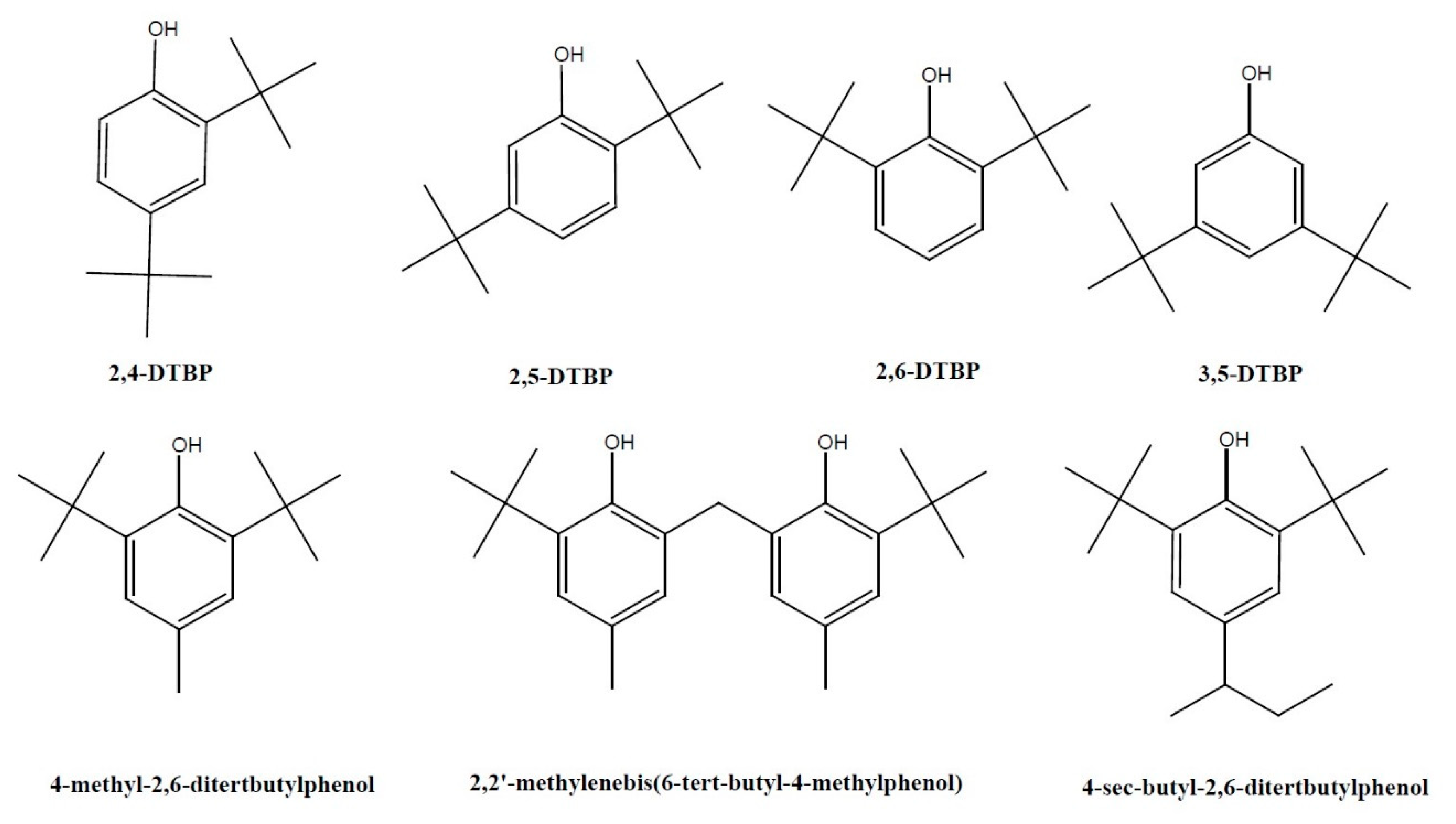
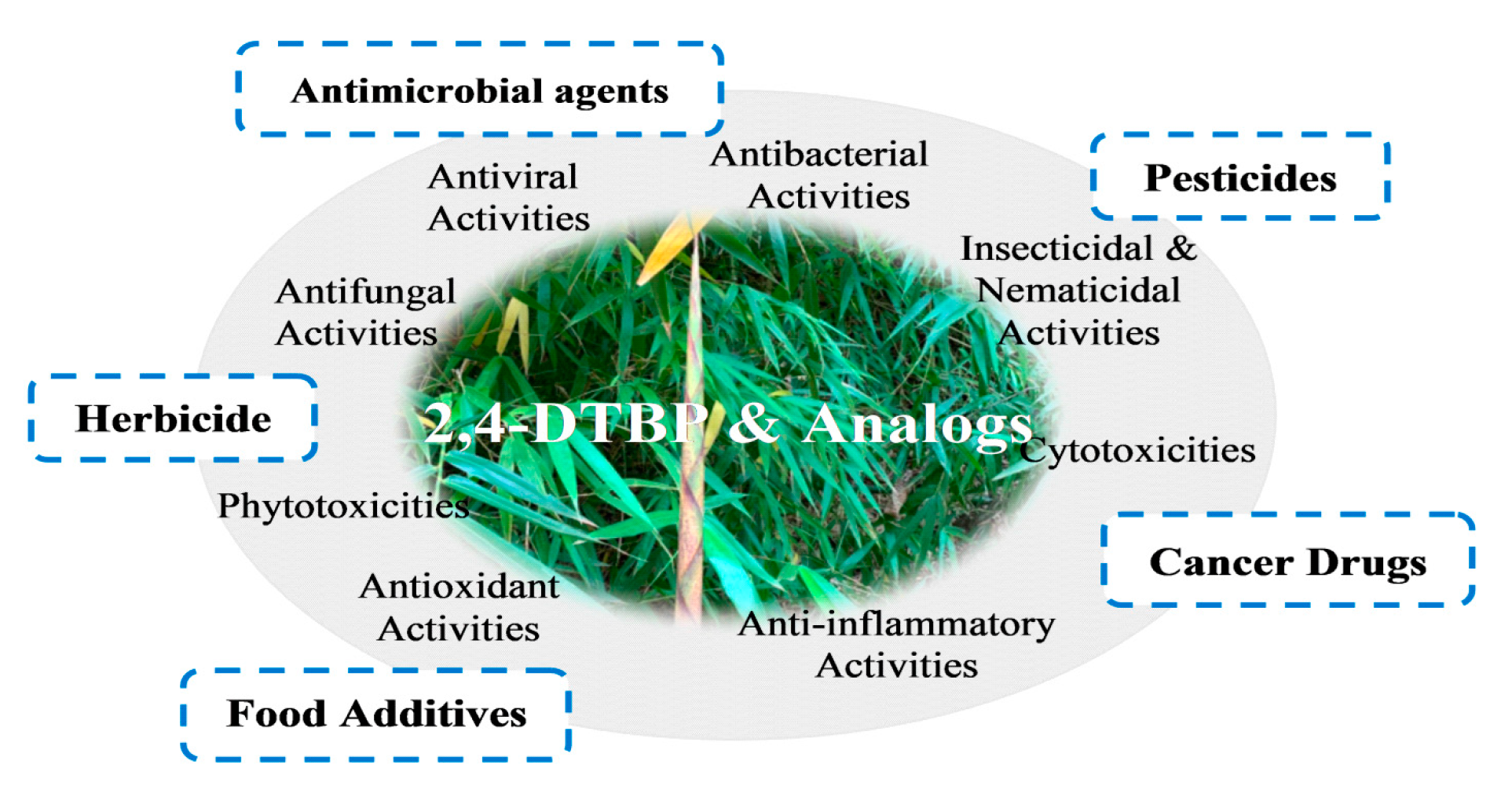
Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs
Abstract
1. Introduction
2. Natural Sources
3. Antioxidant Activities
4. Anti-Inflammatory Activities
5. Cytotoxicities
6. Insecticidal and Nematicidal Activities
7. Antibacterial Activities
8. Antiviral Activity
9. Antifungal Activities
10. Phytotoxicity: Allelopathy and Autotoxicity
11. Conclusions
| Family | Biosource | Tissues | Ref. |
|---|---|---|---|
| Bacteria | |||
| Bacillaceae | Bacillus licheniformis | [2] | |
| B. subtilis Ehrenberg | [3] | ||
| Flavobacteriaceae | Flavobacterium johnsoniae (Stanier) Bernardet et al. | [8,9] | |
| Microcystaceae | Microcystis aeruginosa Kützing | [12] | |
| Arthrobacter sp. | [4] | ||
| Nostocaceae | Nostoc spp. | [136] | |
| Anabaena oryzae F.E. Fritsch A. azotica Ley | [136] | ||
| Paenibacillaceae | Paenibacillus polymyxa (Prazmowski) Ash et al. | [137] | |
| Pseudomonadaceae | Pseudomonas monteilii Elomari et al. | [10] | |
| Shewanellaceae | Shewanella algae Simidu et al. | [11] | |
| Streptococcaceae | Lactococcus sp. | Cell-free supernatant | [6] |
| Streptomycetaceae | Streptomyces globosus Waksman | [4] | |
| S. mutabilis Pridham et al. | [7] | ||
| Vibrionaceae | Vibrio alginolyticus Miyamoto et al. | Cell-free culture supernatant | [13] |
| Fungi | |||
| Agaricaceae | Agaricus bisporus (J.E. Lange) Imbach | [14] | |
| Bionectriaceae | Gliomastix murorum (Corda) S. Hughes | [17] | |
| Glomerellaceae | Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. | [22] | |
| Nectriaceae | Fusarium tricinctum (Corda) Saccardo | [23] | |
| Omphalotaceae | Lentinus edodes (Berk.) Pegler | Caps and stipes | [15] |
| Polyporaceae | Trametes suavelens (L.) Fr. | [16] | |
| Tremellaceae | Cryptococcus albidus (Saito) Skinner | Cell-free extract | [24] |
| Trichocomaceae | Aspergillus terreus (Thom) | [18] | |
| Didymium iridis (Ditmar) Fr. | [138] | ||
| Penicillium flavigenum Frisvad & Samson | Cells | [20] | |
| Penicillium sp. | Culture | [21] | |
| Diatom | |||
| Phaeodactylaceae | Phaeodactylum tricornutum Bohlin | Cells | [25] |
| Liverwort | |||
| Marchantiaceae | Marchantia polymorpha L. | Whole thallus | [26] |
