Bacteriocins of Listeria monocytogenes and Their Potential as a Virulence Factor
Abstract
1. Introduction
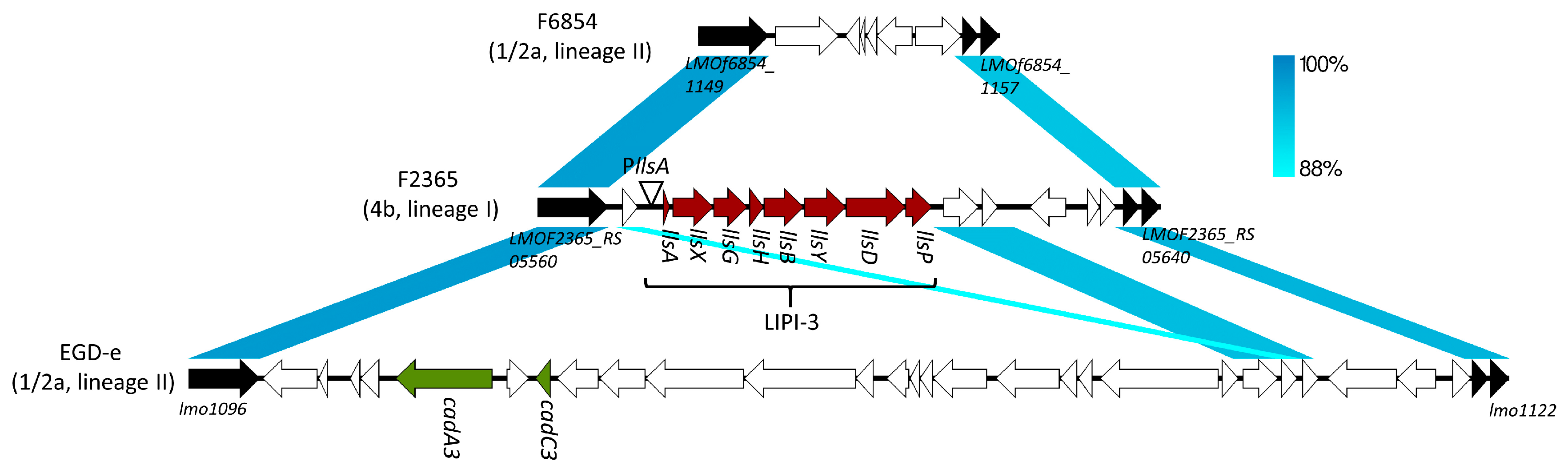
2. LLS, Streptolysin S (SLS)-Like Peptide in L. monocytogenes
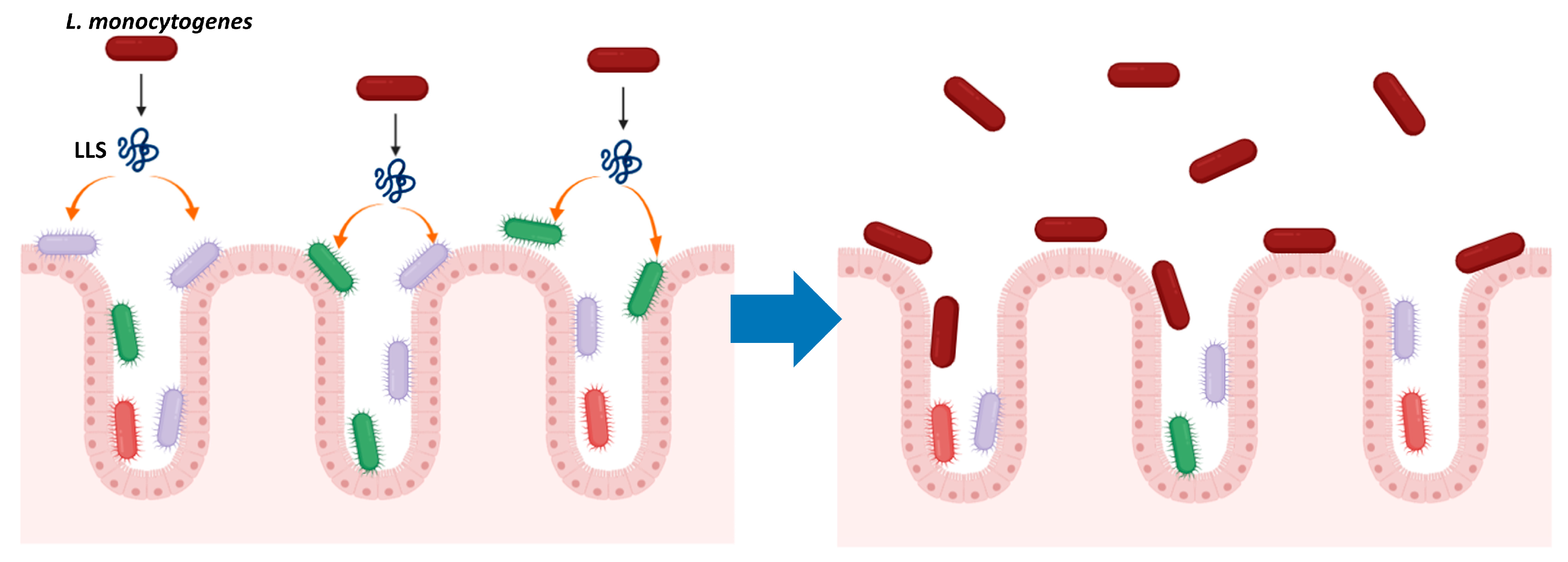
3. LLS as a Virulence Factor
4. LLS, a Bacteriocin That Modulates the Intestinal Microbiota
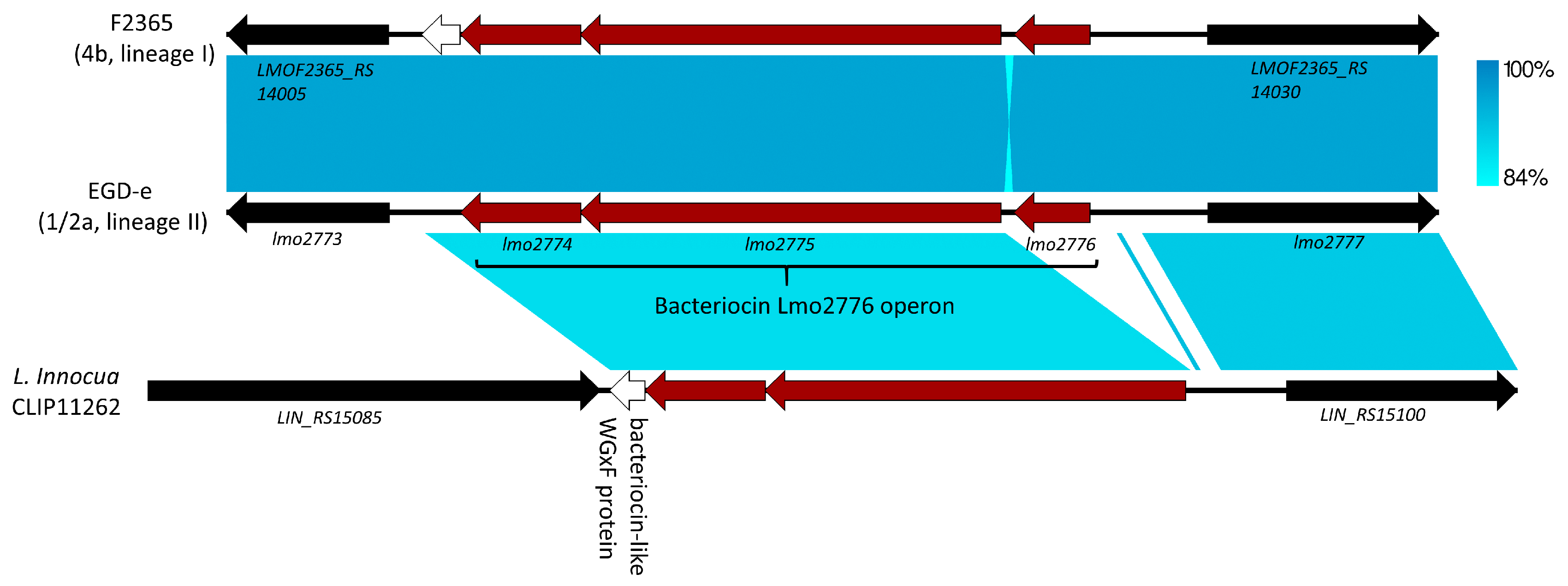
5. Lmo2776, Lactococcin 972-Like Peptide That Specifically Targets an Inflammation-Eliciting Bacterial Species in the Intestinal Microbiota, Leading to Impaired Virulence
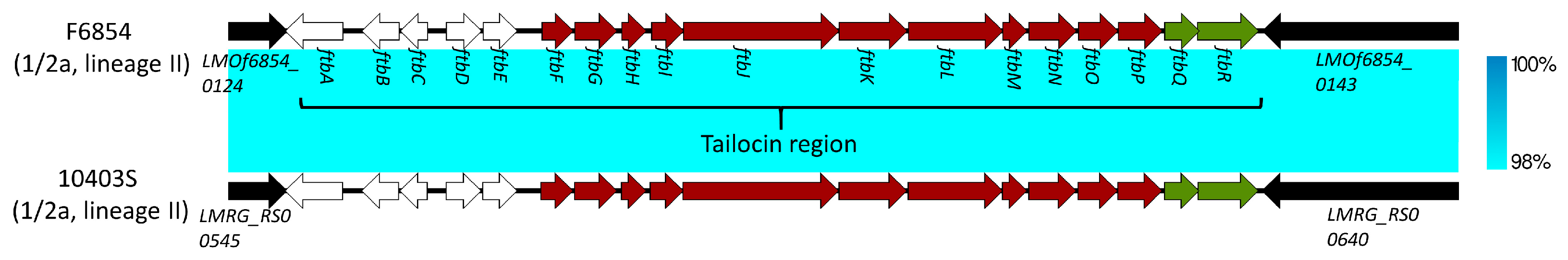
6. Monocins: Tailocins of L. monocytogenes and Potential Virulence Factors
7. Conclusions
Funding
Conflicts of Interest
References
- Painter, J.; Slutsker, L. Listeriosis in Humans. In Listeria, Listeriosis, and Food Safety, 3rd ed.; Ryser, E.T., Marth, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 85–110. [Google Scholar]
- Kathariou, S. Listeria monocytogenes Virulence and Pathogenicity, a Food Safety Perspective. J. Food Prot. 2002, 65, 1811–1829. [Google Scholar] [CrossRef]
- Charlier, C.; Perrodeau, E.; Leclercq, A.; Cazenave, B.; Pilmis, B.; Henry, B.; Lopes, A.; Maury, M.M.; Moura, A.; Goffinet, F.; et al. Clinical Features and Prognostic Factors of Listeriosis: The MONALISA National Prospective Cohort Study. Lancet Infect. Dis. 2017, 17, 510–519. [Google Scholar] [CrossRef]
- de Noordhout, C.M.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The Global Burden of Listeriosis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States-Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A Foodborne Pathogen that Knows how to Survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in Food Industry Equipment and Premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Vazquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Dominguez-Bernal, G.; Goebel, W.; Gonzalez-Zorn, B.; Wehland, J.; Kreft, J. Listeria Pathogenesis and Molecular Virulence Determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a Complete Picture of its Physiology and Pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Cossart, P. Illuminating the Landscape of Host-Pathogen Interactions with the Bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 19484–19491. [Google Scholar] [CrossRef]
