Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of Bothrops jararacussu Snake Venom
Abstract
:1. Introduction
2. Results
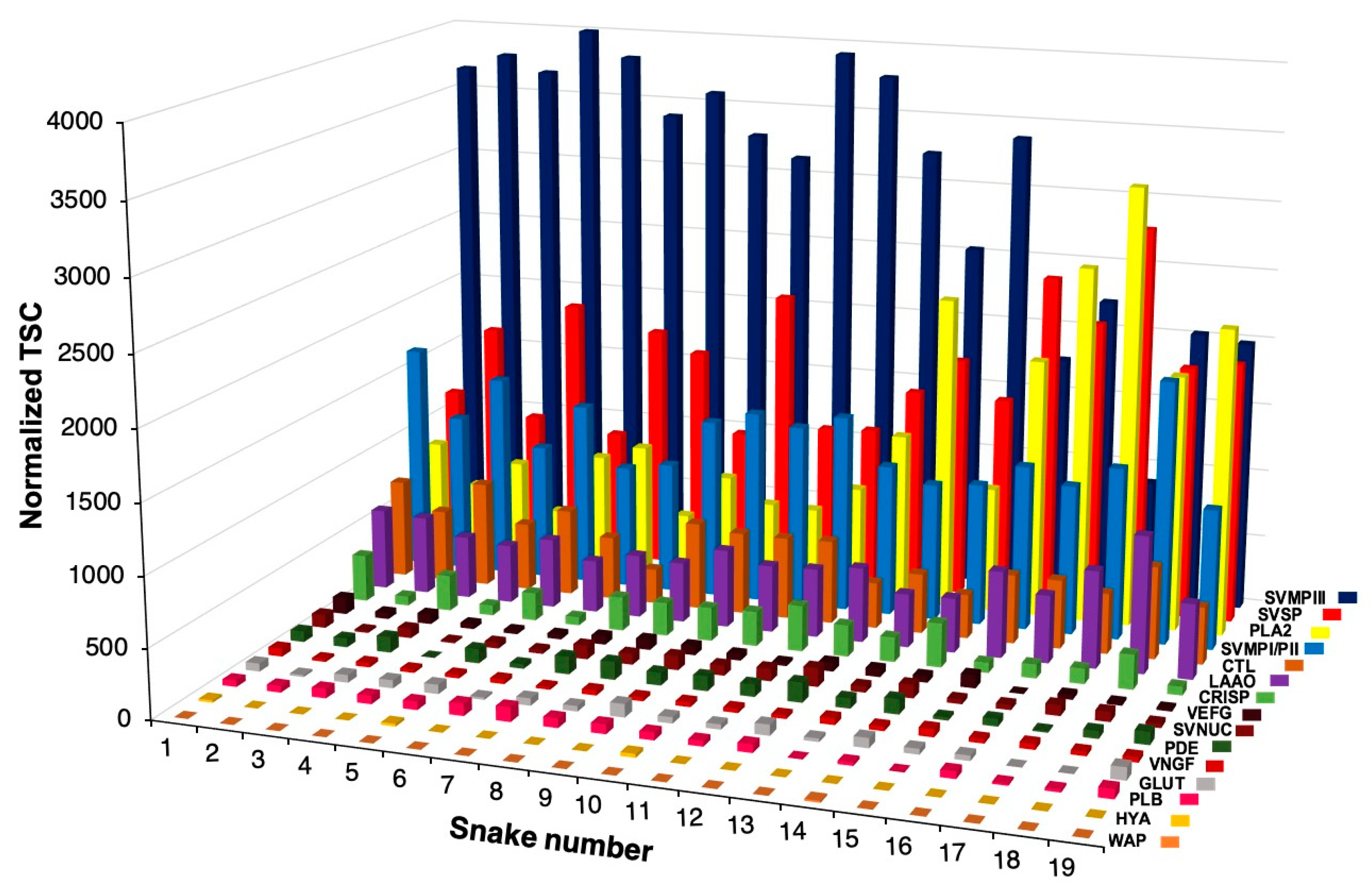
2.1. Intraspecific Variability of B. jararacussu Venom Composition
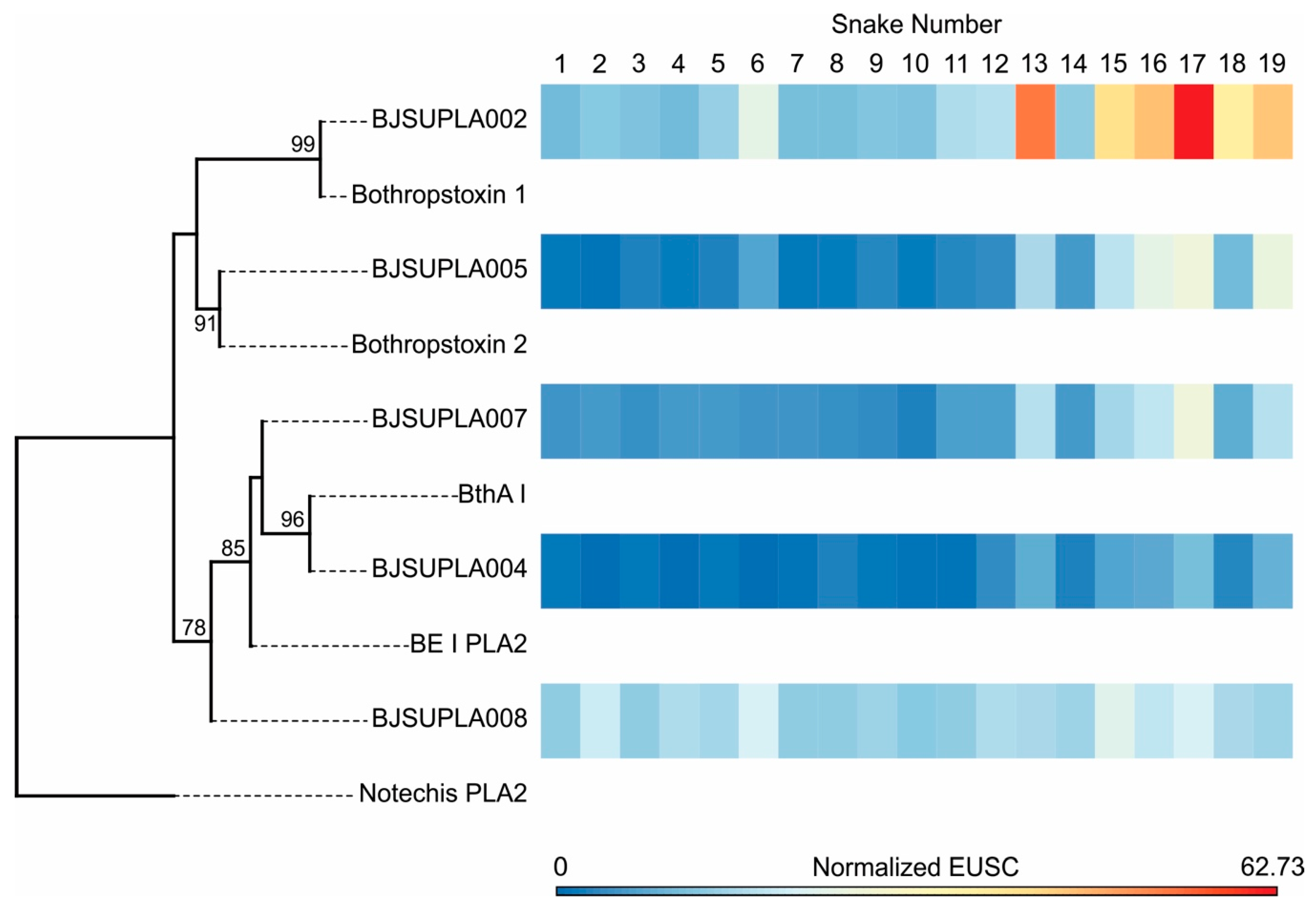
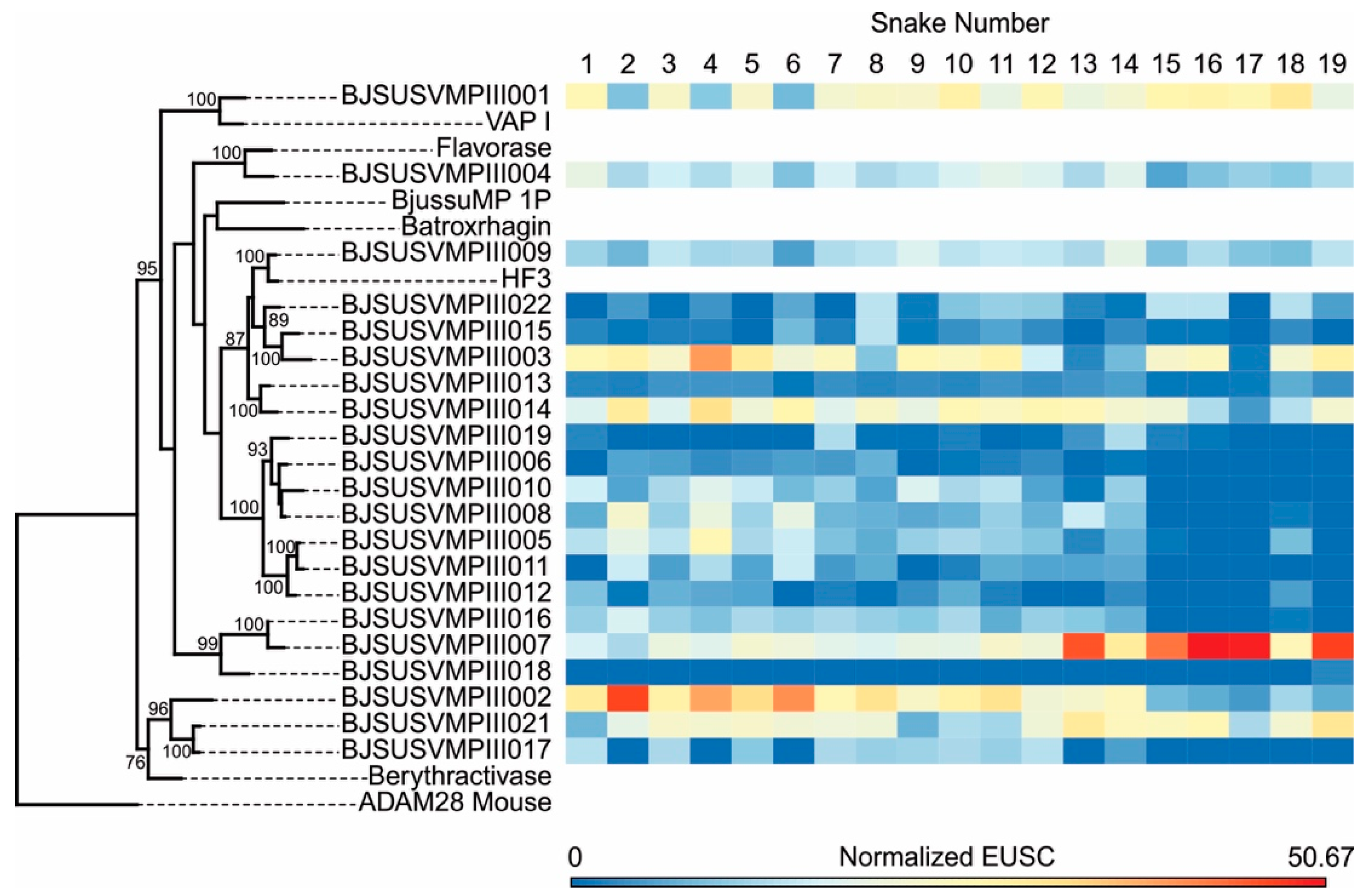
2.2. Specific Isoforms Characterize B. jararacussu Venom Variability
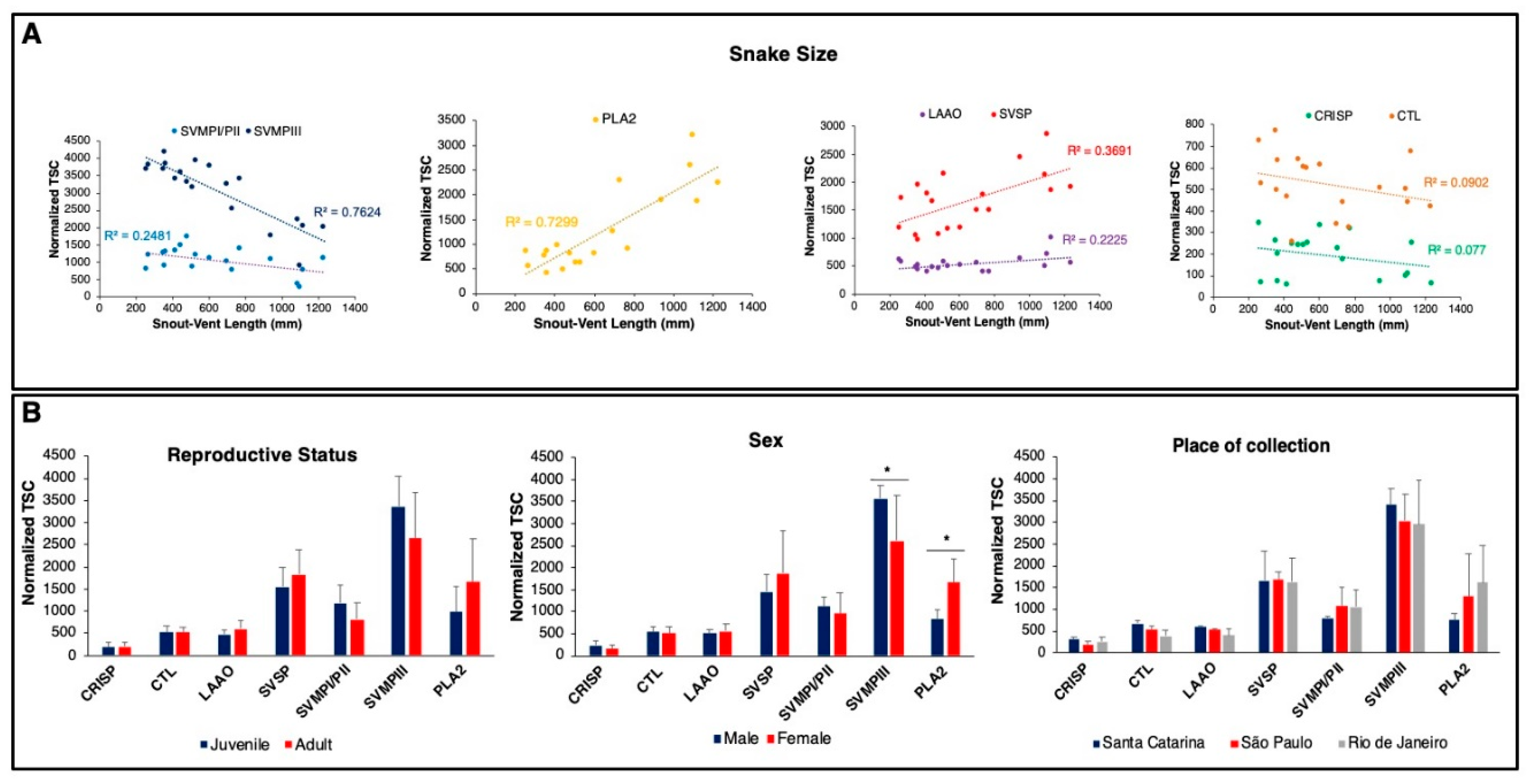
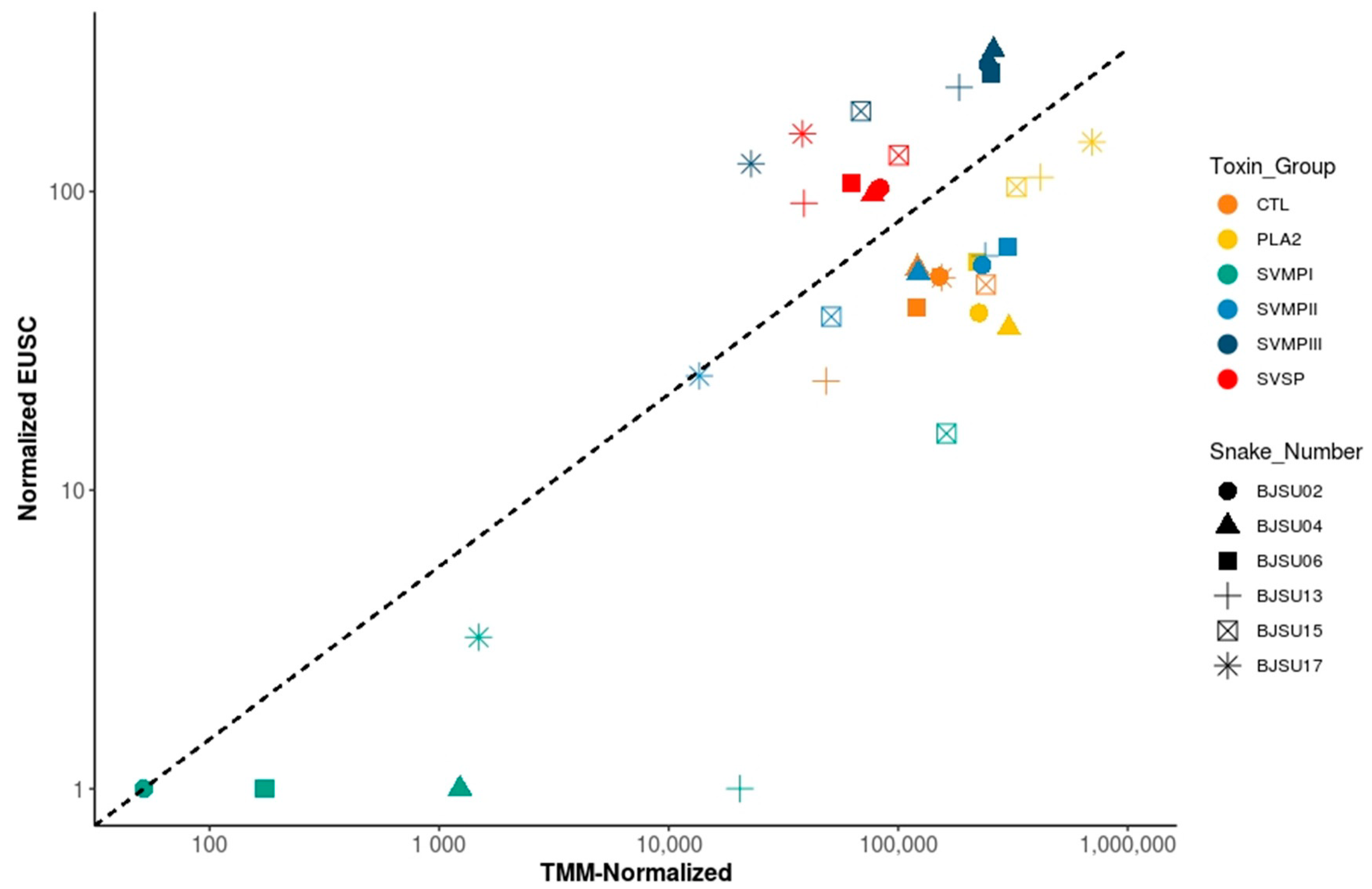
2.3. Insights on the Mechanisms Regulating B. jararacussu Venom Variability
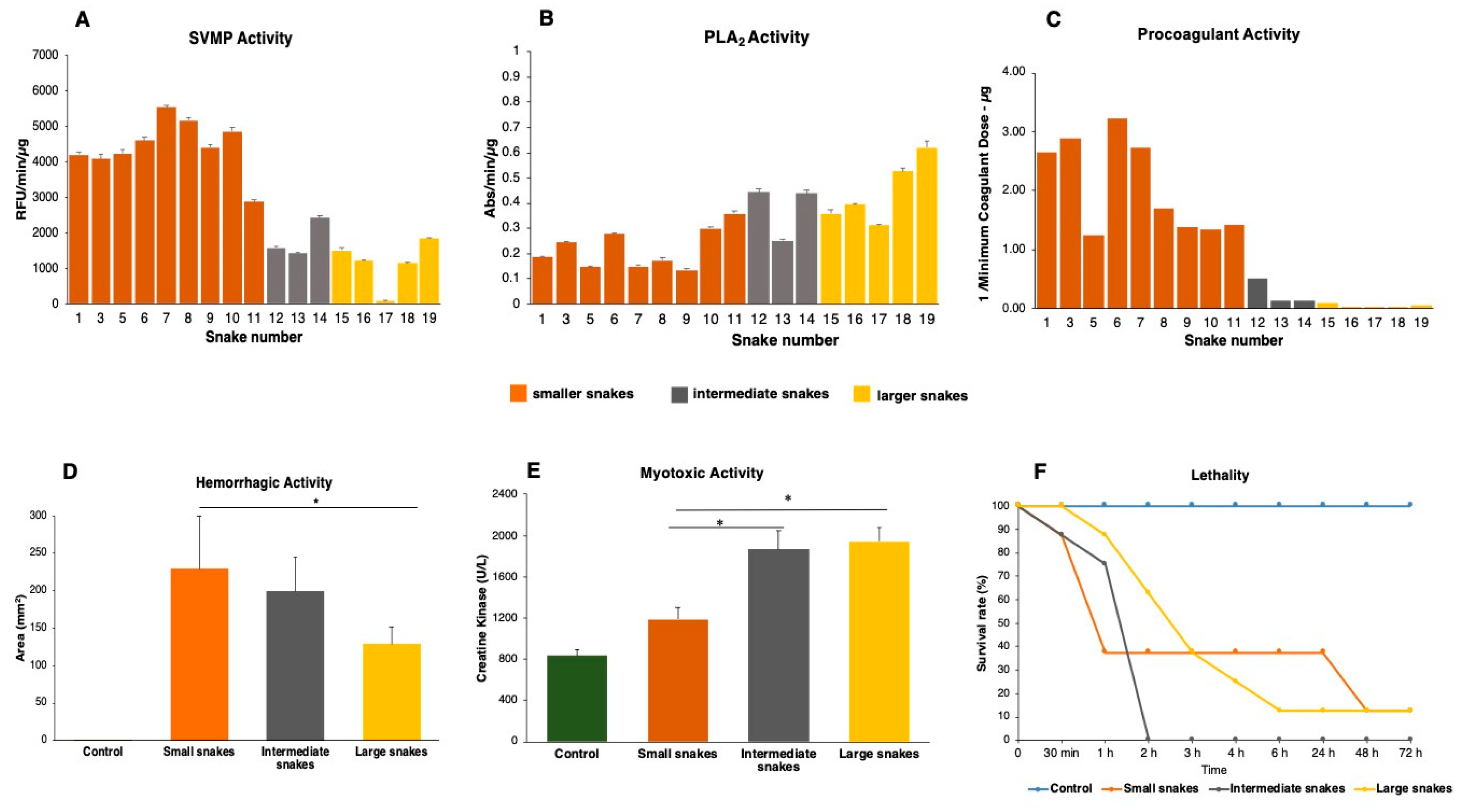
2.4. Compositional Variability Lead to Functional Differences in the Toxic Activities of B. jararacussu Venom
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Snakes and Venoms
5.2. Transcriptome
5.2.1. RNA Extraction and cDNA Library Construction and Sequencing
5.2.2. Transcriptome Assembly
5.2.3. Transcriptome Annotation (Toxins)
5.3. Venom Proteome
5.4. Sequence Alignments and Gene Tree Analyses
5.5. Functional Activities
5.5.1. Enzymatic Assays on Synthetic Substrates
5.5.2. Coagulant Activity
5.5.3. In Vivo Venom Activities
