Quality-Related Properties of Equine Immunoglobulins Purified by Different Approaches
Abstract
:1. Introduction
2. Results
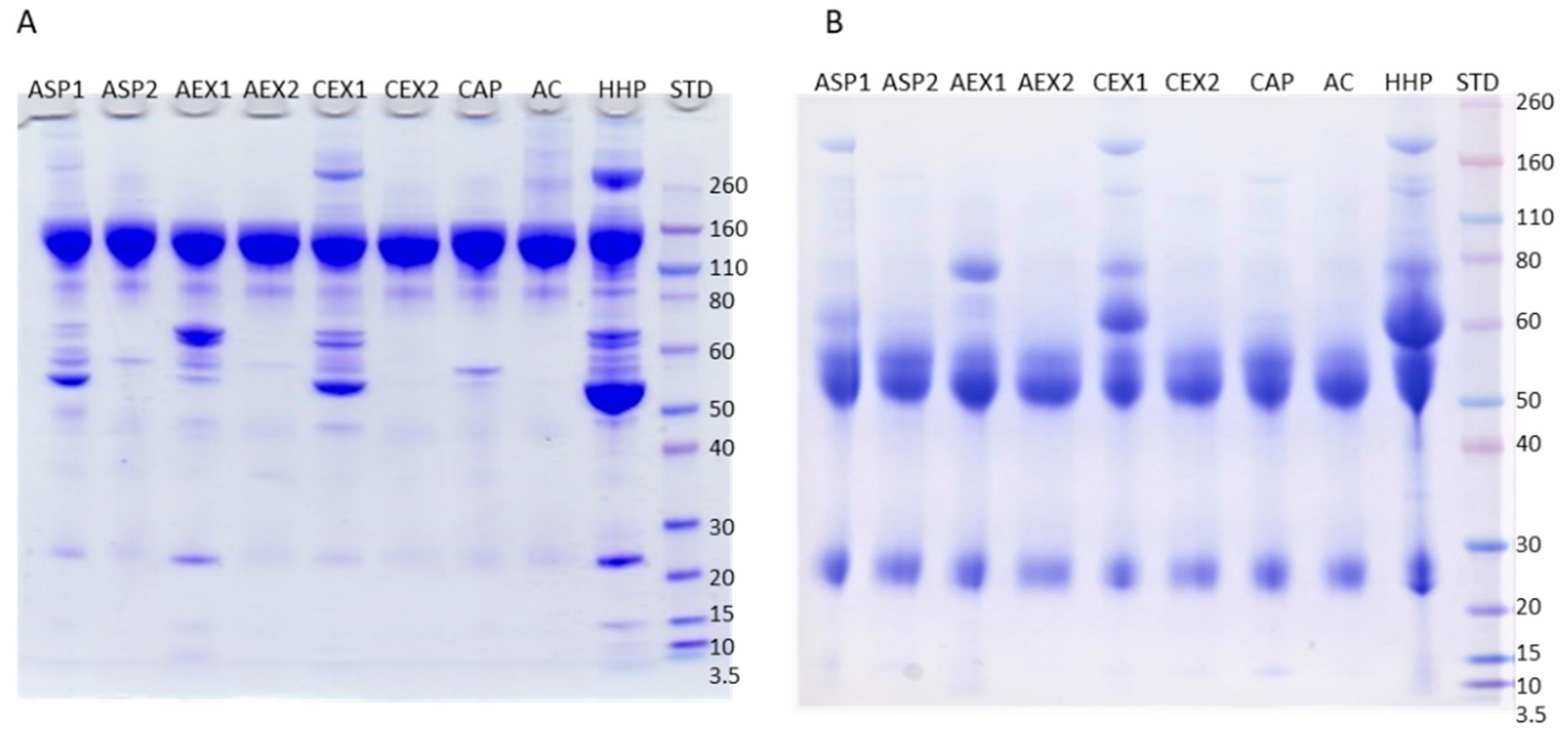
2.1. Purity of IgG Samples
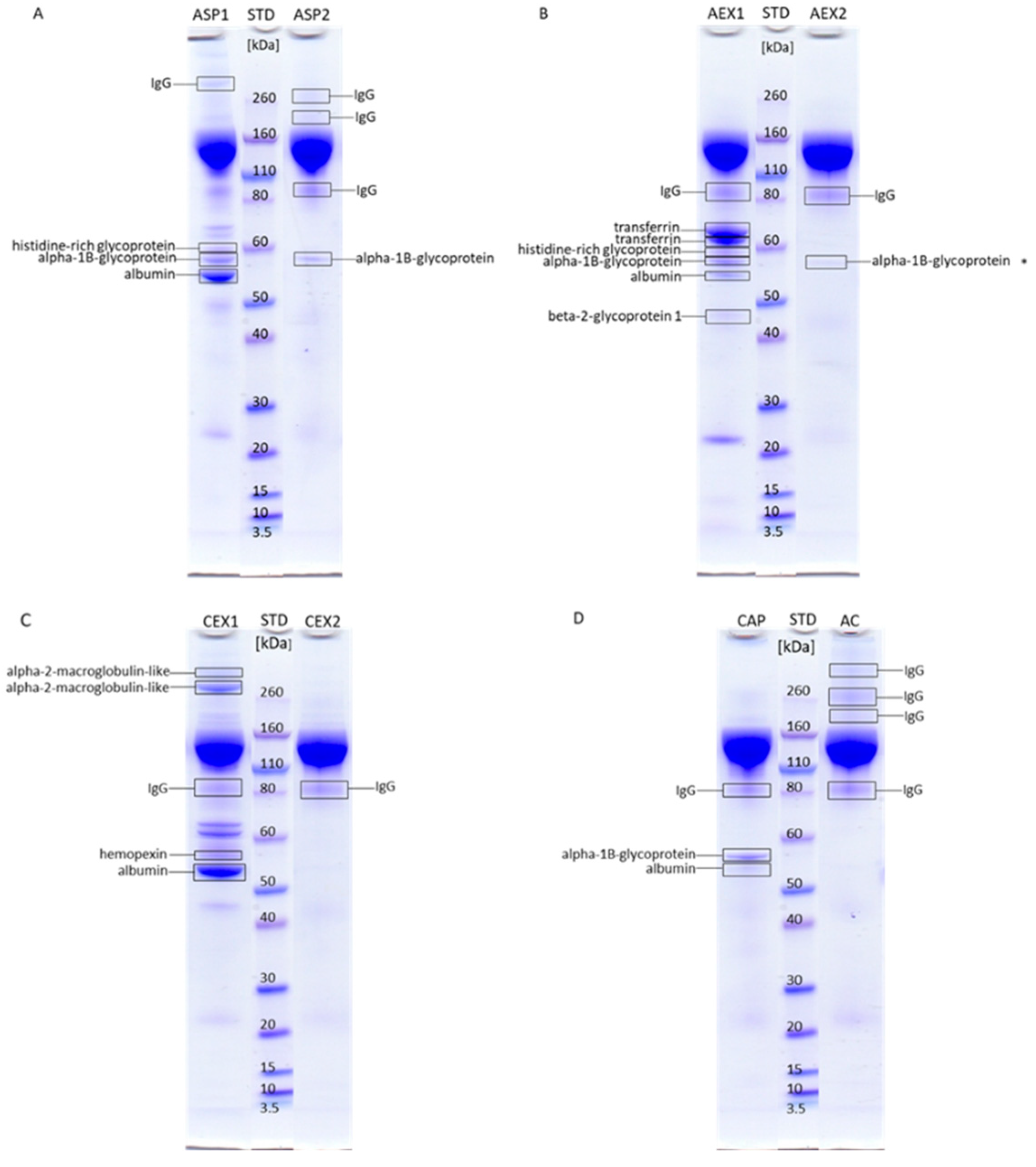
2.2. Impurities Identification
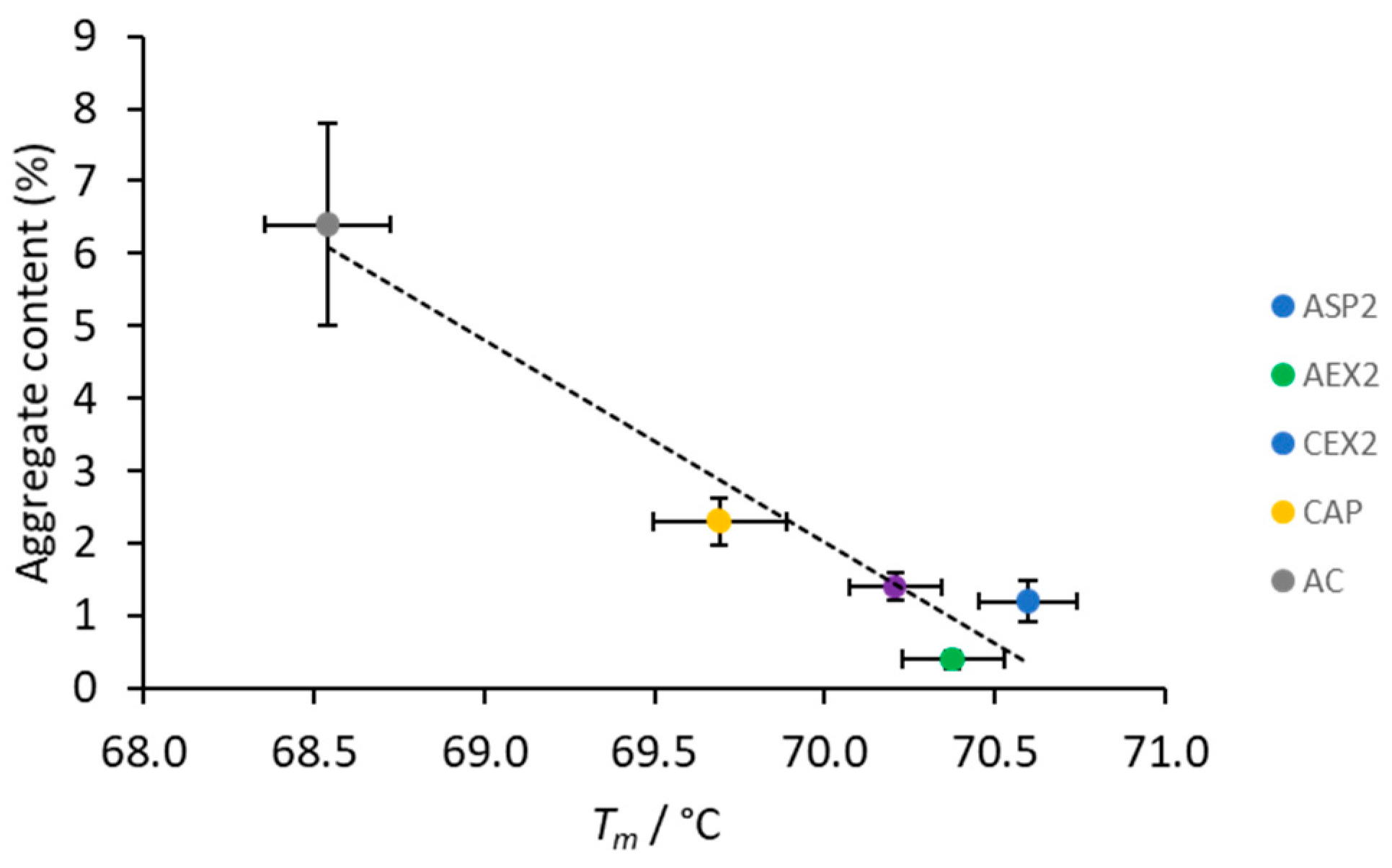
2.3. IgG Thermal Stabiliy
2.4. Aggregate Content
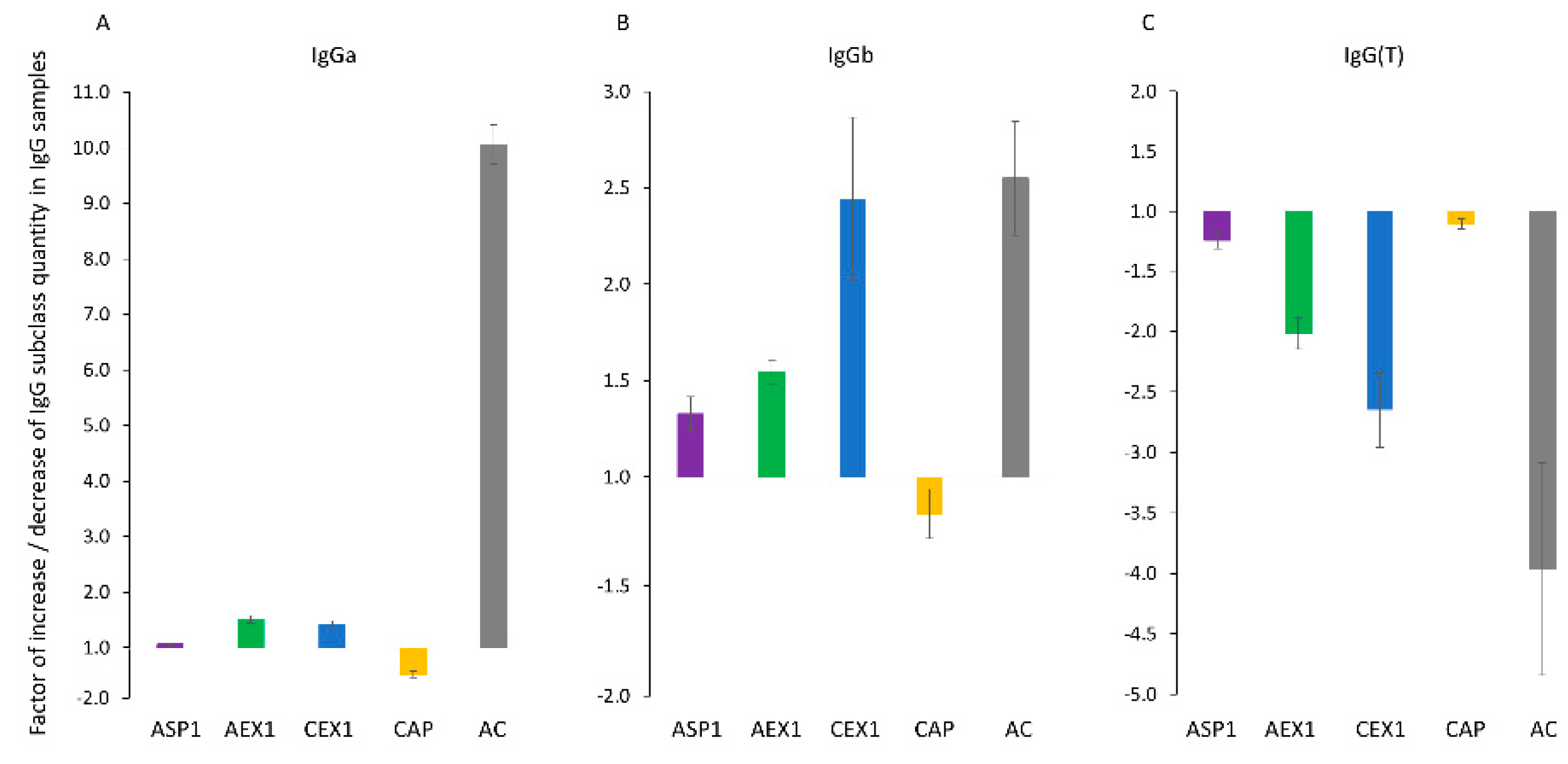
2.5. IgG subclass Distribution
2.6. Vaa Venom-Specific Protective Efficacy
3. Discussion
3.1. Protein A Affinity Chromatography
3.2. Precipitation-Based Purification Procedures
3.3. Ion-Exchange Chromatography
3.4. Thermal Stability
3.5. IgG Subclass Redistribution in Relation to Venom-Neutralising Activity
4. Conclusions
5. Materials and Methods
5.1. Animals, Snake Venom, Plasma Pool, and Reagents
5.2. IgG Purification Procedures
5.2.1. Caprylic Acid Precipitation
5.2.2. Ammonium Sulphate Precipitation
5.2.3. Anion-Exchange Chromatography
5.2.4. Cation-Exchange Chromatography
5.2.5. Protein A Affinity Chromatography
5.3. Diafiltration
5.4. Thermal Shift Assay
5.5. Purity and Aggregate Content Profiling
5.6. Mass Spectrometry for Protein Identification
5.7. Protein and IgG Content Determination
5.8. IgG Subclass Quantity Determination
5.9. Lethal Toxicity Neutralization Assay
5.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef]
- Pucca, M.; Cerni, F.A.; Janke, R.; Bermúdez-Méndez, E.; Ledsgaard, L.; Barbosa, J.E.; Laustsen, A.H. History of envenoming therapy and current perspectives. Front. Immunol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; León, G.; Burnouf, T. Antivenoms for the treatment of snakebite envenomings: The road ahead. Biologicals 2011, 39, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Solano, S.; Segura, Á.; León, G.; Gutiérrez, J.; Burnouf, T. Low pH formulation of whole IgG antivenom: Impact on quality, safety, neutralizing potency and viral inactivation. Biologicals 2012, 40, 129–133. [Google Scholar] [CrossRef]