- Roberts, A.J.; Wiedmann, M. Pathogen, Host and Environmental Factors Contributing to the Pathogenesis of Listeriosis. Cell Mol. Life Sci. 2003, 60, 904–918. [Google Scholar] [CrossRef]
- Hamon, M.; Bierne, H.; Cossart, P. Listeria monocytogenes: A Multifaceted Model. Nat. Rev. Microbiol. 2006, 4, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Littmann, E.R.; Carter, R.A.; Kim, S.G.; Morjaria, S.M.; Ling, L.; Gyaltshen, Y.; Fontana, E.; Taur, Y.; Leiner, I.M.; et al. Commensal Microbes Provide First Line Defense Against Listeria monocytogenes Infection. J. Exp. Med. 2017, 214, 1973–1989. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Kamada, N. Regulation of Virulence: The Rise and Fall of Gastrointestinal Pathogens. J. Gastroenterol. 2016, 51, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Baumler, A.J.; Sperandio, V. Interactions between the Microbiota and Pathogenic Bacteria in the Gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Pamer, E.G. Microbiota-Mediated Colonization Resistance Against Intestinal Pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B. When Pathogenic Bacteria Meet the Intestinal Microbiota. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and Other Fermented Foods as Sources of Health-Promoting Bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef]
- Xie, L.; van der Donk, W.A. Post-Translational Modifications during Lantibiotic Biosynthesis. Curr. Opin. Chem. Biol. 2004, 8, 498–507. [Google Scholar] [CrossRef]
- Jack, R.W.; Jung, G. Lantibiotics and Microcins: Polypeptides with Unusual Chemical Diversity. Curr. Opin. Chem. Biol. 2000, 4, 310–317. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing Innate Immunity for Food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Molloy, E.M.; Cotter, P.D.; Hill, C.; Mitchell, D.A.; Ross, R.P. Streptolysin S-Like Virulence Factors: The Continuing sagA. Nat. Rev. Microbiol. 2011, 9, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Lee, S.W.; Pence, M.A.; Markley, A.L.; Limm, J.D.; Nizet, V.; Dixon, J.E. Structural and Functional Dissection of the Heterocyclic Peptide Cytotoxin Streptolysin S. J. Biol. Chem. 2009, 284, 13004–13012. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Mitchell, D.A.; Markley, A.L.; Hensler, M.E.; Gonzalez, D.; Wohlrab, A.; Dorrestein, P.C.; Nizet, V.; Dixon, J.E. Discovery of a Widely Distributed Toxin Biosynthetic Gene Cluster. Proc. Natl. Acad. Sci. USA 2008, 105, 5879–5884. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Draper, L.A.; Lawton, E.M.; Daly, K.M.; Groeger, D.S.; Casey, P.G.; Ross, R.P.; Hill, C. Listeriolysin S, a Novel Peptide Haemolysin Associated with a Subset of Lineage I Listeria monocytogenes. PLoS Pathog. 2008, 4, e1000144. [Google Scholar] [CrossRef]
- Gonzalez, D.J.; Lee, S.W.; Hensler, M.E.; Markley, A.L.; Dahesh, S.; Mitchell, D.A.; Bandeira, N.; Nizet, V.; Dixon, J.E.; Dorrestein, P.C. Clostridiolysin S, a Post-Translationally Modified Biotoxin from Clostridium botulinum. J. Biol. Chem. 2010, 285, 28220–28228. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.M.; Casjens, S.R.; Cox, C.L.; Maxson, T.; Ethridge, N.A.; Margos, G.; Fingerle, V.; Mitchell, D.A. Identification of the Minimal Cytolytic Unit for Streptolysin S and an Expansion of the Toxin Family. BMC Microbiol. 2015, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Meza-Torres, J.; Cossart, P.; Pizarro-Cerda, J. Listeriolysin S: A Bacteriocin from Epidemic Listeria monocytogenes Strains that Targets the Gut Microbiota. Gut Microbes 2017, 8, 384–391. [Google Scholar] [CrossRef]
- Clayton, E.M.; Hill, C.; Cotter, P.D.; Ross, R.P. Real-Time PCR Assay to Differentiate Listeriolysin S-Positive and -Negative Strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2011, 77, 163–171. [Google Scholar] [CrossRef]
- Datta, V.; Myskowski, S.M.; Kwinn, L.A.; Chiem, D.N.; Varki, N.; Kansal, R.G.; Kotb, M.; Nizet, V. Mutational Analysis of the Group A Streptococcal Operon Encoding Streptolysin S and its Virulence Role in Invasive Infection. Mol. Microbiol. 2005, 56, 681–695. [Google Scholar] [CrossRef]
- Nizet, V.; Beall, B.; Bast, D.J.; Datta, V.; Kilburn, L.; Low, D.E.; De Azavedo, J.C. Genetic Locus for Streptolysin S Production by Group A Streptococcus. Infect. Immun. 2000, 68, 4245–4254. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Schwartz, L.L. Lysis of Bacterial Protoplasts and Spheroplasts by Staphylococcal Alpha-Toxin and Streptolysin S. J. Bacteriol. 1965, 89, 1387–1392. [Google Scholar] [CrossRef]
