 2007, 370, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Gronlund, H.; Bergman, T.; Sandstrom, K.; Alvelius, G.; Reininger, R.; Verdino, P.; Hauswirth, A.; Liderot, K.; Valent, P.; Spitzauer, S.; et al. Formation of disulfide bonds and homodimers of the major cat allergen Fel d 1 equivalent to the natural allergen by expression in Escherichia coli. J. Biol. Chem. 2003, 278, 40144–40151. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.K.; Leung, D.Y.M. Dog and Cat Allergies: Current State of Diagnostic Approaches and Challenges. Allergy Asthma Immunol. Res. 2018, 10, 97–105. [Google Scholar] [CrossRef]
- Smith, W.; O’Neil, S.E.; Hales, B.J.; Chai, T.L.; Hazell, L.A.; Tanyaratsrisakul, S.; Piboonpocanum, S.; Thomas, W.R. Two newly identified cat allergens: The von Ebner gland protein Fel d 7 and the latherin-like protein Fel d 8. Int. Arch. Allergy Immunol. 2011, 156, 159–170. [Google Scholar] [CrossRef]
- Smith, W.; Butler, A.J.; Hazell, L.A.; Chapman, M.D.; Pomes, A.; Nickels, D.G.; Thomas, W.R. Fel d 4, a cat lipocalin allergen. Clin. Exp. Allergy 2004, 34, 1732–1738. [Google Scholar] [CrossRef]
- Ohman, J.L.; Lowell, F.C.; Bloch, K.J. Allergens of mammalian origin: Characterization of allergen extracted from cat pelts. J. Allergy Clin. Immunol. 1973, 52, 231–241. [Google Scholar] [CrossRef]
- van Ree, R.; van Leeuwen, W.A.; Bulder, I.; Bond, J.; Aalberse, R.C. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J. Allergy Clin. Immunol. 1999, 104, 1223–1230. [Google Scholar] [CrossRef]
- Custovic, A.; Simpson, A.; Pahdi, H.; Green, R.M.; Chapman, M.D.; Woodcock, A. Distribution, aerodynamic characteristics, and removal of the major cat allergen Fel d 1 in British homes. Thorax 1998, 53, 33–38. [Google Scholar] [CrossRef]
- Jalil-Colome, J.; de Andrade, A.D.; Birnbaum, J.; Casanova, D.; Mege, J.L.; Lanteaume, A.; Charpin, D.; Vervloet, D. Sex difference in Fel d 1 allergen production. J. Allergy Clin. Immunol. 1996, 98, 165–168. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Morgenstern, J.P.; Griffith, I.J.; Brauer, A.W.; Rogers, B.L.; Bond, J.F.; Chapman, M.D.; Kuo, M.C. Amino acid sequence of Fel dI, the major allergen of the domestic cat: Protein sequence analysis and cDNA cloning. Proc. Nat. Acad. Sci. USA 1991, 88, 9690–9694. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.J.; Nordlund-Moller, L.; Nord, M.; Gustafsson, J.; Lund, J.; Gillner, M. Structural basis for calcium binding by uteroglobins. J. Mol. Biol. 1996, 256, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Beato, M. Binding of steroids to uteroglobin. J. Steroid Biochem 1976, 7, 327–334. [Google Scholar] [CrossRef]
- Emes, R.D.; Riley, M.C.; Laukaitis, C.M.; Goodstadt, L.; Karn, R.C.; Ponting, C.P. Comparative evolutionary genomics of androgen-binding protein genes. Genome Res. 2004, 14, 1516–1529. [Google Scholar] [CrossRef]
- Hard, T.; Barnes, H.J.; Larsson, C.; Gustafsson, J.A.; Lund, J. Solution structure of a mammalian PCB-binding protein in complex with a PCB. Nat. Struct. Biol. 1995, 2, 983–989. [Google Scholar] [CrossRef]
- Lopez de Haro, M.S.; Perez Martinez, M.; Garcia, C.; Nieto, A. Binding of retinoids to uteroglobin. FEBS Lett. 1994, 349, 249–251. [Google Scholar] [CrossRef]
- Negi, S.S.; Schein, C.H.; Oezguen, N.; Power, T.D.; Braun, W. InterProSurf: A web server for predicting interacting sites on protein surfaces. Bioinformatics 2007, 23, 3397–3399. [Google Scholar] [CrossRef]
- Gould, H.J.; Sutton, B.J.; Beavil, A.J.; Beavil, R.L.; McCloskey, N.; Coker, H.A.; Fear, D.; Smurthwaite, L. The biology of IGE and the basis of allergic disease. Annu. Rev. Immunol. 2003, 21, 579–628. [Google Scholar] [CrossRef]
- Kleine-Tebbe, J.; Kleine-Tebbe, A.; Jeep, S.; Schou, C.; Lowenstein, H.; Kunkel, G. Role of the major allergen (Fel d I) in patients sensitized to cat allergens. Int. Arch. Allergy Immunol. 1993, 100, 256–262. [Google Scholar] [CrossRef]