| Pteridophyta | |||
| Osmundaceae | Osmunda regalis L. | [27] | |
| Pteridaceae | Adiantum venustum D. Don | [28] | |
| Gyumnasperms | |||
| Pinaceae | Pinus kesiya var. langbianensis (A.chev.) Gavssen. | Cones | [31] |
| P. massoniana Lamb. | Rhizosphere soil | [33] | |
| P. tabulaeformis Carr. | Needles | [139] | |
| P. yunnanensis Franch. | Cones and bark | [129,140] | |
| Dicots | |||
| Amaryllidaceae | Allium fistulosum L. | Root exudates | [141] |
| Apiaceae | Anethum graveolens L. | [142] | |
| Centella asiatica (L.) Urban | Leaves | [143] | |
| Araliaceae | Panax quinquefolius L. | Leaves and roots | [144] |
| Asclepiadaceae | Metaplexis japonica (Thunb.) Makino | Seeds | [60] |
| Asteraceae | Acroptilon repens (L.) D.C. | Aerial part | [145] |
| Artemisia annua L. | Leaves | [34] | |
| A. apiacea Hance | |||
| A. japonica Thunb. | |||
| A. capillaris Thunb. | |||
| A. argyi H.Lév. & Vaniot | |||
| A. eriopoda Bunge | |||
| A. tschernieviana Besser | Aerial parts | [146] | |
| Atractylodes coreana (Nakai) Kitam | Rhizomes | [147] | |
| A. macrocephala Koidz | Rhizomes | [132] | |
| Chrysanthemum indicum L. | Leaves, stem, rot exudates, and rhizosphere soils | [63] | |
| Gynura cusimbua (D. Don) S. Moore | Aerial parts | [148] | |
| Xanthium sibiricum Patr. | Fruits and aerial parts | [149] | |
| Begoniaceae | Begonia malabarica Lam. | Fresh plants | [150] |
| Boraginaceae | Heliotropium indicum L. | Aerial parts | [151] |
| Brassicaceae | Brassica oleracea var. capitata F. Rubra | Leaves | [152] |
| B. napus L. | Seeds | [153] | |
| Cactaceae | Pereskia bleo (Kunth) de Candolle | Leaves | [154] |
| Caeselpiniaceae | Bauhininia variegata (L.) Benth. | Leaves | [155] |
| Calycanthaceae | Chimonanthus Lindl. | [156] | |
| C. praecox (L.) Link. | Leaves | [82] | |
| C. zhejiangensis M.C. Liu | |||
| C. salicifolius S.Y. Hu | |||
| C. nittens Oliv. | |||
| C. grammatus M.C. Liu | |||
| C. campanulatus R.H. | |||
| Cannabaceae | Humulus lupulus L. | Rhizosphere soils | [131] |
| Capparaceae | Crateva religiosa G. Forst. | Stems | [157] |
| Caprifoliaceae | Lonicera maackii (Rupr.) Maxim. | Fruits | [64] |
| Caricaceae | Carica papaya L. | Seeds | [158] |
| Caryophyllaceae | Spergularia marina (L.) Besser | Aerial part | [159] |