- Clayton, E.M.; Daly, K.M.; Guinane, C.M.; Hill, C.; Cotter, P.D.; Ross, P.R. Atypical Listeria innocua Strains Possess an Intact LIPI-3. BMC Microbiol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Cheng, Y.; Kim, J.W.; Lee, S.; Siletzky, R.M.; Kathariou, S. DNA Probes for Unambiguous Identification of Listeria monocytogenes Epidemic Clone II Strains. Appl. Environ. Microbiol. 2010, 76, 3061–3068. [Google Scholar] [CrossRef][Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A Genome Comparison Visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Solovyev, V.; Salamov, A. Automatic Annotation of Microbial Genomes and Metagenomic Sequences. In Metagenomics and Its Applications in Agriculture, Biomedicine and Environmental Studies; Li, R.W., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 61–78. [Google Scholar]
- Quereda, J.J.; Andersson, C.; Cossart, P.; Johansson, J.; Pizarro-Cerda, J. Role in Virulence of Phospholipases, Listeriolysin O and Listeriolysin S from Epidemic Listeria monocytogenes using the Chicken Embryo Infection Model. Vet. Res. 2018, 49, 13. [Google Scholar] [CrossRef]
- Quereda, J.J.; Dussurget, O.; Nahori, M.A.; Ghozlane, A.; Volant, S.; Dillies, M.A.; Regnault, B.; Kennedy, S.; Mondot, S.; Villoing, B.; et al. Bacteriocin from Epidemic Listeria Strains Alters the Host Intestinal Microbiota to Favor Infection. Proc. Natl. Acad. Sci. USA 2016, 113, 5706–5711. [Google Scholar] [CrossRef]
- Quereda, J.J.; Nahori, M.A.; Meza-Torres, J.; Sachse, M.; Titos-Jimenez, P.; Gomez-Laguna, J.; Dussurget, O.; Cossart, P.; Pizarro-Cerda, J. Listeriolysin S is a Streptolysin S-Like Virulence Factor that Targets Exclusively Prokaryotic Cells in Vivo. mBio 2017, 8, e00259-17. [Google Scholar] [CrossRef]
- Cheng, Y.; Siletzky, R.M.; Kathariou, S. Genomic Divisions/Lineages, Epidemic Clones, and Population Structure. In Handbook of Listeria monocytogenes, 1st ed.; Liu, D., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 337–358. [Google Scholar]
- McLauchlin, J. Distribution of Serovars of Listeria monocytogenes Isolated from Different Categories of Patients with Listeriosis. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 210–213. [Google Scholar] [CrossRef]
