Defence Against Desiccation and Predation in Lophyohylini Casque-Headed Tree Frogs
Abstract
1. Introduction
2. Results
2.1. Natural History Features
2.2. Head Morphology
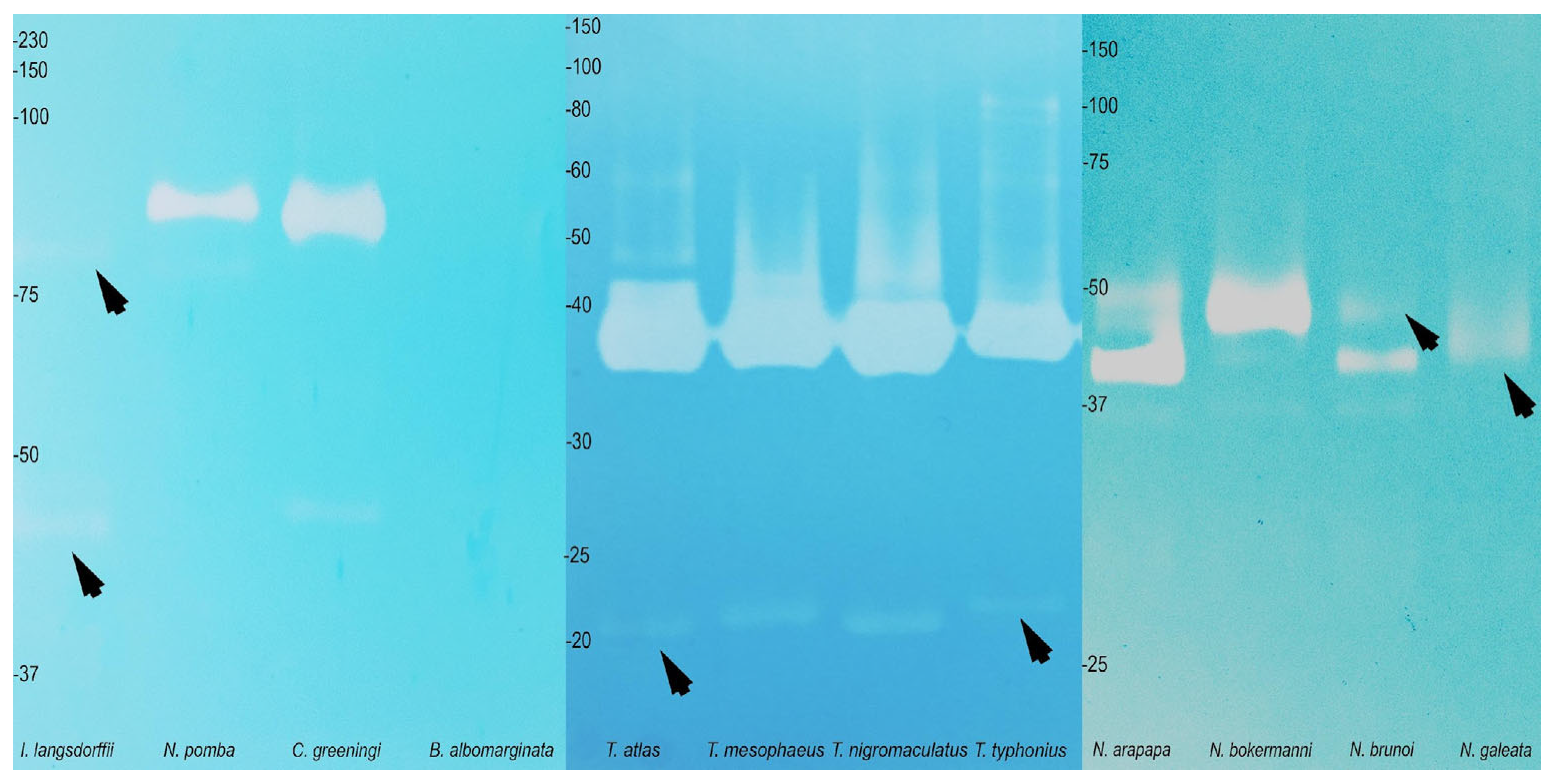
2.3. Biological Activity and Biochemistry of the Cutaneous Poison
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Fieldwork
5.2. Cranial Anatomy
5.3. Histology
5.4. Collection of Cutaneous Secretion
5.5. Cytotoxic Activity
5.6. Polyacrylamide Gel Electrophoresis in the Presence of Sodium Dodecyl Sulfate (SDS-PAGE)
5.7. Zymography
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADS | Attack and Defence System |
| SDS-PAGE J | Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate |
References
- Arbuckle, K. Evolutionary Context of Venom in Animals. Evol. Venom. Anim. Their Toxins 2015, 24, 3–31. [Google Scholar] [CrossRef]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The Diversity of Venom: The Importance of Behaviour and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Jared, C.; Luiz Mailho-Fontana, P.; Maria Antoniazzi, M. Differences between Poison and Venom: An Attempt at an Integrative Biological Approach. Acta Zool. 2021, 102, 337–350. [Google Scholar] [CrossRef]
- Toledo, R.C.; Jared, C. Cutaneous Granular Glands and Amphibian Venoms. Comp. Biochem. Physiol. Part A Physiol. 1995, 111, 1–29. [Google Scholar] [CrossRef]
- Jared, C.; Antoniazzi, M.M.; Jordão, A.E.C.; Silva, J.R.M.C.; Greven, H.; Rodrigues, M.T. Parotoid Macroglands in Toad (Rhinella jimi): Their Structure and Functioning in Passive Defence. Toxicon 2009, 54, 197–207. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Sciani, J.M.; Pimenta, D.C.; Barbaro, K.C.; Jared, C. Morphological and Biochemical Characterization of the Cutaneous Poison Glands in Toads (Rhinella marina Group) from Different Environments. Front. Zool. 2018, 15, 46. [Google Scholar] [CrossRef]
- Daly, J.W. The Chemistry of Poisons in Amphibian Skin. Proc. Natl. Acad. Sci. USA 1995, 92, 9–13. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Lourenço-de-Moraes, R.; Zocca, C.; Duca, C.; Beard, K.H.; Brodie, E.D. Antipredator Mechanisms of Post-Metamorphic Anurans: A Global Database and Classification System. Behav. Ecol. Sociobiol. 2019, 73, 69. [Google Scholar] [CrossRef]
- Regis-Alves, E.; Jared, S.G.S.; Maurício, B.; Mailho-Fontana, P.L.; Antoniazzi, M.M.; Fleury-Curado, M.C.; Brodie, E.D.; Jared, C. Structural Cutaneous Adaptations for defence in Toad (Rhinella icterica) Parotoid Macroglands. Toxicon 2017, 137, 128–134. [Google Scholar] [CrossRef]