| Combretaceae | Terminalia travancorensis Wight & Arn. | Bark | [160] |
| Convolvulaceae | Ipomoea batatas (L.) Lam. | Tubers | [97] |
| Cornaceae | Cornus officinalis Sieb. Et Zucc. | Fruits | [161] |
| Cucurtibitaceae | Cucurbita moschata (Duch. ex Lam.) Duch. ex Poiret | Fruits | [56] |
| Crassulaceae | Rhodiola imbricata Edgew. | Roots | [162] |
| Equisetaceae | Equisetum arvense L. | Whole plant | [163] |
| Ericaceae | Rhododendron dauricum L. | Leaves | [48] |
| Euphorbiaceae | Croton bonplandianum Baill | Leaves | [164] |
| Phyllanthus debilis Klein ex Willd. | Leaves | [165] | |
| Sauropus rostratus Miq. | Leaves | [55] | |
| Fabaceae | Albizia julibrissin Durazz | Leaves and stems | [49] |
| Dalbergia odorifera T. Chen | Wood | [166] | |
| Humboldtia unijuga Bedd. | Roots | [103] | |
| Glycine max (L.) Merr | Root secretion | [167] | |
| Mucuna pruriens (L.) DC. | Seeds | [168] | |
| Vigna radiata (L.) R. Wilczek | Seeds | [169] | |
| Gentianaceae | Gentiana apiata N. E. Br. | Whole plants | [46] |
| G. tibetica King ex J.D. Hooker | Flowers | [170] | |
| Hydrocharitaceae | Hydrilla verticillata (L.f.) Royle | Exudates | [171] |
| Juglandaceae | Juglans regia L. | Root exudates | [172] |
| Lamiaceae | Sphenodesme involucrata var. paniculata (C. B. Clarke) Munir | Leaves | [173] |
| Perilla frutescens (L.) Britton | Leaves | [174] | |
| Salvia miltiorrhiza Bunge | Leaves and roots | [175] | |
| Lauraceae | Cinnamomum longepaniculatum (Gamble) N. Chao ex H. W. Li | Leaves | [176] |
| C. loureirii Nees | Bark | [177] | |
| Lindera aggregata (Sims) Kosterm | Roots | [178] | |
| L. angustifolia (W. C. Cheng) Nakai. L. rubronervia (Gamble) Rehder. | Xylem | [179] | |
| Persea americana Mill. | Roots | [120] | |
| Loranthaceae | Loranthus micranthus L. | Fresh leaves | [180] |
| L. pentapetalus Roxb. | Leaves | [181] | |
| Viscum ovalifolium Wallich ex Candolle | Leaves | [181] | |
| Malvaceae | Cola nitida (Vent.) Schott & Endl. | Fruits | [182] |
| Melastomataceae | Memecylon umbellatum Burm. f | Leaves | [183] |
| Menispermaceae | Tinospora cordifolia (Willd.) Hook. f. & Thoms. | Embryogenic callus | [184] |
| Myrtaceae | Eucalyptus globulus L. | Leaves | [185] |