- Gray, M.J.; Zadoks, R.N.; Fortes, E.D.; Dogan, B.; Cai, S.; Chen, Y.; Scott, V.N.; Gombas, D.E.; Boor, K.J.; Wiedmann, M. Listeria monocytogenes Isolates from Foods and Humans Form Distinct but Overlapping Populations. Appl. Environ. Microbiol. 2004, 70, 5833–5841. [Google Scholar] [CrossRef]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes Lineages: Genomics, Evolution, Ecology, and Phenotypic Characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- Jacquet, C.; Doumith, M.; Gordon, J.I.; Martin, P.M.; Cossart, P.; Lecuit, M. A Molecular Marker for Evaluating the Pathogenic Potential of Foodborne Listeria monocytogenes. J. Infect. Dis. 2004, 189, 2094–2100. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes Hypervirulence by Harnessing its Biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef]
- Johnson, J.; Jinneman, K.; Stelma, G.; Smith, B.G.; Lye, D.; Messer, J.; Ulaszek, J.; Evsen, L.; Gendel, S.; Bennett, R.W.; et al. Natural Atypical Listeria innocua Strains with Listeria monocytogenes Pathogenicity Island 1 Genes. Appl. Environ. Microbiol. 2004, 70, 4256–4266. [Google Scholar] [CrossRef]
- Volokhov, D.V.; Duperrier, S.; Neverov, A.A.; George, J.; Buchrieser, C.; Hitchins, A.D. The Presence of the Internalin Gene in Natural Atypically Hemolytic Listeria innocua Strains Suggests Descent from L. monocytogenes. Appl. Environ. Microbiol. 2007, 73, 1928–1939. [Google Scholar] [CrossRef]
- Greetham, H.L.; Gibson, G.R.; Giffard, C.; Hippe, H.; Merkhoffer, B.; Steiner, U.; Falsen, E.; Collins, M.D. Allobaculum stercoricanis Gen. Nov., Sp. Nov., Isolated from Canine Feces. Anaerobe 2004, 10, 301–307. [Google Scholar] [CrossRef]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.; Wade, W.G. Description of Alloprevotella Rava Gen. Nov., Sp. Nov., Isolated from the Human Oral Cavity, and Reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae Gen. Nov., Comb. Nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar] [CrossRef]
- Sun, Y.; Wilkinson, B.J.; Standiford, T.J.; Akinbi, H.T.; O’Riordan, M.X. Fatty Acids Regulate Stress Resistance and Virulence Factor Production for Listeria monocytogenes. J. Bacteriol. 2012, 194, 5274–5284. [Google Scholar] [CrossRef]
- Ostling, C.E.; Lindgren, S.E. Inhibition of Enterobacteria and Listeria Growth by Lactic, Acetic and Formic Acids. J. Appl. Bacteriol. 1993, 75, 18–24. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B.; Nahori, M.A.; de Bodt, J.; Moura, A.; Lecuit, M.; Dussurget, O.; Berard, M.; Marzorati, M.; Fehlner-Peach, H.; et al. A Listeria monocytogenes Bacteriocin can Target the Commensal Prevotella copri and Modulate Intestinal Infection. Cell Host Microbe 2019, 26, 691–701.e5. [Google Scholar] [CrossRef]