- Kurtović, T.; Lang Balija, M.; Brgles, M.; Sviben, D.; Tunjić, M.; Cajner, H.; Marchetti-Deschmann, M.; Allmaier, G.; Halassy, B. Refinement strategy for antivenom preparation of high yield and quality. PLoS Negl. Trop. Dis. 2019, 13, e0007431. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutiérrez, J.M.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef]
- World Health Organisation. Snakebite Envenoming: A Strategy for Prevention and Control; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/324838/9789241515641-eng.pdf (accessed on 8 September 2020).
- León, G.; Herrera, M.; Segura, Á.; Villalta, M.; Vargas, M.; Gutiérrez, J.M. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon 2013, 76, 63–76. [Google Scholar] [CrossRef]
- Raweerith, R.; Ratanabanangkoon, K. Fractionation of equine antivenom using caprylic acid precipitation in combination with cationic ion-exchange chromatography. J. Immunol. Methods 2003, 282, 63–72. [Google Scholar] [CrossRef]
- Fernandes, A.; Kaundinya, J.O.; Daftary, G.; Saxena, L.; Banerjee, S.; Pattnaik, P. Chromatographic purification of equine immunoglobulin G F(ab)2 from plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 876, 109–115. [Google Scholar] [CrossRef]
- Vargas, M.; Segura, Á.; Villalta, M.; Herrera, M.; Gutiérrez, J.M.; León, G. Purification of equine whole IgG snake antivenom by using an aqueous two phase system as a primary purification step. Biologicals 2015, 43, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Gutiérrez, J.M.; Rojas, G.; Núñez, V.; Díaz, A.; Miranda, E.; Uribe, J.F.; Silva, J.F.; Ospina, J.G.; Medina, Y. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: Correlation between safety and biochemical characteristics of antivenoms. Toxicon 1999, 37, 895–908. [Google Scholar] [CrossRef]
