ATP-Binding Cassette Subfamily a Member 2 Is a Functional Receptor for Bacillus thuringiensis Cry2A Toxins in Bombyx mori, But Not for Cry1A, Cry1C, Cry1D, Cry1F, or Cry9A Toxins
Abstract
1. Introduction
2. Results
2.1. Creation of Silkworm Strains with C-Terminal Half-Deleted BmABCA2s and Mutants with C-Terminal Deletions in TM7 by TALEN-Mediated Mutagenesis
2.2. BmABCA2 Activity against Cry2A Toxins
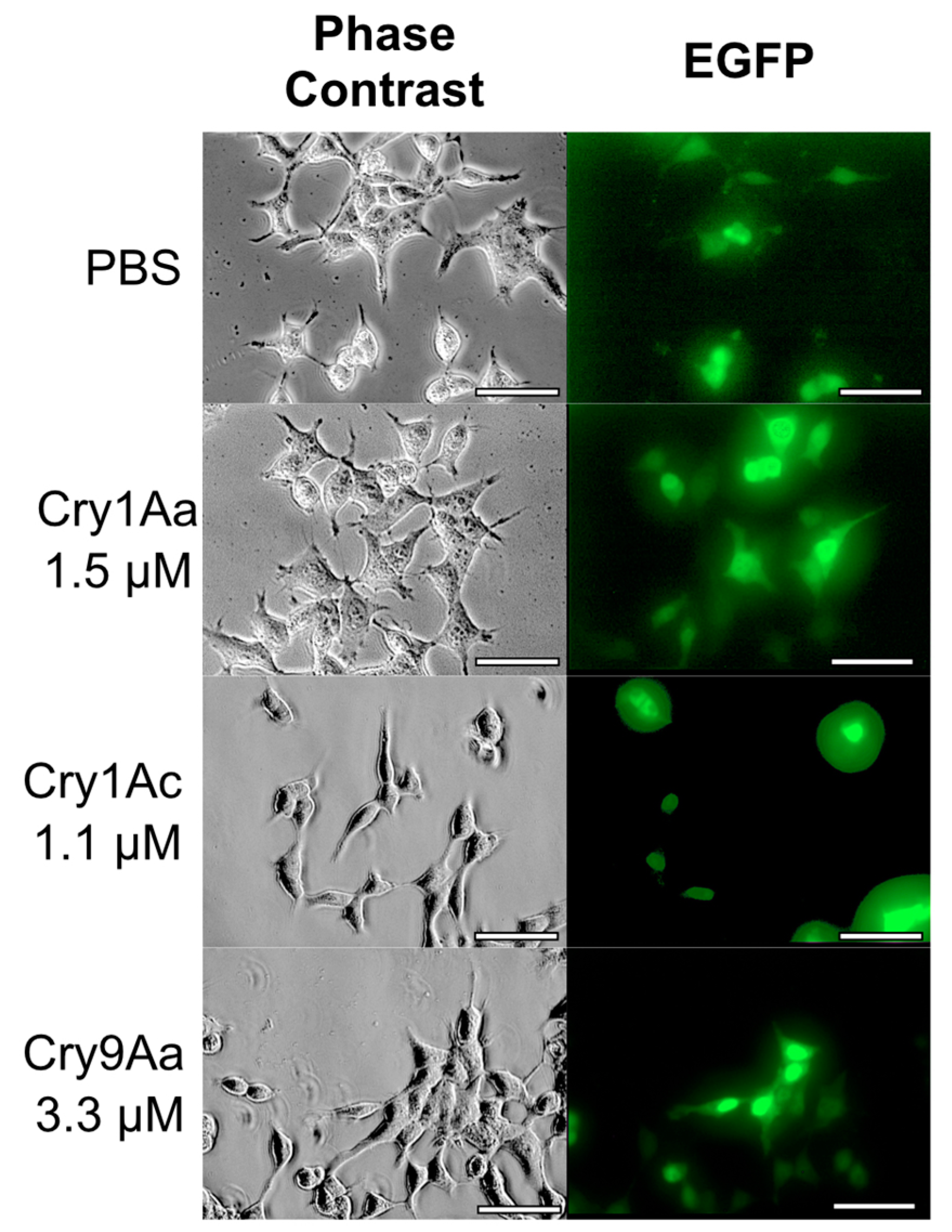
2.3. BmABCA2-Dependent Cry2A Toxins Induce Cell Swelling
3. Discussion
4. Materials and Methods
4.1. Silkworm Strains and Rearing
4.2. DNA Target Site Selection and Preparation of TALEN mRNA
4.3. Egg Microinjection
4.4. Identification of BmABCA2 Mutation Induced by TALENs
4.5. Cry Toxins Preparation
4.6. Diet Overlay Bioassays
4.7. cDNA Cloning of BmABCA2 and Construction of Expression Vectors for HEK293T Cells
4.8. Expression of BmABCA2 and BmABCC2 in HEK293T Cells and Cell Swelling Assay with Cry Toxins
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Soberón, M.; Pardo, L.; Muñóz-Garay, C.; Sánchez, J.; Gómez, I.; Porta, H.; Bravo, A. Pore formation by Cry toxins. In Proteins Membrane Binding and Pore Formation; Springer: New York, NY, USA, 2010; pp. 127–142. [Google Scholar]
- Pigott, C.R.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Villalon, M.; Vachon, V.; Brousseau, R.; Schwartz, J.L.; Laprade, R. Video imaging analysis of the plasma membrane permeabilizing effects of Bacillus thuringiensis insecticidal toxins in Sf9 cells. Biochim. Biophys. Acta (BBA) Biomembr. 1998, 1368, 27–34. [Google Scholar] [CrossRef]
- Endo, H.; Tanaka, S.; Imamura, K.; Adegawa, S.; Kikuta, S.; Sato, R. Cry toxin specificities of insect ABCC transporters closely related to lepidopteran ABCC2 transporters. Peptides 2017, 98, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.L.D.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef]
- Roh, J.Y.; Choi, J.Y.; Li, M.S.; Jin, B.R.; Je, Y.H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 2007, 17, 547. [Google Scholar]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.S.; Hernández-Martínez, P.; Van Rie, J.; Escriche, B.; Ferré, J. Shared midgut binding sites for Cry1A. 105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS ONE 2013, 8, e68164. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.S.; Van Vliet, A.; Bautsoens, N.; Van Rie, J.; Ferré, J. Specific binding of Bacillus thuringiensis Cry2A insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl. Environ. Microbiol. 2008, 74, 7654–7659. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Liu, Y.B.; de Maagd, R.A.; Dennehy, T.J. Cross-resistance of pink bollworm (Pectinophora gossypiella) to Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 2000, 66, 4582–4584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Tabashnik, B.E.; Meyer, S.K.; Crickmore, N. Cross-resistance and stability of resistance to Bacillus thuringiensis toxin Cry1C in diamondback moth. Appl. Environ. Microbiol. 2001, 67, 3216–3219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) Transporters: Roles in Xenobiotic Detoxification and Bt Insecticidal Activity. Int. J. Mol. Sci. 2019, 20, 2829. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Adegawa, S.; Li, X.; Tanaka, S.; Endo, H. Function and role of ATP-binding cassette transporters as receptors for 3D-Cry toxins. Toxins 2019, 11, 124. [Google Scholar] [CrossRef]
- Tanaka, S.; Miyamoto, K.; Noda, H.; Jurat-Fuentes, J.L.; Yoshizawa, Y.; Endo, H.; Sato, R. The ATP-binding cassette transporter subfamily C member 2 in B ombyx mori larvae is a functional receptor for C ry toxins from B acillus thuringiensis. FEBS J. 2013, 280, 1782–1794. [Google Scholar] [CrossRef]
- Tanaka, S.; Endo, H.; Adegawa, S.; Kikuta, S.; Sato, R. Functional characterization of Bacillus thuringiensis Cry toxin receptors explains resistance in insects. FEBS J. 2016, 283, 4474–4490. [Google Scholar] [CrossRef]
- Obata, F.; Tanaka, S.; Kashio, S.; Tsujimura, H.; Sato, R.; Miura, M. Induction of rapid and selective cell necrosis in Drosophila using Bacillus thuringiensis Cry toxin and its silkworm receptor. BMC Biol. 2015, 13, 48. [Google Scholar] [CrossRef]
- Pauchet, Y.; Bretschneider, A.; Augustin, S.; Heckel, D.G. A P-glycoprotein is linked to resistance to the Bacillus thuringiensis Cry3Aa toxin in a leaf beetle. Toxins 2016, 8, 362. [Google Scholar] [CrossRef]
- Tay, W.T.; Mahon, R.J.; Heckel, D.G.; Walsh, T.K.; Downes, S.; James, W.J.; Lee, S.F.; Reineke, A.; Williams, A.K.; Gordon, K.H. Insect resistance to Bacillus thuringiensis toxin Cry2Ab is conferred by mutations in an ABC transporter subfamily a protein. PLoS Genet. 2015, 11, e1005534. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Liu, S.; Liu, L.; Tay, W.T.; Walsh, T.K.; Yang, Y.; Wu, Y. CRISPR/Cas9 mediated genome editing of Helicoverpa armigera with mutations of an ABC transporter gene HaABCA2 confers resistance to Bacillus thuringiensis Cry2A toxins. Insect Biochem. Mol. Biol. 2017, 87, 147–153. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Song, X.; Ma, X.; Cotto-Rivera, R.O.; Kain, W.; Chu, H.; Chen, Y.R.; Fei, Z.; Wang, P. Mutation of ABC transporter ABCA2 confers resistance to Bt toxin Cry2Ab in Trichoplusia ni. Insect Biochem. Mol. Biol. 2019, 112, 103209. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.G.; Ponnuraj, J.; Mallappa, B.; Chowdary, L.R.; Zhang, J.; Tay, W.T.; Walsh, T.K.; Gordon, K.H.; Heckel, D.G.; Carrière, Y. ABC transporter mis-splicing associated with resistance to Bt toxin Cry2Ab in laboratory-and field-selected pink bollworm. Sci. Rep. 2018, 8, 13531. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, A.; Heckel, D.G.; Pauchet, Y. Three toxins, two receptors, one mechanism: Mode of action of Cry1A toxins from Bacillus thuringiensis in Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 76, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.; Song, S.; Bruning, J.B.; Choo, A.; Baxter, S.W. Expressing a moth abcc2 gene in transgenic Drosophila causes susceptibility to Bt Cry1Ac without requiring a cadherin-like protein receptor. Insect Biochem. Mol. Biol. 2017, 80, 61–70. [Google Scholar] [CrossRef]
- Endo, H.; Tanaka, S.; Adegawa, S.; Ichino, F.; Tabunoki, H.; Kikuta, S.; Sato, R. Extracellular loop structures in silkworm ABCC transporters determine their specificities for Bacillus thuringiensis Cry toxins. J. Biol. Chem. 2018, 293, 8569–8577. [Google Scholar] [CrossRef]
- Adegawa, S.; Nakama, Y.; Endo, H.; Shinkawa, N.; Kikuta, S.; Sato, R. The domain II loops of Bacillus thuringiensis Cry1Aa form an overlapping interaction site for two Bombyx mori larvae functional receptors, ABC transporter C2 and cadherin-like receptor. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2017, 1865, 220–231. [Google Scholar] [CrossRef]
- Takasu, Y.; Sajwan, S.; Daimon, T.; Osanai-Futahashi, M.; Uchino, K.; Sezutsu, H.; Tamura, T.; Zurovec, M. Efficient TALEN construction for Bombyx mori gene targeting. PLoS ONE 2013, 8, e73458. [Google Scholar] [CrossRef]
- Takasu, Y.; Kobayashi, I.; Tamura, T.; Uchino, K.; Sezutsu, H.; Zurovec, M. Precise genome editing in the silkworm Bombyx mori using TALENs and ds-and ssDNA donors-A practical approach. Insect Biochem. Mol. Biol. 2016, 78, 29–38. [Google Scholar] [CrossRef]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Eappen, A.; Kamba, M.; Kômoto, N.; Thomas, J.L.; Mauchamp, B.; Shirk, P. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81. [Google Scholar]
- Atsumi, S.; Mizuno, E.; Hara, H.; Nakanishi, K.; Kitami, M.; Miura, N.; Tabunoki, H.; Watanabe, A.; Sato, R. Location of the Bombyx mori aminopeptidase N type 1 binding site on Bacillus thuringiensis Cry1Aa toxin. Appl. Environ. Microbiol. 2005, 71, 3966–3977. [Google Scholar] [CrossRef]
- Sasaki, J.; Asano, S.; Hashimoto, N.; Lay, B.W.; Hastowo, S.; Bando, H.; Iizuka, T. Characterization of a cry2A Gene Cloned from an Isolate of Bacillus thuringiensis serovar sotto. Curr. Microbiol. 1997, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.; Mazza, A.; Sangadala, S.; Adang, M.J.; Brousseau, R. Polydispersity of Bacillus thuringiensis Cry1 toxins in solution and its effect on receptor binding kinetics. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2002, 1594, 266–275. [Google Scholar] [CrossRef]