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.F.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an arsenal: Structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell Proteom. 2008, 7, 215–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura-da-Silva, A.M.; Theakston, R.D.; Crampton, J.M. Evolution of disintegrin cysteine-rich and mammalian matrix-degrading metalloproteinases: Gene duplication and divergence of a common ancestor rather than convergent evolution. J. Mol. Evol. 1996, 43, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.L.; Sanz, L.; Chiucchi, J.E.; Farrell, T.M.; Calvete, J.J. Proteomic analysis of ontogenetic and diet-related changes in venom composition of juvenile and adult Dusky Pigmy rattlesnakes (Sistrurus miliarius barbouri). J. Proteom. 2011, 74, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef] [Green Version]
- Amazonas, D.R.; Portes-Junior, J.A.; Nishiyama-Jr, M.Y.; Nicolau, C.A.; Chalkidis, H.M.; Mourão, R.H.V.; Grazziotin, F.G.; Rokyta, D.R.; Gibbs, H.L.; Valente, R.H.; et al. Molecular mechanisms underlying intraspecific variation in snake venom. J. Proteom. 2018, 181, 60–72. [Google Scholar] [CrossRef]
- Sanz, L.; Gibbs, H.L.; Mackessy, S.P.; Calvete, J.J. Venom proteomes of closely related Sistrurus rattlesnakes with divergent diets. J. Proteome Res. 2006, 5, 2098–2112. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Wray, K.P.; Margres, M.J. The genesis of an exceptionally lethal venom in the timber rattlesnake (Crotalus horridus) revealed through comparative venom-gland transcriptomics. BMC Genom. 2013, 14, 394. [Google Scholar] [CrossRef] [Green Version]
- Saldarriaga, M.M.; Otero, R.; Núñez, V.; Toro, M.F.; Díaz, A.; Gutiérrez, J.M. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 2003, 42, 405–411. [Google Scholar] [CrossRef]
- Guércio, R.A.; Shevchenko, A.; López-Lozano, J.L.; Paba, J.; Sousa, M.V.; Ricart, C.A. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteome Sci. 2006, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelanis, A.; Tashima, A.K.; Rocha, M.M.; Furtado, M.F.; Camargo, A.C.; Ho, P.L.; Serrano, S.M. Analysis of the ontogenetic variation in the venom proteome/peptidome of Bothrops jararaca reveals different strategies to deal with prey. J. Proteome Res. 2010, 9, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.C.; Furtado, M.F.; Travaglia-Cardoso, S.R.; Camargo, A.C.; Serrano, S.M. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 2006, 47, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrops asper: Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Pérez, A.; Borges, A.; Vargas, A.M.; Lomonte, B.; Angulo, Y.; Gutiérrez, J.M.; Chalkidis, H.M.; Mourão, R.H.; et al. Snake population venomics and antivenomics of Bothrops atrox: Paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteom. 2011, 74, 510–527. [Google Scholar] [CrossRef]
- Moretto Del-Rei, T.H.; Sousa, L.F.; Rocha, M.M.T.; Freitas-de-Sousa, L.A.; Travaglia-Cardoso, S.R.; Grego, K.; Sant’Anna, S.S.; Chalkidis, H.M.; Moura-da-Silva, A.M. Functional variability of Bothrops atrox venoms from three distinct areas across the Brazilian Amazon and consequences for human envenomings. Toxicon 2019, 164, 61–70. [Google Scholar] [CrossRef]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourão, R.H.; Chalkidis, H.M.; Valente, R.H.; et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 9 October 2020).