- Satyaraj, E.; Gardner, C.; Filipi, I.; Cramer, K.; Sherrill, S. Reduction of active Fel d1 from cats using an antiFel d1 egg IgY antibody. Immun. Inflamm. Dis. 2019, 7, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Uermosi, C.; Zabel, F.; Manolova, V.; Bauer, M.; Beerli, R.R.; Senti, G.; Kundig, T.M.; Saudan, P.; Bachmann, M.F. IgG-mediated down-regulation of IgE bound to mast cells: A potential novel mechanism of allergen-specific desensitization. Allergy 2014, 69, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kepley, C.L.; Zhang, K.; Terada, T.; Yamada, T.; Saxon, A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat. Med. 2005, 11, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, S.A.; Nekaris, K.A.I. Vertical clingers and gougers: Rapid acquisition of adult limb proportions facilitates feeding behaviours in young Javan slow lorises (Nycticebus javanicus). Mammalian Biology-Zeitschrift für Säugetierkunde 2017, 87, 40–49. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30 (Suppl. 1), S162–S173. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput Chem 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Negri, L.; Lattanzi, R.; Giannini, E.; Melchiorri, P. Bv8/Prokineticin proteins and their receptors. Life Sci. 2007, 81, 1103–1116. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Nat. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

| Enzyme Digest | Initial de novo Sequence Returned by Peaks Studio | ALC (%) | m/z | z | Final Correct Sequence |
|---|---|---|---|---|---|
| Trypsin | N(+42.01) TLFYGVFGALVTGDK | 96 | 872.4631 | 2 | CPIFYGVFGAIVTGDK |
| Trypsin | NLLDSFLDKVSGTEPEK | 96 | 631.3182 | 2 | NIIDSFIDKVSGTEPEK |
| Trypsin | T(+42.01)TC(+71.06)KLQM(+15.99)AAFNEEGLTGK | 92 | 762.8611 | 2 | IQECFNEEGITGK |
| Trypsin | LTEEDQDNVQSGLDK | 97 | 564.2603 | 3 | LTEEDKDNVQSGLDK |
| Trypsin | Edman degradation | VKSSAVLENAK | |||
| Trypsin | Edman degradation | IYSSNFCPIFYGVFGAIVTGDK | |||
| AspN | DNKLTEEDKN(+.98)NVQSGL | 94 | 902.9345 | 2 | DNKLTEEDKDNVQSGL |
| LysC | LTEEDKDNVQSGLDK | 98 | 845.91 | 2 | LTEEDKDNVQSGLDK |
| LysC | AAFENLKEC (+57.02) FNEEGLTGK | 96 | 1028.9913 | 2 | AAFENIQECFNEEGITGK |
| Chain | Polar Area/Energy | Apolar Area/Energy | Total Area/Energy | Surface Atoms | Buried Atoms |
|---|---|---|---|---|---|
| Domain A | 2602.53 | 4497.99 | 7100.53 | 609 | 439 |
| Domain B | 2602.62 | 4497.99 | 7100.61 | 609 | 439 |
| Homodimer | 4730.58 | 7466.48 | 12197.06 | 1170 | 926 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheib, H.; Nekaris, K.A.-I.; Rode-Margono, J.; Ragnarsson, L.; Baumann, K.; Dobson, J.S.; Wirdateti, W.; Nouwens, A.; Nijman, V.; Martelli, P.; et al. The Toxicological Intersection between Allergen and Toxin: A Structural Comparison of the Cat Dander Allergenic Protein Fel d1 and the Slow Loris Brachial Gland Secretion Protein. Toxins 2020, 12, 86. https://doi.org/10.3390/toxins12020086
Scheib H, Nekaris KA-I, Rode-Margono J, Ragnarsson L, Baumann K, Dobson JS, Wirdateti W, Nouwens A, Nijman V, Martelli P, et al. The Toxicological Intersection between Allergen and Toxin: A Structural Comparison of the Cat Dander Allergenic Protein Fel d1 and the Slow Loris Brachial Gland Secretion Protein. Toxins. 2020; 12(2):86. https://doi.org/10.3390/toxins12020086
Chicago/Turabian StyleScheib, Holger, K. Anne-Isola Nekaris, Johanna Rode-Margono, Lotten Ragnarsson, Kate Baumann, James S. Dobson, Wirdateti Wirdateti, Amanda Nouwens, Vincent Nijman, Paolo Martelli, and et al. 2020. "The Toxicological Intersection between Allergen and Toxin: A Structural Comparison of the Cat Dander Allergenic Protein Fel d1 and the Slow Loris Brachial Gland Secretion Protein" Toxins 12, no. 2: 86. https://doi.org/10.3390/toxins12020086
APA StyleScheib, H., Nekaris, K. A.-I., Rode-Margono, J., Ragnarsson, L., Baumann, K., Dobson, J. S., Wirdateti, W., Nouwens, A., Nijman, V., Martelli, P., Ma, R., Lewis, R. J., Kwok, H. F., & Fry, B. G. (2020). The Toxicological Intersection between Allergen and Toxin: A Structural Comparison of the Cat Dander Allergenic Protein Fel d1 and the Slow Loris Brachial Gland Secretion Protein. Toxins, 12(2), 86. https://doi.org/10.3390/toxins12020086




_Kwok.png)