- Rojas, G.; Jimenez, M.J.; Gutiérrez, J.M. Caprylic acid fractionation of hyperimmune horse plasma: Description of a simple procedure production. Toxicon 1994, 32, 351–363. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rojas, E.; Quesada, L.; León, G.; Núñez, J.; Laing, G.D.; Sasa, J.M.; Renjifo, J.M.; Nasidi, A.; Warrell, D.A.; et al. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: An alternative to the antivenom crisis in Africa. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 468–475. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.; Chennamsetty, N.; Xu, X.; Li, Z.J. Insights in understanding aggregate formation and dissociation in cation exchange chromatography for a structurally unstable Fc-fusion protein. J. Chromatogr. A 2016, 1460, 110–122. [Google Scholar] [CrossRef]
- Hari, S.B.; Lau, H.; Razinkov, V.I.; Chen, S.; Latypov, R.F. Acid-induced aggregation of human monoclonal IgG1 and IgG2: Molecular mechanism and the effect of solution composition. Biochemistry 2010, 49, 9328–9338. [Google Scholar] [CrossRef]
- Gagnon, P.; Nian, R.; Leong, D.; Hoi, A. Transient conformational modification of immunoglobulin G during purification by protein A affinity chromatography. J. Chromatogr. A 2015, 1395, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Tarrant, R.D.R.; Velez-Suberbie, M.L.; Tait, A.; Smales, C.M.; Bracewell, D.G. Host cell protein adsorption characteristics during protein a chromatography. Biotechnol. Prog. 2012, 28, 1037–1044. [Google Scholar] [CrossRef]
- Tao, Y.; Ibraheem, A.; Conley, L.; Cecchini, D.; Ghose, S. Evaluation of high-capacity cation exchange chromatography for direct capture of monoclonal antibodies from high-titer cell culture processes. Biotechnol. Bioeng. 2014, 111, 1354–1364. [Google Scholar] [CrossRef]