- Campbell, J.A.; Lamar, W.W. The Venomous Reptiles of the Western Hemisphere; Cornell University Press: Ithaca, NY, USA, 2004; Volume 1. [Google Scholar]
- Martins, M.; Araujo, M.S.; Sawaya, R.J.; Nunes, R. Diversity and evolution of macrohabitat use, body sizeand morphology in a monophyletic group of Neotropical pitvipers (Bothrops). J. Zool. 2001, 254, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, C.C.; Argôlo, A.J.S.; Arzamendia, V.; Azevedo, J.A.; Barbo, F.E.; Bérnils, R.S.; Bolochio, B.E.; Borges-Martins, M.; Brasil-Godinho, M.; Braz, H.; et al. Atlas of Brazilian snakes: Verified point-locality maps to mitigate the Wallacean shortfall in a megadiverse snake fauna. South Am. J. Herpetol. 2019, 14, 1–274. [Google Scholar] [CrossRef]
- Silva, K.M.P.; Braz, H.B.; Kasperoviczus, K.N.; Marques, O.A.V.; Almeida-Santos, S.M. Reproduction in the pitviper Bothrops jararacussu: Large females increase their reproductive output while small males increase their potential to mate. Zoology 2020, 142, 125816. [Google Scholar] [CrossRef]
- Melgarejo, A.R. Serpentes peçonhentas do Brasil. In Animais Peçonhentos no Brasil: Biologia, Clínica e Terapêutica dos Acidentes; Cardoso, J.L.C., Ed.; Sarvier: São Paulo, Brasil, 2009; pp. 42–70. [Google Scholar]
- Martins, M.; Marques, O.; Sazima, I. Ecological and phylogenetic correlates of feeding habits in neotropical pitvipers of the genus Bothrops. In Biology of the Vipers; Schuett, G.W., Hoggren, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2002; pp. 307–328. [Google Scholar]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourão, R.H.; Lima-dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of phylogeny, venom composition and neutralization by antivenom in diverse species of Bothrops complex. PLoS Negl. Trop. Dis. 2013, 7, e2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Aguiar, W.; da Costa Galizio, N.; Sant’Anna, S.S.; Silveira, G.P.M.; de Souza Rodrigues, F.; Grego, K.F.; de Morais-Zani, K.; Tanaka-Azevedo, A.M. Ontogenetic study of Bothrops jararacussu venom composition reveals distinct profiles. Toxicon 2020, 186, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Bernardoni, J.L.; Sousa, L.F.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.; Zingali, R.B.; Moura-da-Silva, A.M. Functional variability of snake venom metalloproteinases: Adaptive advantages in targeting different prey and implications for human envenomation. PLoS ONE 2014, 9, e109651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, P.; Kuharev, J.; Gillet, L.C.; Bernhardt, O.M.; MacLean, B.; Röst, H.L.; Tate, S.A.; Tsou, C.C.; Reiter, L.; Distler, U.; et al. A multicenter study benchmarks software tools for label-free proteome quantification. Nat. Biotechnol. 2016, 34, 1130–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokyta, D.R.; Margres, M.J.; Calvin, K. Post-transcriptional mechanisms contribute little to phenotypic variation in snake venoms. G3 2015, 5, 2375–2382. [Google Scholar] [CrossRef] [Green Version]
- Holding, M.L.; Margres, M.J.; Mason, A.J.; Parkinson, C.L.; Rokyta, D.R. Evaluating the performance of De Novo assembly methods for venom-gland transcriptomics. Toxins 2018, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.W.; Serrano, S.M. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef]
- Homsi-Brandeburgo, M.I.; Queiroz, L.S.; Santo-Neto, H.; Rodrigues-Simioni, L.; Giglio, J.R. Fractionation of Bothrops jararacussu snake venom: Partial chemical characterization and biological activity of bothropstoxin. Toxicon 1988, 26, 615–627. [Google Scholar] [CrossRef]
- Pereira, M.F.; Novello, J.C.; Cintra, A.C.; Giglio, J.R.; Landucci, E.T.; Oliveira, B.; Marangoni, S. The amino acid sequence of bothropstoxin-II, an Asp-49 myotoxin from Bothrops jararacussu (Jararacuçu) venom with low phospholipase A2 activity. J. Protein Chem. 1998, 17, 381–386. [Google Scholar] [CrossRef]
- Roberto, P.G.; Kashima, S.; Marcussi, S.; Pereira, J.O.; Astolfi-Filho, S.; Nomizo, A.; Giglio, J.R.; Fontes, M.R.; Soares, A.M.; França, S.C. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A2 from Bothrops jararacussu venom. Protein J. 2004, 23, 273–285. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque Modesto, J.C.; Spencer, P.J.; Fritzen, M.; Valença, R.C.; Oliva, M.L.; da Silva, M.B.; Chudzinski-Tavassi, A.M.; Guarnieri, M.C. BE-I-PLA2, a novel acidic phospholipase A2 from Bothrops erythromelas venom: Isolation, cloning and characterization as potent anti-platelet and inductor of prostaglandin I2 release by endothelial cells. Biochem. Pharmacol. 2006, 72, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.C.; Paes Leme, A.F.; Melo, R.L.; Silva, C.A.; Della Casa, M.; Bruni, F.M.; Lima, C.; Lopes-Ferreira, M.; Camargo, A.C.; Fox, J.W.; et al. Activation of leukocyte rolling by the cysteine-rich domain and the hyper-variable region of HF3, a snake venom hemorrhagic metalloproteinase. FEBS Lett. 2008, 582, 3915–3921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shioi, N.; Nishijima, A.; Terada, S. Flavorase, a novel non-haemorrhagic metalloproteinase in Protobothrops flavoviridis venom, is a target molecule of small serum protein-3. J. Biochem. 2015, 158, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Masuda, S.; Maeda, H.; Ying, M.J.; Hayashi, H. Involvement of specific integrins in apoptosis induced by vascular apoptosis-inducing protein 1. Toxicon 2002, 40, 535–542. [Google Scholar] [CrossRef]