- Zink, R.; Loessner, M.J.; Scherer, S. Characterization of Cryptic Prophages (Monocins) in Listeria and Sequence Analysis of a holin/endolysin Gene. Microbiology 1995, 141 Pt 10, 2577–2584. [Google Scholar] [CrossRef]
- Lee, G.; Chakraborty, U.; Gebhart, D.; Govoni, G.R.; Zhou, Z.H.; Scholl, D. F-Type Bacteriocins of Listeria monocytogenes: A New Class of Phage Tail-Like Structures Reveals Broad Parallel Coevolution between Tailed Bacteriophages and High-Molecular-Weight Bacteriocins. J. Bacteriol. 2016, 198, 2784–2793. [Google Scholar] [CrossRef]
- Ghequire, M.G.K.; De Mot, R. The Tailocin Tale: Peeling Off Phage Tails. Trends Microbiol. 2015, 23, 587–590. [Google Scholar] [CrossRef]
- Hamon, Y.; Peron, Y. Study of the Bacteriocinogenic Potency in the Genus Listeria. II. Individuality and Classification of the Bacteriocins in Question. Ann. Inst. Pasteur (Paris) 1963, 104, 55–65. [Google Scholar]
- Ortel, S. Studies about Monocines (Author’s Transl). Zentralbl. Bakteriol. Orig A 1978, 242, 72–78. [Google Scholar]
- Wilhelms, D.; Sandow, D. Preliminary Studies on Monocine Typing of Listeria monocytogenes Strains. Acta Microbiol. Hung. 1989, 36, 235–238. [Google Scholar]
- Curtis, G.D.; Mitchell, R.G. Bacteriocin (Monocin) Interactions among Listeria monocytogenes Strains. Int. J. Food Microbiol. 1992, 16, 283–292. [Google Scholar] [CrossRef]
- Zink, R.; Loessner, M.J.; Glas, I.; Scherer, S. Supplementary Listeria-Typing with Defective Listeria Phage Particles (Monocins). Lett. Appl. Microbiol. 1994, 19, 99–101. [Google Scholar] [CrossRef]
- Bannerman, E.; Boerlin, P.; Bille, J. Typing of Listeria monocytogenes by Monocin and Phage Receptors. Int. J. Food Microbiol. 1996, 31, 245–262. [Google Scholar] [CrossRef]
- Buchrieser, C. Biodiversity of the Species Listeria monocytogenes and the Genus Listeria. Microbes Infect. 2007, 9, 1147–1155. [Google Scholar] [CrossRef]
- Argov, T.; Rabinovich, L.; Sigal, N.; Herskovits, A.A. An Effective Counterselection System for Listeria monocytogenes and its use to Characterize the Monocin Genomic Region of Strain 10403S. Appl. Environ. Microbiol. 2017, 83, e02927-16. [Google Scholar] [CrossRef]