- Kittipongwarakarn, S.; Hawe, A.; Tantipolphan, R.; Limsuwun, K.; Khomvilai, S.; Puttipipatkhachorn, S.; Jiskoot, W. New method to produce equine antirabies immunoglobulin F(ab’) 2 fragments from crude plasma in high quality and yield. Eur. J. Pharm. Biopharm. 2011, 78, 189–195. [Google Scholar] [CrossRef]
- Li, Y.; Stern, D.; Lock, L.L.; Mills, J.; Ou, S.H.; Morrow, M.; Xu, X.; Ghose, S.; Li, Z.J.; Cui, H. Emerging biomaterials for downstream manufacturing of therapeutic proteins. Acta Biomater. 2019, 95, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.A.; Landon, J. Enhanced pepsin digestion: A novel process for purifying antibody F(ab’)2 fragments in high yield from serum. J. Immunol. Methods 2002, 263, 57–74. [Google Scholar] [CrossRef]
- León, G.; Vargas, M.; Segura, Á.; Herrera, M.; Villalta, M.; Sánches, A.; Solano, G.; Gómez, A.; Sánches, M.; Estrada, R.; et al. Current technology for the industrial manufacture of snake antivenoms. Toxicon 2018, 151, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Nail, S.L. The influence of ion exchange chromatography conditions on aggregation of equine IgG. Process. Biochem. 1997, 32, 279–283. [Google Scholar] [CrossRef]
- Khamehchian, S.; Zolfagharian, H.; Dounighi, N.M.; Tebianian, M.; Madani, R. Study on camel IgG purification: A new approach to prepare Naja naja oxiana antivenom as passive immunization for therapy. Hum. Vaccines Immunother. 2014, 10, 1633–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Zhang, S.; Carta, G. Unfolding and aggregation of a glycosylated monoclonal antibody on a cation exchange column. Part I. Chromatographic elution and batch adsorption behavior. J. Chromatogr. A 2014, 1356, 117–128. [Google Scholar] [CrossRef]