- Albuquerque-Pinna, J.; Jeckel, A.M.; Nakamura, D.Y.M.; Bernarde, P.S.; Kocheff, S.; Saporito, R.A.; Grant, T. Defensive Alkaloid Variation and Palatability in Sympatric Poison Frogs. Chemoecology 2024, 34, 83–94. [Google Scholar] [CrossRef]
- Smith, K.E.; Halpin, C.G.; Rowe, C. Body Size Matters for Aposematic Prey during Predator Aversion Learning. Behav. Process. 2014, 109, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.K.; Corbit, A.G.; Hayes, W.K. Poisons, Toxungens, and Venoms: Redefining and Classifying Toxic Biological Secretions and the Organisms That Employ Them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.K.; Gren, E.C.K.; Nelsen, D.R.; Corbit, A.G.; Cooper, A.M.; Fox, G.A.; Streit, M.B. It’s a Small World After All: The Remarkable but Overlooked Diversity of Venomous Organisms, with Candidates Among Plants, Fungi, Protists, Bacteria, and Viruses. Toxins 2025, 17, 99. [Google Scholar] [CrossRef]
- Brodie, E.D.B., Jr.; Nussbaum, R.A.; Digiovanni, M. Antipredator Adaptations of Asian Salamanders (Salamandridae). Herpetologica 1984, 40, 56–68. [Google Scholar]
- Heiss, E.; Natchev, N.; Salaberger, D.; Gumpenberger, M.; Rabanser, A.; Weisgram, J. Hurt Yourself to Hurt Your Enemy: New Insights on the Function of the Bizarre Antipredator Mechanism in the Salamandrid Pleurodeles waltl. J. Zool. 2009, 280, 156–162. [Google Scholar] [CrossRef]
- Jared, C.; Mailho-Fontana, P.L.; Antoniazzi, M.M.; Mendes, V.A.; Barbaro, K.C.; Rodrigues, M.T.; Brodie, E.D. Venomous Frogs Use Heads as Weapons. Curr. Biol. 2015, 25, 2166–2170. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Alexandre, C.; Pimenta, D.C.; Sciani, J.M.; Brodie, E.D.; Jared, C. Morphological Evidence for an Oral Venom System in Caecilian Amphibians. iScience 2020, 23, 101234. [Google Scholar] [CrossRef]
- Cajade, R.; Hermida, G.; Piñeiro, J.M.; Regueira, E.; Alcalde, L.; Fusco, L.S.; Marangoni, F. Multiple Anti-Predator Mechanisms in the Red-Spotted Argentina Frog (Amphibia: Hylidae). J. Zool. 2017, 302, 94–107. [Google Scholar] [CrossRef]
- Blotto, B.L.; Lyra, M.L.; Cardoso, M.C.S.; Trefaut Rodrigues, M.; Dias, I.R.; Marciano, E., Jr.; Dal Vechio, F.; Orrico, V.G.D.; Brandão, R.A.; Lopes de Assis, C.; et al. The Phylogeny of the Casque-Headed Treefrogs (Hylidae: Hylinae: Lophyohylini). Cladistics 2021, 37, 36–72. [Google Scholar] [CrossRef]