| E. grandis W. Hill ex Maiden | Root | [186] | |
| Eugenia dysenterica D.C. | Fruits | [187] | |
| Nelumbonaceae | Nelumbo nucifera Gaertn. | Rhizomes | [188] |
| Oleaceae | Olea europaea L. | Stems | [117] |
| Paeioniaaceae | Paeionia lactiflora Pall. | Root | [189] |
| Papaveraceae | Eomecon chionantha Hance | [67] | |
| Phyllanthaceae | Phyllanthus emblica L. | Fruits | [61] |
| Sauropus rostratus Miq. | Leaves | [55] | |
| Piperaceae | Piper nigrum L. | Seeds | [190] |
| Plumbaginaceae | Plumbago zeylanica L. | Roots | [191] |
| Polygonaceae | Calligonum polygonoides L. | Fruits and stems | [192] |
| Polygonum viscosum Buch-ham | Leaves | [193] | |
| Primulaceae | Lysimachia foenum-graecum Hance | [194] | |
| Ranunculaceae | Aconitum carmichaeli Dibx. | Root | [68] |
| Clematis connata D.C. | Whole plant | [195] | |
| Consolida regalis Gray | Stem and leaves | [196] | |
| Rosaceae | Chaenomeles sinensis C.K. Schneid. | Fruits | [197] |
| Prunus persica (L.) Batsch | Roots | [198] | |
| Rosa iberica Stev. | Hips | [199] | |
| Sibiraea angustata (Rehd.) Hand.-Mazz. | Infructescence | [54] | |
| Rubiaceae | Rubia cordifolia L. | Stems | [200] |
| Rutaceae | Zanthoxylum planispinum Sieb. et Zucc. | Litters | [201] |
| Nauclea diderrichii (De Wild. & T. Durand) Merrill | Leaves | [202] | |
| Sapindaceae | Koelreuteria paniculata Laxm. | Leaves | [203] |
| Saururaceae | Houttuynia cordata Thunb. | Aerial part | [66] |
| Scrophulariaceae | Verbascum phlomoides L. | Flowers | [204] |
| Solanaceae | Capsicum annuum L. | Root exudates | [133,205] |
| Solanum lycopersicum var. cerasiforme (Dunal) A.Gray | Fruits | [206] | |
| S. melongena L. | Root exudates | [207] | |
| Withania coagulans (Stocks) Dunal | Leaves and micropropagated plant | [208] | |
| Styracaceae | Sinojackia sarcocarpa L.Q. Lou | Drupes | [209] |
| Theaceae | Camellia sinensis (L.) Kuntze | Leaves | [210] |
| Thymelaeaceae | Aquilaria sinensis (Loureiro) Sprengel | Resin | [211] |
| Urticaceae | Boehmeria nivea (L.) Gaudich. | Rhizosphere soil | [77] |
| Urtica dioica L. | Leaves | [212] | |
| Violaceae | Viola betonicifolia Sm. | Whole plant | [213] |
| Vitaceae | Ampelopsis grossedentata (Hand.-Mazz.) W.T. Wang | [214] | |