- Silva, M.B.; Schattner, M.; Ramos, C.R.; Junqueira-de-Azevedo, I.L.; Guarnieri, M.C.; Lazzari, M.A.; Sampaio, C.A.; Pozner, R.G.; Ventura, J.S.; Ho, P.L.; et al. A prothrombin activator from Bothrops erythromelas (jararaca-da-seca) snake venom: Characterization and molecular cloning. Biochem. J. 2003, 369, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Mazzi, M.V.; Magro, A.J.; Amui, S.F.; Oliveira, C.Z.; Ticli, F.K.; Stábeli, R.G.; Fuly, A.L.; Rosa, J.C.; Braz, A.S.; Fontes, M.R.; et al. Molecular characterization and phylogenetic analysis of BjussuMP-I: A RGD-P-III class hemorrhagic metalloprotease from Bothrops jararacussu snake venom. J. Mol. Graph. Model 2007, 26, 69–85. [Google Scholar] [CrossRef]
- Schield, D.R.; Card, D.C.; Hales, N.R.; Perry, B.W.; Pasquesi, G.M.; Blackmon, H.; Adams, R.H.; Corbin, A.B.; Smith, C.F.; Ramesh, B.; et al. The origins and evolution of chromosomes, dosage compensation, and mechanisms underlying venom regulation in snakes. Genome Res. 2019, 29, 590–601. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Núñez, J.; Díaz, C.; Cintra, A.C.; Homsi-Brandeburgo, M.I.; Giglio, J.R. Skeletal muscle degeneration and regeneration after injection of bothropstoxin-II, a phospholipase A2 isolated from the venom of the snake Bothrops jararacussu. Exp. Mol. Pathol. 1991, 55, 217–229. [Google Scholar] [CrossRef]
- De Carvalho, D.D.; Marangoni, S.; Novello, J.C. Primary structure characterization of Bothrops jararacussu snake venom lectin. J. Protein Chem. 2002, 21, 43–50. [Google Scholar] [CrossRef]
- Pires, W.L.; de Castro, O.B.; Kayano, A.M.; da Silva Setúbal, S.; Pontes, A.S.; Nery, N.M.; Paloschi, M.V.; Dos Santos Pereira, S.; Stábeli, R.G.; Fernandes, C.F.C.; et al. Effect of BjcuL, a lectin isolated from Bothrops jararacussu, on human peripheral blood mononuclear cells. Toxicol. In Vitro 2017, 41, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Bortoleto, R.K.; Murakami, M.T.; Watanabe, L.; Soares, A.M.; Arni, R.K. Purification, characterization and crystallization of Jararacussin-I, a fibrinogen-clotting enzyme isolated from the venom of Bothrops jararacussu. Toxicon 2002, 40, 1307–1312. [Google Scholar] [CrossRef]
- Kashima, S.; Roberto, P.G.; Soares, A.M.; Astolfi-Filho, S.; Pereira, J.O.; Giuliati, S.; Faria, M.; Xavier, M.A.; Fontes, M.R.; Giglio, J.R.; et al. Analysis of Bothrops jararacussu venomous gland transcriptome focusing on structural and functional aspects: I--gene expression profile of highly expressed phospholipases A2. Biochimie 2004, 86, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, C.D.; Bernardes, C.P.; Izidoro, L.F.; Mazzi, M.V.; Soares, S.G.; Fuly, A.L.; Zingali, R.B.; Magro, A.J.; Braz, A.S.; Fontes, M.R.; et al. Molecular characterization of BjussuSP-I, a new thrombin-like enzyme with procoagulant and kallikrein-like activity isolated from Bothrops jararacussu snake venom. Biochimie 2008, 90, 500–507. [Google Scholar] [CrossRef]
- Marcussi, S.; Bernardes, C.P.; Santos-Filho, N.A.; Mazzi, M.V.; Oliveira, C.Z.; Izidoro, L.F.; Fuly, A.L.; Magro, A.J.; Braz, A.S.; Fontes, M.R.; et al. Molecular and functional characterization of a new non-hemorrhagic metalloprotease from Bothrops jararacussu snake venom with antiplatelet activity. Peptides 2007, 28, 2328–2339. [Google Scholar] [CrossRef]
- Freitas-de-Sousa, L.A.; Amazonas, D.R.; Sousa, L.F.; Sant’Anna, S.S.; Nishiyama, M.Y.; Serrano, S.M.; Junqueira-de-Azevedo, I.L.; Chalkidis, H.M.; Moura-da-Silva, A.M.; Mourão, R.H. Comparison of venoms from wild and long-term captive Bothrops atrox snakes and characterization of Batroxrhagin, the predominant class PIII metalloproteinase from the venom of this species. Biochimie 2015, 118, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Paine, M.J.; Desmond, H.P.; Theakston, R.D.; Crampton, J.M. Purification, cloning, and molecular characterization of a high molecular weight hemorrhagic metalloprotease, jararhagin, from Bothrops jararaca venom. Insights into the disintegrin gene family. J. Biol. Chem. 1992, 267, 22869–22876. [Google Scholar]
- Gonçalves-Machado, L.; Pla, D.; Sanz, L.; Jorge, R.J.B.; Leitão-De-Araújo, M.; Alves, M.L.M.; Alvares, D.J.; De Miranda, J.; Nowatzki, J.; de Morais-Zani, K.; et al. Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteom. 2016, 135, 73–89. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Contreras-Bernal, J.C.; Bisneto, P.F.; Sachett, J.; Mendonça da Silva, I.; Lacerda, M.; Guimarães da Costa, A.; Val, F.; Brasileiro, L.; Sartim, M.A.; et al. Bothrops atrox, the most important snake involved in human envenomings in the amazon: How venomics contributes to the knowledge of snake biology and clinical toxinology. Toxicon X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Zelanis, A.; Andrade-Silva, D.; Rocha, M.M.; Furtado, M.F.; Serrano, S.M.; Junqueira-de-Azevedo, I.L.; Ho, P.L. A transcriptomic view of the proteome variability of newborn and adult Bothrops jararaca snake venoms. PLoS Negl. Trop. Dis. 2012, 6, e1554. [Google Scholar] [CrossRef] [Green Version]