- Paluh, D.J.; Stanley, E.L.; Blackburn, D.C. Evolution of Hyperossification Expands Skull Diversity in Frogs. Proc. Natl. Acad. Sci. USA 2020, 117, 8554–8562. [Google Scholar] [CrossRef]
- Trueb, L. Evolutionary Relationships of Casque-Headed Tree Frogs with Co-Ossified Skulls (Family Hylidae). Univ. Kans. Publ. Mus. Nat. Hist. 1970, 18, 547–716. [Google Scholar] [CrossRef]
- Wheeler, W.M. The Physiognomy of Insects. Q. Rev. Biol. 1927, 2, 1–36. [Google Scholar] [CrossRef]
- Ebel, R.; Herrel, A.; Scheyer, T.M.; Keogh, J.S. Review of Osteoderm Function and Future Research Directions. J. Zool. 2024, 325, 1–24. [Google Scholar] [CrossRef]
- De Andrade, D.V.; Abe, A.S. Evaporative Water Loss and Oxygen Uptake in Two Casque-Headed Tree Frogs, Aparasphenodon brunoi and Corythomantis greeningi (Anura, Hylidae). Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.R. Amphibian Species of the World: An Online Reference, version 6.2; American Museum of Natural History: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Jared, C.; Antoniazzi, M.M.; Navas, C.A.; Katchburian, E.; Freymüller, E.; Tambourgi, D.V.; Rodrigues, M.T. Head Co-Ossification, Phragmosis and Defence in the Casque-Headed Tree Frog Corythomantis greeningi. J. Zool. 2005, 265, 1–8. [Google Scholar] [CrossRef]
- Neto, A.M.; Teixeira, M., Jr. Checklist of the Genus Aparasphenodon Miranda-Ribeiro, 1920 (Anura: Hylidae): Distribution Map, and New Record from São Paulo State, Brazil. Check List 2012, 8, 1303–1307. [Google Scholar] [CrossRef]
- Ferro, J.M.; Cardozo, D.E.; Suárez, P.; Boeris, J.M.; Blasco-Zúñiga, A.; Barbero, G.; Gomes, A.; Gazoni, T.; Costa, W.; Nagamachi, C.Y.; et al. Chromosome Evolution in Cophomantini (Amphibia, Anura, Hylinae). PLoS ONE 2018, 13, e0192861. [Google Scholar] [CrossRef]
- Faivovich, J.; Pereyra, M.O.; Luna, M.C.; Hertz, A.; Blotto, B.L.; Vásquez-Almazán, C.R.; McCranie, J.R.; Sánchez, D.A.; Baêta, D.; Araujo-Vieira, K.; et al. On the Monophyly and Relationships of Several Genera of Hylini (Anura: Hylidae: Hylinae), with Comments on Recent Taxonomic Changes in Hylids. South Am. J. Herpetol. 2018, 13, 1–32. [Google Scholar] [CrossRef]
- Licht, L.E.; Sever, D.M. Structure and Development of the Parotoid Gland in Metamorphosed and Neotenic Ambystoma gracile. Copeia 1993, 1, 116–123. [Google Scholar] [CrossRef]