| B. mori Strains | N 1 | Cry Toxin LC50 (ppm, 95%CI) 2 | Slope 3 | RR 4 | |
|---|---|---|---|---|---|
| 2Aa | A2T14 | 180 | >332 | - | >9.182 |
| Wild-type | 210 | 36.155 (26.431–49.518) | 2.173 | 1.000 | |
| 2Ab | A2T14 | 180 | >4096 | - | >9990.244 |
| Wild-type | 270 | 0.410 (0.302–0.799) | 2.114 | 1.000 | |
| 1Aa | A2T14 | 240 | 1.382 (1.152–1.730) | 4.009 | 1.544 |
| Wild-type | 240 | 0.895 (0.705–1.128) | 2.410 | 1.000 | |
| 1Ab | A2T14 | 210 | 4.808 (3.818–6.344) | 1.994 | 1.057 |
| Wild-type | 210 | 4.616 (3.515–6.771) | 2.119 | 1.000 | |
| 1Ac | A2T14 | 210 | 22.078 (17.150–28.664) | 2.116 | 4.212 |
| Wild-type | 210 | 5.242 (3.579–9.043) | 1.746 | 1.000 | |
| 1Ca | A2T14 | 240 | 0.450 (0.365–0.567) | 2.916 | 0.569 |
| Wild-type | 210 | 0.791 (0.530–1.627) | 1.478 | 1.000 | |
| 1Da | A2T14 | 210 | 0.836 (0.600–1.301) | 1.498 | 1.101 |
| Wild-type | 210 | 0.759 (0.596–0.981) | 2.497 | 1.000 | |
| 1Fa | A2T14 | 210 | 57.116 (47.210–73.042) | 3.801 | 2.514 |
| Wild-type | 240 | 22.719 (17.757–30.446) | 1.816 | 1.000 | |
| 9Aa | A2T14 | 180 | 0.264 (0.183–0.467) | 1.492 | 0.708 |
| Wild-type | 180 | 0.373 (0.311–0.475) | 3.985 | 1.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Miyamoto, K.; Takasu, Y.; Wada, S.; Iizuka, T.; Adegawa, S.; Sato, R.; Watanabe, K. ATP-Binding Cassette Subfamily a Member 2 Is a Functional Receptor for Bacillus thuringiensis Cry2A Toxins in Bombyx mori, But Not for Cry1A, Cry1C, Cry1D, Cry1F, or Cry9A Toxins. Toxins 2020, 12, 104. https://doi.org/10.3390/toxins12020104
Li X, Miyamoto K, Takasu Y, Wada S, Iizuka T, Adegawa S, Sato R, Watanabe K. ATP-Binding Cassette Subfamily a Member 2 Is a Functional Receptor for Bacillus thuringiensis Cry2A Toxins in Bombyx mori, But Not for Cry1A, Cry1C, Cry1D, Cry1F, or Cry9A Toxins. Toxins. 2020; 12(2):104. https://doi.org/10.3390/toxins12020104
Chicago/Turabian StyleLi, Xiaoyi, Kazuhisa Miyamoto, Yoko Takasu, Sanae Wada, Tetsuya Iizuka, Satomi Adegawa, Ryoichi Sato, and Kenji Watanabe. 2020. "ATP-Binding Cassette Subfamily a Member 2 Is a Functional Receptor for Bacillus thuringiensis Cry2A Toxins in Bombyx mori, But Not for Cry1A, Cry1C, Cry1D, Cry1F, or Cry9A Toxins" Toxins 12, no. 2: 104. https://doi.org/10.3390/toxins12020104
APA StyleLi, X., Miyamoto, K., Takasu, Y., Wada, S., Iizuka, T., Adegawa, S., Sato, R., & Watanabe, K. (2020). ATP-Binding Cassette Subfamily a Member 2 Is a Functional Receptor for Bacillus thuringiensis Cry2A Toxins in Bombyx mori, But Not for Cry1A, Cry1C, Cry1D, Cry1F, or Cry9A Toxins. Toxins, 12(2), 104. https://doi.org/10.3390/toxins12020104