- Guo, J.; Carta, G. Unfolding and aggregation of a glycosylated monoclonal antibody on a cation exchange column. Part II. Protein structure effects by hydrogen deuterium exchange mass spectrometry. J. Chromatogr. A 2014, 1356, 129–137. [Google Scholar] [CrossRef]
- Morais, V.; Massaldi, H. Snake antivenoms: Adverse reactions and production technology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Wagner, B. Immunoglobulins and immunoglobulin genes of the horse. Dev. Comp. Immunol. 2006, 30, 155–164. [Google Scholar] [CrossRef]
- European Pharmacopoeia 10.0. Human Normal Immunoglobulin for Intravenous Administration; European Pharmacopoeia: Strasbourg, France, 2020; pp. 2862–2863. [Google Scholar]
- Lewis, M.J.; Wagner, B.; Woof, J.M. The different effector function capabilities of the seven equine IgG subclasses have implications for vaccine strategies. Mol. Immunol. 2008, 45, 818–827. [Google Scholar] [CrossRef]
- Brodsky, Y.; Zhang, C.; Yigzaw, Y.; Vedantham, G. Caprylic acid precipitation method for impurity reduction: An alternative to conventional chromatography for monoclonal antibody purification. Biotechnol. Bioeng. 2012, 109, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Trapp, A.; Faude, A.; Hörold, N.; Schubert, S.; Faust, S.; Grob, T.; Schmidt, S. Multiple functions of caprylic acid-induced impurity precipitation for process intensification in monoclonal antibody purification. J. Biotechnol. 2018, 279, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Halassy, B.; Kurtović, T.; Lang Balija, M.; Brgles, M.; Tunjić, M.; Sviben, D. Concept of sample-specific correction of immunoassay results for precise and accurate IgG quantification in horse plasma. J. Pharm. Biomed. Anal. 2019, 164, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Zolfagharian, H. Progress and improvement of the manufacturing process of snake antivenom. Arch. Razi Inst. 2013, 68, 1–10. [Google Scholar]