- Pimenta, B.V.S.; Napoli, M.F.; Haddad, C.F.B. A New Species of Casque-Headed Tree Frog, Genus Aparasphenodon Miranda-Ribeiro (Amphibia: Anura: Hylidae), from the Atlantic Rainforest of Southern Bahia, Brazil. Zootaxa 2009, 2123, 46–54. [Google Scholar] [CrossRef]
- Jared, C.; Antoniazzi, M.M.; Katchburian, E.; Toledo, R.C.; Freymüller, E. Some Aspects of the Natural History of the Casque-Headed Tree Frog Corythomantis greeningi Boulenger (Hylidae). Ann. Sci. Nat. 1999, 20, 105–115. [Google Scholar] [CrossRef]
- Toledo, L.F.; Sazima, I.; Haddad, C.F.B. Behavioural Defences of Anurans: An Overview. Ethol. Ecol. Evol. 2011, 23, 1–25. [Google Scholar] [CrossRef]
- Assis, C.D.; Santana, D.J.; Da Silva, F.A.; Quintela, F.M.; Feio, R.N. A New and Possibly Critically Endangered Species of Casque-Headed Tree Frog Aparasphenodon Miranda-Ribeiro, 1920 (Anura, Hylidae) from Southeastern Brazil. Zootaxa 2013, 3716, 583–591. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. The Systematic Status and Relationships of the Hylid Frog Nyctimantis rugiceps Boulenger. Occas. Pap. Mus. Nat. Hist. Univ. Kans. 1976, 58, 1–14. [Google Scholar]
- Lantyer-Silva, A.S.F.; Solé, M.; Zina, J. Reproductive Biology of a Bromeligenous Frog Endemic to the Atlantic Forest: Aparasphenodon arapapa Pimenta, Napoli and Haddad, 2009 (Anura: Hylidae). An. Acad. Bras. Cienc. 2014, 86, 867–880. [Google Scholar] [CrossRef]
- Cisneros-Heredia, D.F. Notes on the Natural History of the Casque-Headed Treefrog Trachycephalus jordani (Stejneger & Test, 1891). Herpetozoa 2007, 20, 92–94. [Google Scholar]
- Katchburian, E.; Antoniazzi, M.M.; Jared, C.; Faria, F.P.; Souza Santos, H.; Freymüller, E. Mineralized Dermal Layer of the Brazilian Tree-Frog Corythomantis greeningi. J. Morphol. 2001, 248, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Navas, C.A.; Jared, C.; Antoniazzi, M.M. Water Economy in the Casque-Headed Tree-Frog Corythomantis greeningi (Hylidae): Role of Behaviour, Skin, and Skull Skin Co-Ossification. J. Zool. 2002, 257, 525–532. [Google Scholar] [CrossRef]
- Toledo, R.C.; Jared, C. Cutaneous Adaptations to Water Amphibians Balance. Comp. Biochem. Pysiol. 1993, 105, 593–608. [Google Scholar] [CrossRef]
- Bala, E.; Hazarika, R.; Singh, P.; Yasir, M.; Shrivastava, R. A Biological Overview of Hyaluronidase: A Venom Enzyme and Its Inhibition with Plants Materials. Mater. Today Proc. 2018, 5, 6406–6412. [Google Scholar] [CrossRef]