| Monocots | |||
| Araceae | Amorphophallus campanulatus (Dennst.) Nicolson | Tuber | [215] |
| Arecaceae | Cocos nucifera L. (coconut) | Fruit juice | [216] |
| Commelinaceae | Murdannia nudiflora (L.) Brenan | Whole plant | [62] |
| Cyperaceae | Cyperus rotundus L. | Rhizomes | [217] |
| Heleocharis dulcis (Burm. f.) Trin. | Rhizomes | [136] | |
| Kyllinga triceps Rottbøll | [218] | ||
| Liliaceae | Lilium davidii var. willmottiae (E.H. Wilson) Raffill | Bulb | [134] |
| Musaceae | Musa spp. | Root | [219] |
| Orchidaceae | Dendrobium moniliforme (L.) Sw. | Flowers | [220] |
| Gastrodia elata Blume | Rhizomes | [125] | |
| Palmae | Phoenix canariensis Chabaud Washingtonia filifera (Lind.) H. Wendl. Phoenix roebelenii O’Brien | Leaves | [221] |
| Poaceae | Echinochloa crusgalli (L.) Beauv | Root exudates | [222] |
| Imperata cylindrica (L.) Beauv | Rhizome and root exudates | [123] | |
| Oryza sativa L. | Root exudate | [223] | |
| Pennisetum orientale Rich. | Aerial part | [47] | |
| Pennisetum purpureum Schumach. | Culm and leaves | [127,129] | |
| Phyllostachys pubescens (Pradelle) Mazel ex J. Houz. | Fresh parenchyma | [224] | |
| Sorghum bicolor (L.) Moench | Root exudate | [65] | |
| Spartina cynosuroides (L.) Roth | Fresh grass | [225] | |
| Triticum durum L. | Seeds | [226] | |
| Zingiberaceae | Zingiber cassumunar Roxb. | Rhizomes and leaves | [227] |
| Animals | |||
| Mantidae | Mantidis ootheca | Egg cases | [75] |
| Mycalidae | Zygomycale sp. | [71] | |
| Scolopendridae | Scolopendra subspinipes Leach | Dried bodies | [72] |
| Styelidae | Styela clava Herdman | [74] | |
| Tetranychidae | Tetranychus cinnabarinus (Boisduval) | [73] | |
| Bioactivities | Chemical Name | Experimental Model | Treatment Doses | Cellular and Molecular Targets | Ref. |
|---|---|---|---|---|---|
| Antioxidant Activities | 2,4-DTBP | TBARS assay | IC50: 8.20 mM | LDL-oxidation | [72] |
| Human plasma LDL | IC50: 9.9 mM | AAPH-mediated oxidation | [72] | ||
| Human plasma LDL | 5.0 mM | SIN-1-mediated oxidation | [72] | ||
| PheochromocytomPC12 cells and mice | 2–10 mg/100mL | Hydrogen-peroxide-induced oxidative stress | [97] | ||
| Mice injected with amyloid-beta peptide (Ab1-42) | 5–40 mg/kg | Alternation behavior | [97] | ||