- Miller, I.; Friedlein, A.; Tsangaris, G.; Maris, A.; Fountoulakis, M.; Gemeniner, M. The serum proteome of Equus caballus. Proteomics 2004, 4, 3227–3234. [Google Scholar] [CrossRef]
- Lepczyński, A.; Ozgo, M.; Dratwa-Chałupnik, A.; Robak, P.; Pyć, A.; Zaborski, D.; Herosimczyk, A. An update on medium-and low-abundant blood plasma proteome of horse. Animal 2018, 12, 76–87. [Google Scholar] [CrossRef]
- Xu, L.; Glatz, C.E. Predicting protein retention time in ion-exchange chromatography based on three-dimensional protein characterization. J. Chromatogr. A 2009, 1216, 274–280. [Google Scholar] [CrossRef]
- Pabst, T.M.; Carta, G. pH transitions in cation exchange chromatographic columns containing weak acid groups. J. Chromatogr. A 2007, 1142, 19–31. [Google Scholar] [CrossRef]
- Ghose, S.; McNerney, T.M.; Hubbard, B. pH transitions in ion-exchange systems: Role in the development of a cation-exchange process for a recombinant protein. Biotechnol. Prog. 2002, 18, 530–537. [Google Scholar] [CrossRef]
- Laptoš, T.; Omersel, J. The importance of handling high-value biologicals: Physico-chemical instability and immunogenicity of monoclonal antibodies (review). Exp. Ther. Med. 2018, 15, 3161–3168. [Google Scholar] [CrossRef] [Green Version]
- Sviben, D.; Bertoša, B.; Hloušek-Kasun, A.; Forcic, D.; Halassy, B.; Brgles, M. Investigation of the thermal shift assay and its power to predict protein and virus stabilizing conditions. J. Pharm. Biomed. Anal. 2018, 161, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Segura, Á.; Herrera, M.; González, E.; Vargas, M.; Solano, G.; Gutiérrez, J.M.; Léon, G. Stability of equine IgG antivenoms obtained by caprylic acid precipitation: Towards a liquid formulation stable at tropical room temperature. Toxicon 2009, 53, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, I.; Takehara, H.A.; Mota, I. Isolation of IgGT from hyperimmune horse anti-snake venom serum: Its protective ability. Toxicon 1991, 29, 1373–1379. [Google Scholar] [CrossRef]