- Bordon, K.C.F.; Wiezel, G.A.; Amorim, F.G.; Arantes, E.C. Arthropod Venom Hyaluronidases: Biochemical Properties and Potential Applications in Medicine and Biotechnology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 43. [Google Scholar] [CrossRef]
- Dias, I.R.; Silva, G.T.; Solé, M.; de Mira-Mendes, C.V. The Advertisement Call of the Rare Casque-Headed Frog Nyctimantis galeata (Anura: Hylidae) from Its Type Locality, Morro Do Chapéu, Bahia, Brazil. Zootaxa 2020, 4853, 447–450. [Google Scholar] [CrossRef]
- Zanette-Silva, L.; Lemos Farias, D.; Ghizoni, I.R. New Records of Aparasphenodon bokermanni (Pombal, 1993) from Santa Catarina, Southern Brazil, and Extension of Genus Range (Anura: Hylidae). Check List 2016, 12, 1–4. [Google Scholar] [CrossRef]
- Aggarwal, N.; Gupta, M.; Goyal, P.K.; Kaur, J. An Alternative Approach to Bone Cleaning Methods for Anatomical Purposes. Int. J. Anat. Res. 2016, 4, 2216–2221. [Google Scholar] [CrossRef][Green Version]
- Prado, G.M.; Pombal, J.P., Jr. Espécies de Proceratophrys Miranda-Ribeiro, 1920 com apêndices palpebrais (Anura; Cycloramphidae). Arq. Zool. 2008, 39, 1–85. [Google Scholar] [CrossRef]
- Cho, A.; Suzuki, S.; Hatakeyama, J.; Haruyama, N.; Kulkarni, A.B. A Method for Rapid Demineralization of Teeth and Bones. Open Dent. J. 2010, 4, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Fiala, J.C. Reconstruct: A Free Editor for Serial Section Microscopy. J. Microsc. 2005, 218, 52–61. [Google Scholar] [CrossRef]
- Conceição, K.; Konno, K.; Richardson, M.; Antoniazzi, M.M.; Jared, C.; Daffre, S.; Camargo, A.C.M.; Pimenta, D.C. Isolation and Biochemical Characterization of Peptides Presenting Antimicrobial Activity from the Skin of Phyllomedusa hypochondrialis. Peptides 2006, 27, 3092–3099. [Google Scholar] [CrossRef]
- Mossmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Heussen, C.; Dowdle, E.B. Electrophoretic Analysis of Plasminogen Activators in Polyacrylamide Gels Containing Sodium Dodecyl Sulfate and Copolymerized Substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Valdujo, P.H.; Camacho, A.; Recoder, R.S.; Teixeira Junior, M.; Ghellere, J.M.B.; Mott, T.; Nunes, P.M.S.; Nogueira, C.; Rodrigues, M.T. Anfíbios Da Estação Ecológica Serra Geral Do Tocantins, Região Do Jalapão, Estados Do Tocantins e Bahia. Biota Neotrop. 2011, 11, 251–261. [Google Scholar] [CrossRef]