- Mora-Obando, D.; Guerrero-Vargas, J.A.; Prieto-Sánchez, R.; Beltrán, J.; Rucavado, A.; Sasa, M.; Gutiérrez, J.M.; Ayerbe, S.; Lomonte, B. Proteomic and functional profiling of the venom of Bothrops ayerbei from Cauca, Colombia, reveals striking interspecific variation with Bothrops asper venom. J. Proteom. 2014, 96, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Margres, M.J.; Wray, K.P.; Seavy, M.; McGivern, J.J.; Sanader, D.; Rokyta, D.R. Phenotypic integration in the feeding system of the eastern diamondback rattlesnake (Crotalus adamanteus). Mol. Ecol. 2015, 24, 3405–3420. [Google Scholar] [CrossRef] [PubMed]
- Borja, M.; Neri-Castro, E.; Pérez-Morales, R.; Strickland, J.L.; Ponce-López, R.; Parkinson, C.L.; Espinosa-Fematt, J.; Sáenz-Mata, J.; Flores-Martínez, E.; Alagón, A.; et al. Ontogenetic change in the venom of Mexican black-tailed rattlesnakes. Toxins 2018, 10, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, K.M.P.; Silva, K.B.; Sueiro, L.R.; Oliveira, M.E.; Almeida-Santos, S.M. Reproductive biology of Bothrops atrox (Serpentes, Viperidae, Crotalinae) from the Brazilian Amazon. Herpetologica 2019, 75, 198–207. [Google Scholar] [CrossRef]
- Schonour, R.B.; Huff, E.M.; Holding, M.L.; Claunch, N.M.; Ellsworth, S.A.; Hogan, M.P.; Wray, K.; McGivern, J.; Margres, M.J.; Colston, T.J.; et al. Gradual and discrete ontogenetic shifts in rattlesnake venom composition and assessment of hormonal and ecological correlates. Toxins 2020, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Durban, J.; Pérez, A.; Sanz, L.; Gómez, A.; Bonilla, F.; Rodríguez, S.; Chacón, D.; Sasa, M.; Angulo, Y.; Gutiérrez, J.M.; et al. Integrated "omics" profiling indicates that miRNAs are modulators of the ontogenetic venom composition shift in the Central American rattlesnake, Crotalus simus simus. BMC Genom. 2013, 14, 234. [Google Scholar] [CrossRef] [Green Version]
- Durban, J.; Sanz, L.; Trevisan-Silva, D.; Neri-Castro, E.; Alagón, A.; Calvete, J.J. Integrated venomics and venom gland transcriptome analysis of juvenile and adult Mexican rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 2017, 16, 3370–3390. [Google Scholar] [CrossRef]
- Mason, A.J.; Margres, M.J.; Strickland, J.L.; Rokyta, D.R.; Sasa, M.; Parkinson, C.L. Trait differentiation and modular toxin expression in palm-pitvipers. BMC Genom. 2020, 21, 147. [Google Scholar] [CrossRef] [Green Version]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- López-Lozano, J.L.; de Sousa, M.V.; Ricart, C.A.; Chávez-Olortegui, C.; Flores Sanchez, E.; Muniz, E.G.; Bührnheim, P.F.; Morhy, L. Ontogenetic variation of metalloproteinases and plasma coagulant activity in venoms of wild Bothrops atrox specimens from Amazonian rain forest. Toxicon 2002, 40, 997–1006. [Google Scholar] [CrossRef]
- Furtado, M.F.; Travaglia-Cardoso, S.R.; Rocha, M.M. Sexual dimorphism in venom of Bothrops jararaca(Serpentes: Viperidae). Toxicon 2006, 48, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.; de Barros, L.C.; Biscola, N.P.; Pimenta, D.C.; Barraviera, S.R.S.B.; Barraviera, B.; Ferreira, R.S. Intraspecific variation of biological activities in venoms from wild and captive Bothrops jararaca. J. Toxicol. Environ. Health Part A 2012, 75, 1081–1090. [Google Scholar] [CrossRef]
- Milani Júnior, R.; Jorge, M.T.; de Campos, F.P.; Martins, F.P.; Bousso, A.; Cardoso, J.L.; Ribeiro, L.A.; Fan, H.W.; França, F.O.; Sano-Martins, I.S.; et al. Snake bites by the jararacuçu (Bothrops jararacussu): Clinicopathological studies of 29 proven cases in São Paulo State, Brazil. QJM 1997, 90, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, J.C.C.; Bisneto, P.F.; Pereira, J.P.T.; Ibiapina, H.N.D.S.; Sarraff, L.K.S.; Monteiro-Júnior, C.; da Silva Pereira, H.; Santos, B.; de Moura, V.M.; de Oliveira, S.S.; et al. "Bad things come in small packages": Predicting venom-induced coagulopathy in Bothrops atrox bites using snake ontogenetic parameters. Clin. Toxicol. 2020, 58, 388–396. [Google Scholar] [CrossRef]
- Maruyama, M.; Kamiguti, A.S.; Cardoso, J.L.; Sano-Martins, I.S.; Chudzinski, A.M.; Santoro, M.L.; Morena, P.; Tomy, S.C.; Antonio, L.C.; Mihara, H. Studies on blood coagulation and fibrinolysis in patients bitten by Bothrops jararaca (jararaca). Thromb. Haemost. 1990, 63, 449–453. [Google Scholar] [CrossRef]
- Cardoso, J.L.; Fan, H.W.; França, F.O.; Jorge, M.T.; Leite, R.P.; Nishioka, S.A.; Avila, A.; Sano-Martins, I.S.; Tomy, S.C.; Santoro, M.L. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med. 1993, 86, 315–325. [Google Scholar]
- Sajevic, T.; Leonardi, A.; Križaj, I. Haemostatically active proteins in snake venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Chaves, F.; Bolaños, R. Comparative study of venoms of newborn and adult specimens of Bothrops asper. Rev. Biol. Trop. 1980, 28, 341–351. [Google Scholar]