- Gohmann, S.; Leimeister-Wachter, M.; Schiltz, E.; Goebel, W.; Chakraborty, T. Characterization of a Listeria monocytogenes-Specific Protein Capable of Inducing Delayed Hypersensitivity in Listeria-Immune Mice. Mol. Microbiol. 1990, 4, 1091–1099. [Google Scholar] [CrossRef]
- Samuels, A.N.; Roggiani, M.; Zhu, J.; Goulian, M.; Kohli, R.M. The SOS Response Mediates Sustained Colonization of the Mammalian Gut. Infect. Immun. 2019, 87, e00711-18. [Google Scholar] [CrossRef]
| Name | Mechanism of Action | Gene Expression Condition | Targets | Effect on Virulence | Further Questions | References |
|---|---|---|---|---|---|---|
| LLS | ND 1 | Host intestine | Alloprevotella and Allobaculum | + 2 | What signals induce LLS expression? What is the structure of the mature LLS? What is the mechanism of action as a bacteriocin? Is LLS expressed outside the host? What functions does LLS play in the environment? | [22,25,29,33,38,39,40] |
| Lmo2776 | ND | Stationary phase in the laboratory condition | P. copri and B. subtilis | − 3 | Why do L. monocytogenes strains harbor lmo2776? What signals induce the expression of Lmo2776? What is the structure of Lmo2776? What is the mechanism of action as a bacteriocin? What functions does it play in the environment? | [53] |
| Monocins including M35152 | ND | SOS response-inducing conditions | L. monocytogenes | ND | Outside L. monocytogenes, what bacteria do monocins target? Do monocins contribute to the virulence of L. monocytogenes? Are monocins expressed inside the human intestine? | [54,55,57,58,59,60,61,62,63,64] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S. Bacteriocins of Listeria monocytogenes and Their Potential as a Virulence Factor. Toxins 2020, 12, 103. https://doi.org/10.3390/toxins12020103
Lee S. Bacteriocins of Listeria monocytogenes and Their Potential as a Virulence Factor. Toxins. 2020; 12(2):103. https://doi.org/10.3390/toxins12020103
Chicago/Turabian StyleLee, Sangmi. 2020. "Bacteriocins of Listeria monocytogenes and Their Potential as a Virulence Factor" Toxins 12, no. 2: 103. https://doi.org/10.3390/toxins12020103
APA StyleLee, S. (2020). Bacteriocins of Listeria monocytogenes and Their Potential as a Virulence Factor. Toxins, 12(2), 103. https://doi.org/10.3390/toxins12020103