- Godinho, L.B.; Moura, M.R.; Feio, R.N. New Records and Geographic Distribution of Corythomantis greeningi Boulenger, 1896 (Amphibia: Hylidae). Check List 2013, 9, 148–150. [Google Scholar] [CrossRef]
- de Freitas, M.A.; Abegg, A.D.; Dias, I.R.; de Figueiredo Moraes, E.P. Herpetofauna from Serra Da Jibóia, an Atlantic Rainforest Remnant in the State of Bahia, Northeastern Brazil. Herpetol. Notes 2018, 11, 59–72. [Google Scholar]
- Arzabe, C.; Loebmann, D. Amphibia, Hylidae, Itapotihyla langsdorffii: Distribution Extension. Check List 2006, 2, 33–34. [Google Scholar] [CrossRef]
- Lingnau, R.; Zank, C.; Colombo, P.; Vinciprova, G. Amphibia, Hylidae, Itapotihyla langsdorffii: Distribution Extension. Check List 2006, 2, 38–39. [Google Scholar] [CrossRef]
- Airaldi-Wood, K.; Molas, L.P.; González-Soria, L. First Record of Itapotihyla langsdorffii for Caaguazu Department (Paraguay) and Observations on Another Endemic Species of The Atlantic Forest. Kempffiana 2021, 17, 22–29. [Google Scholar]
- Lourenço-de-Moraes, R.; Lantyer-Silva, A.S.F.; Toledo, L.F.; Solé, M. Tadpole, Oophagy, Advertisement Call, and Geographic Distribution of Aparasphenodon arapapa Pimenta, Napoli and Haddad 2009 (Anura, Hylidae). J. Herpetol. 2013, 47, 575–579. [Google Scholar] [CrossRef]
- Pombal, J.P. New Species of Aparasphenodon (Anura: Hylidae) from Southeastern Brazil. Copeia 1993, 1993, 1088–1091. [Google Scholar] [CrossRef]
- Vilela, V.M.d.F.N.; Brassaloti, R.A.; Bertoluci, J. Anurofauna Da Floresta de Restinga Do Parque Estadual Da Ilha Do Cardoso, Sudeste Do Brasil: Composição de Espécies e Uso de Sítios Reprodutivos. Biota Neotrop. 2011, 11, 83–93. [Google Scholar] [CrossRef]
- Ruas, D.S.; Mendes, C.V.d.M.; Del-Grande, M.L.; Solé, M. Aparasphenodon brunoi Miranda-Ribeiro, 1920 (Anura: Hylidae): Distribution Extension and Geographic Distribution Map for Bahia State, Brazil. Check List 2013, 9, 858–859. [Google Scholar] [CrossRef]
- Pombal, J.P.; Menezes, V.A.; Fontes, A.F.; Nunes, I.; Rocha, C.F.D.; van Sluys, M. A Second Species of the Casque-Headed Frog Genus Corythomantis (Anura: Hylidae) from Northeastern Brazil, the Distribution of C. greeningi, and Comments on the Genus. Bol. Mus. Nac. 2012, 530, 1–14. [Google Scholar]
- Magalhães, F.D.M.; Marques, R.; Dos Santos, D.F.; De Magalhães, R.F.; Pezzuti, T.L. Tadpole of Nyctimantis galeata (Anura: Hylidae: Lophyohylini), a Narrow Endemic Casque-Headed Frog from Bahia, Brazil. Zootaxa 2021, 4948, 295–300. [Google Scholar] [CrossRef]