- Chacón, F.; Oviedo, A.; Escalante, T.; Solano, G.; Rucavado, A.; Gutiérrez, J.M. The lethality test used for estimating the potency of antivenoms against Bothrops asper snake venom: Pathophysiological mechanisms, prophylactic analgesia, and a surrogate in vitro assay. Toxicon 2015, 93, 41–50. [Google Scholar] [CrossRef]
- Andrade, D.V.; Abe, A.S. Relationships of venom ontogeny and diet in Bothrops. Herpetologica 1999, 55, 200–204. [Google Scholar]
- Sazima, I. Comportamento alimentar de jararaca, Bothrops jararaca: Encontros provocados na natureza. Ciência Cult. 1989, 41, 500–505. [Google Scholar]
- Hartmann, P.A.; Hartmann, M.T.; Giasson, L.O.M. Uso do hábitat e alimentação em juvenis de Bothrops jararaca (Serpentes, Viperidae) na Mata Atlântica do sudeste do Brasil. Phyllomedusa 2003, 2, 35–41. [Google Scholar] [CrossRef]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops. Toxins 2018, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Mackessy, S.P. Evolutionary trends in venom composition in the western rattlesnakes (Crotalus viridis sensu lato): Toxicity vs. tenderizers. Toxicon 2010, 55, 1463–1474. [Google Scholar] [CrossRef]
- Puorto, G.; Mara da Graça, S.; Laporta-Ferreira, I.L. The quantity of venom produced and injected by juvenile and adult Bothrops jararaca (Viperidae, Crotalinae). Snake 1996, 27, 140–144. [Google Scholar]
- Almeida-Santos, S.M.; Santos, L.C.; Sueiro, L.R.; Barros, V.A.; Rojas, C.A.; Kasperoviczus, K.N. Biologia reprodutiva de serpentes: Recomendações para a coleta e análise de dados. Herpetol. Bras. 2014, 3, 14–24. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Hofmann, E.P.; Rautsaw, R.M.; Strickland, J.L.; Holding, M.L.; Hogan, M.P.; Mason, A.J.; Rokyta, D.R.; Parkinson, C.L. Comparative venom-gland transcriptomics and venom proteomics of four Sidewinder Rattlesnake (Crotalus cerastes) lineages reveal little differential expression despite individual variation. Sci. Rep. 2018, 8, 15534. [Google Scholar] [CrossRef]
- Krueger, F. A Wrapper Tool Around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 7 December 2020).
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate illumina paired-end reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Rokyta, D.R.; Lemmon, A.R.; Margres, M.J.; Aronow, K. The venom-gland transcriptome of the eastern diamondback rattlesnake (Crotalus adamanteus). BMC Genom. 2012, 13, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Bankar, K.G.; Todur, V.N.; Shukla, R.N.; Vasudevan, M. Ameliorated de novo transcriptome assembly using Illumina paired end sequence data with Trinity Assembler. Genom. Data 2015, 5, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Nachtigall, P.G.; Kashiwabara, A.Y.; Durham, A.M. CodAn: Predictive models for precise identification of coding regions in eukaryotic transcripts. Brief Bioinform. 2020, 27, bbaa045. [Google Scholar] [CrossRef]
- Margres, M.J.; Aronow, K.; Loyacano, J.; Rokyta, D.R. The venom-gland transcriptome of the eastern coral snake (Micrurus fulvius) reveals high venom complexity in the intragenomic evolution of venoms. BMC Genom. 2013, 14, 531. [Google Scholar] [CrossRef] [Green Version]
- McGivern, J.J.; Wray, K.P.; Margres, M.J.; Couch, M.E.; Mackessy, S.P.; Rokyta, D.R. RNA-seq and high-definition mass spectrometry reveal the complex and divergent venoms of two rear-fanged colubrid snakes. BMC Genom. 2014, 15, 1061. [Google Scholar] [CrossRef] [Green Version]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Knittel, P.S.; Long, P.F.; Brammall, L.; Marques, A.C.; Almeida, M.T.; Padilla, G.; Moura-da-Silva, A.M. Characterising the enzymatic profile of crude tentacle extracts from the South Atlantic jellyfish Olindias sambaquiensis (Cnidaria: Hydrozoa). Toxicon 2016, 119, 1–7. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas-de-Sousa, L.A.; Nachtigall, P.G.; Portes-Junior, J.A.; Holding, M.L.; Nystrom, G.S.; Ellsworth, S.A.; Guimarães, N.C.; Tioyama, E.; Ortiz, F.; Silva, B.R.; et al. Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of Bothrops jararacussu Snake Venom. Toxins 2020, 12, 791. https://doi.org/10.3390/toxins12120791
Freitas-de-Sousa LA, Nachtigall PG, Portes-Junior JA, Holding ML, Nystrom GS, Ellsworth SA, Guimarães NC, Tioyama E, Ortiz F, Silva BR, et al. Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of Bothrops jararacussu Snake Venom. Toxins. 2020; 12(12):791. https://doi.org/10.3390/toxins12120791
Chicago/Turabian StyleFreitas-de-Sousa, Luciana A., Pedro G. Nachtigall, José A. Portes-Junior, Matthew L. Holding, Gunnar S. Nystrom, Schyler A. Ellsworth, Noranathan C. Guimarães, Emilly Tioyama, Flora Ortiz, Bruno R. Silva, and et al. 2020. "Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of Bothrops jararacussu Snake Venom" Toxins 12, no. 12: 791. https://doi.org/10.3390/toxins12120791
APA StyleFreitas-de-Sousa, L. A., Nachtigall, P. G., Portes-Junior, J. A., Holding, M. L., Nystrom, G. S., Ellsworth, S. A., Guimarães, N. C., Tioyama, E., Ortiz, F., Silva, B. R., Kunz, T. S., Junqueira-de-Azevedo, I. L. M., Grazziotin, F. G., Rokyta, D. R., & Moura-da-Silva, A. M. (2020). Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of Bothrops jararacussu Snake Venom. Toxins, 12(12), 791. https://doi.org/10.3390/toxins12120791