- Fernandes, I.; Takehara, H.A.; Santos, A.C.R.; Cormont, F.; Latinne, D.; Bazin, H.; Mota, I. Neutralization of bothropic and crotalic venom toxic activities by IgG(T) and IgGa subclasses isolated from immune horse serum. Toxicon 1997, 35, 931–936. [Google Scholar] [CrossRef]
- Saetang, T.; Treamwattana, N.; Suttijitpaisal, P.; Ratanabanangkoon, K. Quantitative comparison on the refinement of horse antivenom by salt fractionation and ion-exchange chromatography. J. Chromatogr. B Biomed. Appl. 1997, 700, 233–239. [Google Scholar] [CrossRef]
- Fernandes, I.; Tavares, F.L.; Sano-Martins, I.S.; Takehara, H.A. Efficacy of bothropic antivenom and its IgG(T) fraction in restoring fibrinogen levels of Bothrops jararaca envenomed mice. Toxicon 2000, 38, 995–998. [Google Scholar] [CrossRef]
- Kukongviriyapan, V.; Ratanabanangkoon, K. Some parameters of affinity chromatography in the purification of antibody against Naja naja siamensis toxin 3. J. Immunol. Methods 1982, 49, 97–104. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.; Emerson, M.; Altman, D. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94. [Google Scholar] [CrossRef] [Green Version]
- Ehresmann, B.; Imbault, P.; Well, J.H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA’s and rRNA’s. Anal. Biochem. 1973, 54, 454–463. [Google Scholar] [CrossRef]
- Kurtović, T.; Leonardi, A.; Lang Balija, M.; Brgles, M.; Habjanec, L.; Križaj, I.; Halassy, B. The standard mouse assay of anti-venom quality does not measure antibodies neutralising the haemorrhagic activity of Vipera ammodytes venom. Toxicon 2012, 59, 709–717. [Google Scholar] [CrossRef]


| Purification Method | IgG Purity (%) |
|---|---|
| ASP1 | 83.1 ± 1.5 (n = 2) |