- Assis, C.; Feio, R.N. Aparasphenodon pomba. MG Biota 2017, 10, 49–52. [Google Scholar]
- Bokermann, W.C.A. Una Nueva Especie de Trachycephalus de Bahia, Brasil (Amphibia, Hylidae). Neotropica 1966, 12, 120–124. [Google Scholar]
- Magalhães, A.J.C., Jr.; Barbosa, A.R.; de Azevedo, S.M., Jr.; Pinho, M.S.; Beltrão, M.G.; Alves, B.C.F. Amphibia, Anura, Hylidae, Trachycephalus atlas Bokermann, 1966: Novo Registro e Comentários Sobre a Distribuição Geográfica. Rev. Nord. Zool. 2011, 5, 53–57. [Google Scholar]
- Roberto, I.J.; Ribeiro, S.C.; Bezerra, L.; Carneiro, P.B.M. Amphibia, Anura, Hylidae, Trachycephalus atlas Bokermann, 1966: Distribution Extension and Geographic Distribution Map. Check List 2011, 7, 326–327. [Google Scholar] [CrossRef]
- Santana, D.; Almeida, R.P.S.; Caldas, F.L.S.; Dias, E.J.D.R.; Faria, R. New Records of Trachycephalus mesophaeus (Hensel, 1867) (Anura: Hylidae) from Atlantic Forest in Sergipe State, Brazil. Herpetol. Notes 2016, 9, 255–260. [Google Scholar]
- Gondim-Silva, F.A.T.; Andrade, A.R.S.; Abreu, R.O.; Nascimento, J.S.; Corrêa, G.P.; Menezes, L.; Trevisan, C.C.; Camargo, S.S.; Napoli, M.F. Composition and Diversity of Anurans in the Restinga of the Conde Municipality, Northern Coast of the State of Bahia, Northeastern Brazil. Biota Neotrop. 2016, 16, e20160157. [Google Scholar] [CrossRef]
- Dias, I.R.; Vilaça, T.R.A.; Silva, J.R.d.S.; Barbosa, R.S.; Solé, M. Amphibia, Anura, Hylidae, Trachycephalus nigromaculatus Tschudi, 1838: Distribution Extension. Check List 2010, 6, 412–413. [Google Scholar] [CrossRef]
- Soares, M.D.L.; Iop, S.; Santos, T.G. dos Expansion of the Geographical Distribution of Trachycephalus typhonius (Linnaeus, 1758) (Anura: Hylidae): First Record for the State of Rio Grande Do Sul, Brazil. Check List 2012, 8, 817–818. [Google Scholar] [CrossRef]
- Miranda, D.B.; Venâncio, N.M.; de Albuquerque, S. Rapid Survey of the Herpetofauna in an Area of Forest Management in Eastern Acre, Brazil. Check List 2014, 10, 893–899. [Google Scholar] [CrossRef]
- Campião, K.M.; da Silva, I.C.O.; Dalazen, G.T.; Paiva, F.; Tavares, L.E.R. Helminth Parasites of 11 Anuran Species from the Pantanal Wetland, Brazil. Comp. Parasitol. 2016, 83, 92–100. [Google Scholar] [CrossRef]
- de Araújo-Silva, C.M.; de Castro Araújo, K.; Ávila, R.W.; Andrade, E.B. Unveiling the Diet Composition of Trachycephalus typhonius (Linnaeus, 1758) from a Relictual Mountain in Brazilian Semiarid. Biol. Bull. 2024, 51, 1181–1189. [Google Scholar] [CrossRef]
- Lopes, P.d.N.; Varago, A.; Portilho, J.; Santana, D.J.; Mângia, S. Release Call of Trachycephalus typhonius (Anura, Hylidae) in the Cerrado, Central-Western Brazil. J. Vertebr. Biol. 2024, 73, 24052. [Google Scholar] [CrossRef]

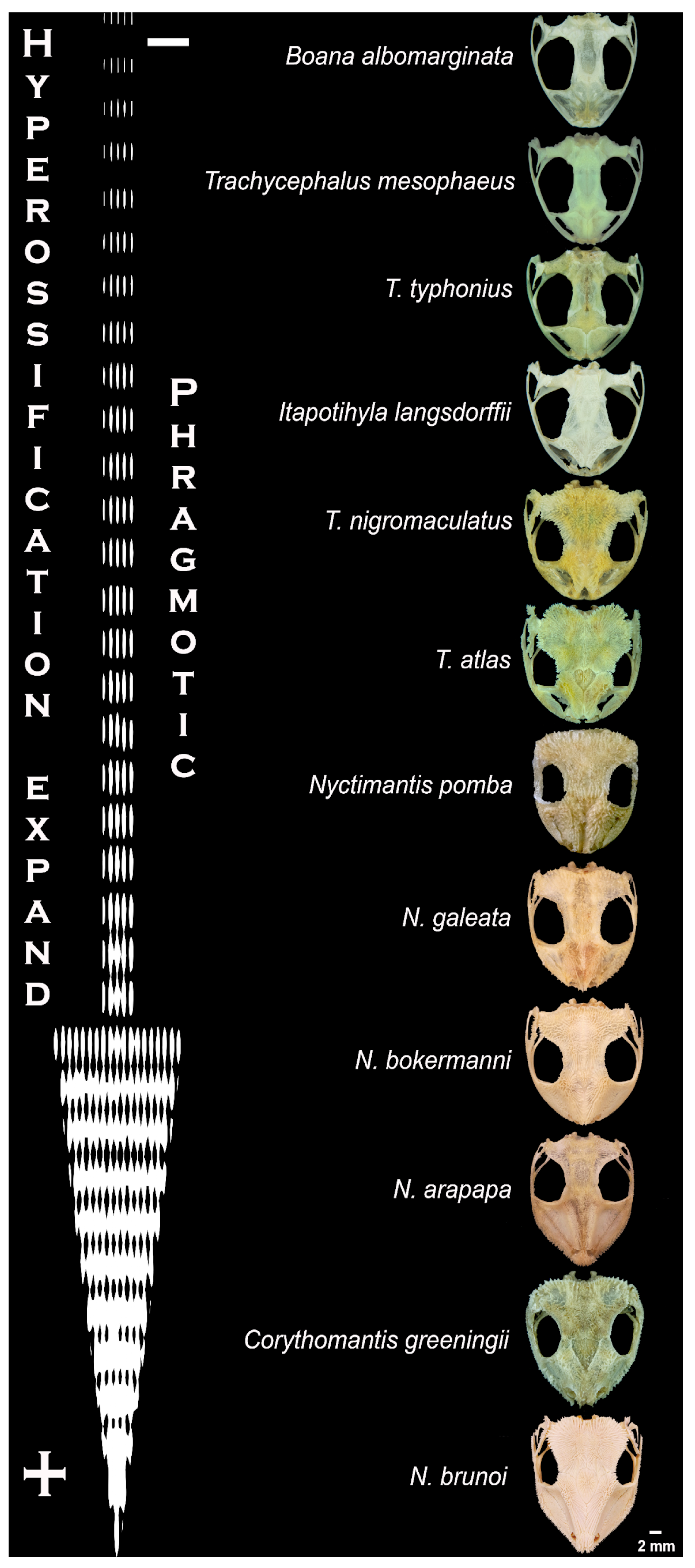

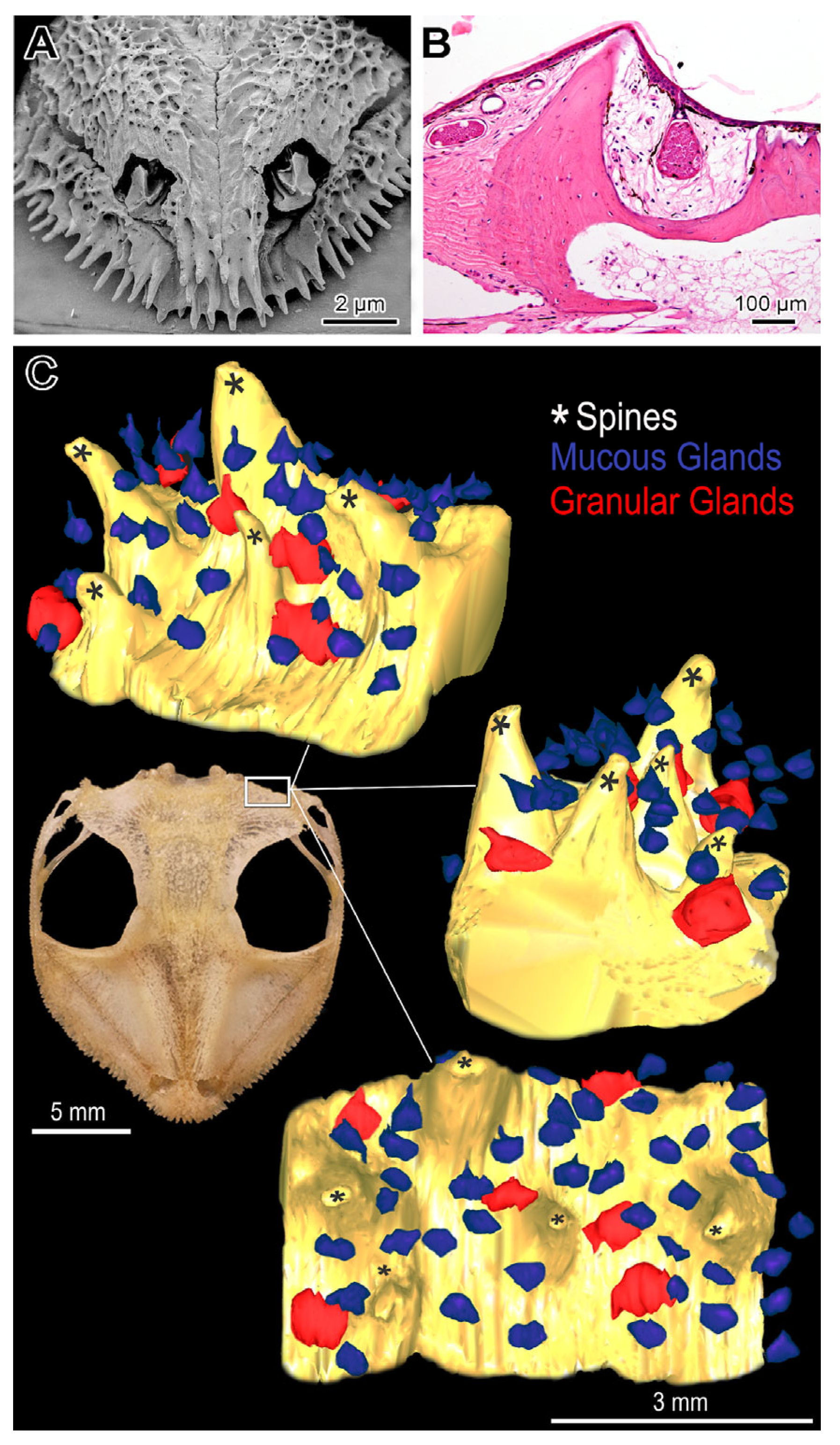
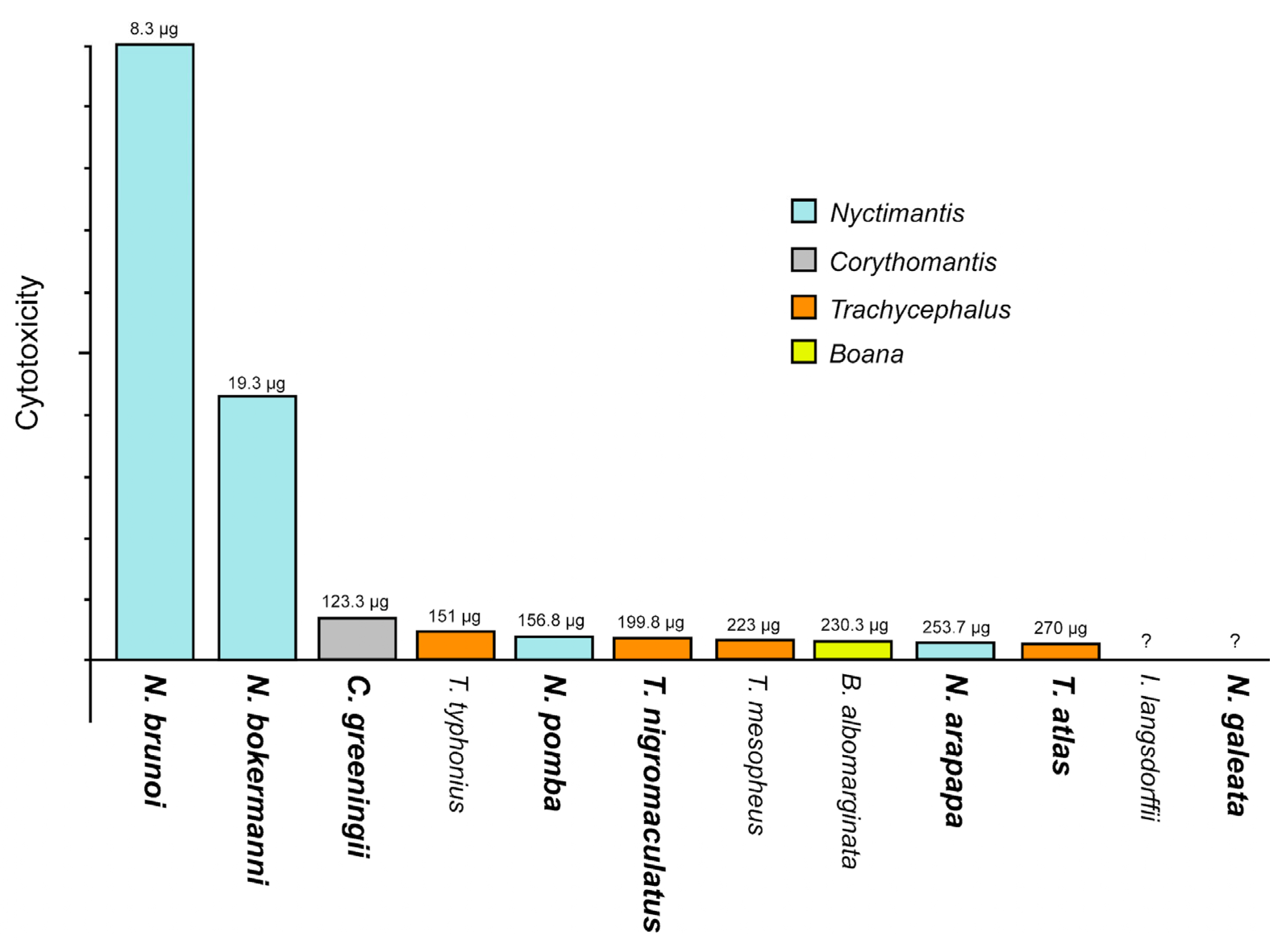
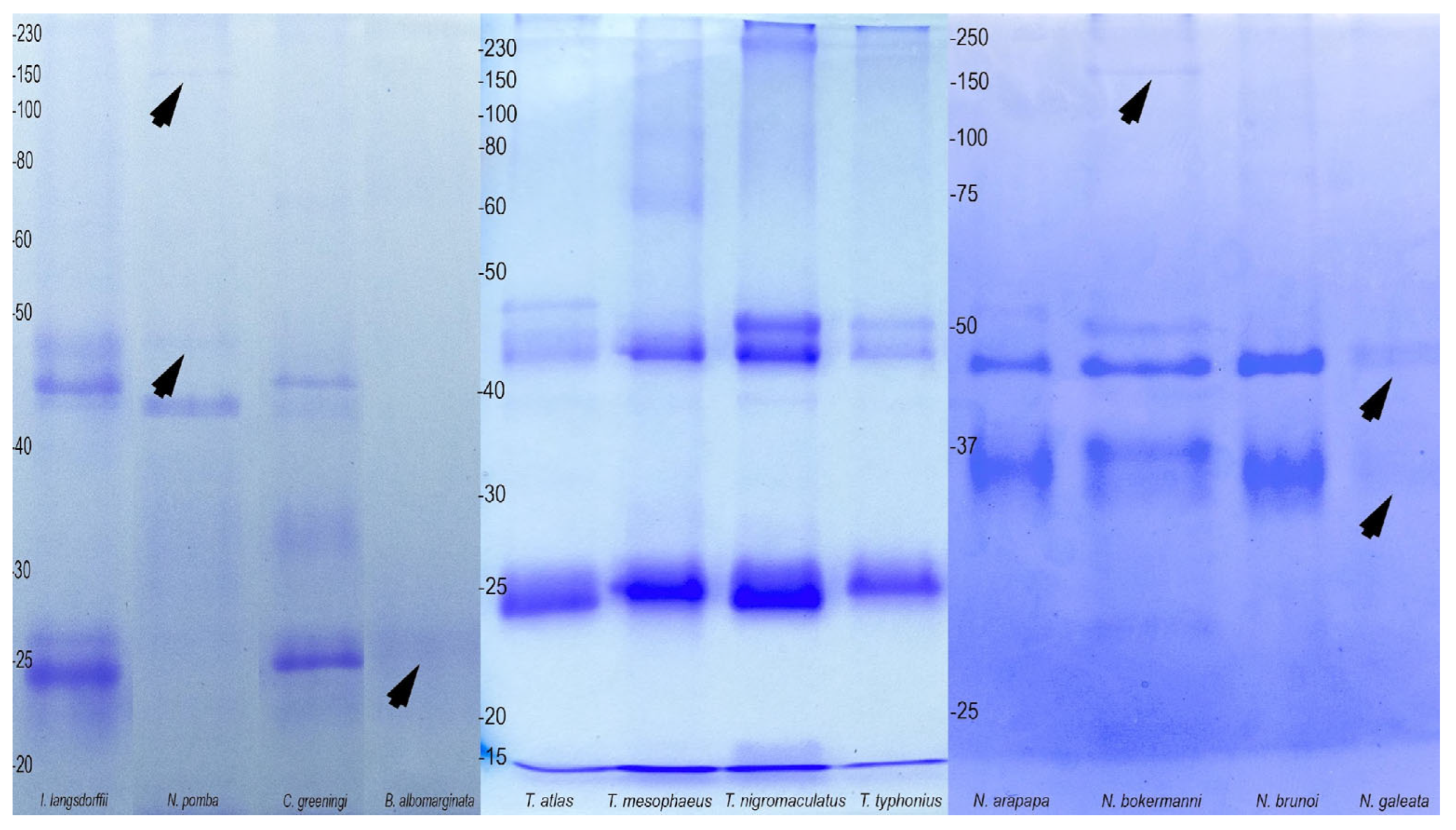
| Species | Biome | Microenvironment | Phragmosis | Head Attack | Cranial Spines |
|---|---|---|---|---|---|
| Boana albomarginata | Atlantic Rainforest * | Not specific | No | No | No |
| Corythomantis greeeningii | Caatinga | Rock crevices | Yes | Yes | Yes |
| Itapotihyla langsdorffii | Atlantic Rainforest * | Not specific | No | No | No |
| Nyctimantis arapapa | Atlantic Rainforest | Bromeliads | Yes | Yes | Yes |
| N. bokermanni | Atlantic Rainforest * | Bromeliads | Yes | Yes | Yes |
| N. brunoi | Atlantic Rainforest * | Bromeliads/tree holes | Yes | Yes | Yes |
| N. galeata | Caatinga | Bromeliads | Yes | Yes | Yes |
| N. pomba | Atlantic Rainforest | Bamboo | Yes | Yes | Yes |
| Trachycephalus atlas | Caatinga | Bromeliads/palm trees | Yes | Yes | Yes |
| T. mesophaeus | Atlantic Rainforest | Not specific | No | No | No |
| T. nigromaculatus | Atlantic Rainforest | Bromeliads/tree holes | Yes | Yes | Yes |
| T. typhonius | Savannah | Not specific | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandre, C.; Mailho-Fontana, P.L.; Távora, B.C.L.F.; Antoniazzi, M.M.; Jared, C. Defence Against Desiccation and Predation in Lophyohylini Casque-Headed Tree Frogs. Toxins 2025, 17, 303. https://doi.org/10.3390/toxins17060303
Alexandre C, Mailho-Fontana PL, Távora BCLF, Antoniazzi MM, Jared C. Defence Against Desiccation and Predation in Lophyohylini Casque-Headed Tree Frogs. Toxins. 2025; 17(6):303. https://doi.org/10.3390/toxins17060303
Chicago/Turabian StyleAlexandre, César, Pedro L. Mailho-Fontana, Bianca C. L. F. Távora, Marta M. Antoniazzi, and Carlos Jared. 2025. "Defence Against Desiccation and Predation in Lophyohylini Casque-Headed Tree Frogs" Toxins 17, no. 6: 303. https://doi.org/10.3390/toxins17060303
APA StyleAlexandre, C., Mailho-Fontana, P. L., Távora, B. C. L. F., Antoniazzi, M. M., & Jared, C. (2025). Defence Against Desiccation and Predation in Lophyohylini Casque-Headed Tree Frogs. Toxins, 17(6), 303. https://doi.org/10.3390/toxins17060303