| ASP2 | 96.6 ± 1.2 (n = 4) |
| AEX1 | 76.7 ± 0.8 (n = 2) |
| AEX2 | 98.0 ± 0.8 (n = 4) |
| CEX1 | 72.6 ± 2.2 (n = 3) |
| CEX2 | 97.8 ± 0.8 (n = 4) |
| CAP | 93.3 ± 1.4 (n = 4) |
| AC | 92.1 ± 1.7 (n = 4) |
| Purification Method | Aggregate Content (%) |
|---|---|
| ASP1 | 4.5 ± 1.4 |
| ASP2 | 1.4 ± 0.2 |
| AEX1 | 0.0 ± 0.0 |
| AEX2 | 0.4 ± 0.1 |
| CEX1 | 5.4 ± 0.9 |
| CEX2 | 1.2 ± 0.3 |
| CAP | 2.3 ± 0.3 |
| AC | 6.4 ± 1.4 |
| Hyperimmune Horse Plasma | CEX2 | AC | CAP | |
|---|---|---|---|---|
| R/LD50 mL−1 | 23.89 | 14.15 | <5.48 | 12.05 |
| γ(IgG)/mg mL−1 | 22.89 | 31.40 | 13.35 | 12.84 |
| Specific activity [R/γ(IgG)] | 1.03 | 0.45 | <0.41 | 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateljak Lukačević, S.; Kurtović, T.; Lang Balija, M.; Brgles, M.; Steinberger, S.; Marchetti-Deschmann, M.; Halassy, B. Quality-Related Properties of Equine Immunoglobulins Purified by Different Approaches. Toxins 2020, 12, 798. https://doi.org/10.3390/toxins12120798
Mateljak Lukačević S, Kurtović T, Lang Balija M, Brgles M, Steinberger S, Marchetti-Deschmann M, Halassy B. Quality-Related Properties of Equine Immunoglobulins Purified by Different Approaches. Toxins. 2020; 12(12):798. https://doi.org/10.3390/toxins12120798
Chicago/Turabian StyleMateljak Lukačević, Sanja, Tihana Kurtović, Maja Lang Balija, Marija Brgles, Stephanie Steinberger, Martina Marchetti-Deschmann, and Beata Halassy. 2020. "Quality-Related Properties of Equine Immunoglobulins Purified by Different Approaches" Toxins 12, no. 12: 798. https://doi.org/10.3390/toxins12120798
APA StyleMateljak Lukačević, S., Kurtović, T., Lang Balija, M., Brgles, M., Steinberger, S., Marchetti-Deschmann, M., & Halassy, B. (2020). Quality-Related Properties of Equine Immunoglobulins Purified by Different Approaches. Toxins, 12(12), 798. https://doi.org/10.3390/toxins12120798