| BHT | Ultra-oxygen-free radical | 600 mg/L | Radical scavenging | [101] | |
| Hydroxyl-free radical | 500 mg/L | Radical scavenging | [101] | ||
| Liver and serum of rat | 100-800 mg/L | MDA, SOD, and GSH-PX content | [101] | ||
| Anti-Inflammatory Activities | 2,4-DTBP | RAW264.7 mouse macrophage cell line | 50 and 100 µg/mL | TNF-α, IL-6, and IL-1b genes | [103] |
| BHT | RAW264.7 cells | 10 μM | Cox2 and TNF-α genes upon stimulation with Pg | [102] | |
| Cytotoxicities | 2,4-DTBP | HeLa cells | IC50 value of 10 μg/mL | Cytotoxicity | [6] |
| MCF-7 and A431 cell lines | 50 and 100 µg/mL | P53 and caspase 7 generation | [103] | ||
| Rats | 5 and 20 mg/kg/day | Respective no-observed-adverse-effect levels (NOAELs) | [104] | ||
| Uteri and vagina ovariectomized (OVX) CD1 mice | 10–250 mg/kg by oral treatment | Uterotrophic effect | [105] | ||
| BHT | 32P-labeled DNA fragments | 50–500 µM | DNA damage | [107] | |
| Small intestinal crypts of mice | Number of mitoses | [108] | |||
| HL-60 and HSC-2 cells | 0.2–0.3 mM | Manganese superoxide dismutase (MnSOD) and reverse transcriptase-polymerase chain reaction (PCR) | [109] | ||
| Insecticidal and Nematicidal Activities | 2,4-DTBP | Spider mite Tetranychus cinnabarinus | LC50 values of 1256.51, 625.39, and 743.64 ppm | Adulticidal, larvicidal, ovicidal, repellent, and oviposition-deterrent activities | [73] |
| Caenorhabditis elegans | 0.5–4 g/L | Nematicidal activity | [101] | ||
| BHT | Trogoderma variabile Ballion and Attagenus megatoma (F.) | 0.5 or 2.0% | Larvicidal and ovicidal activity | [111] | |
| Oryzaephilus surinamensis (L.), and Tribolium castaneum (Herbst) | 10–45 mM | Lethal insecticidal activity | [112] | ||
| A non-toxic aqueous pesticide | 1:10 to about 1:600 | Preservative treatment | [113] | ||
| Paranoplium gracile (Leconte) | 5% test solution | Stabilize a male-produced aggregation-sex pheromone | [114] | ||
| Female Monochamus alternatus | Repellent activity | [115] | |||
| Antibacterial Activities | 2,4-DTBP | Biofilm of Serratia marcescens | 250–300 µg/mL | Secreted etracellular polymeric substances, quorum sensing, and hydration of the cell wall | [13,116] |
| Pseudomonas aeruginosa and Staphylococcus aureus in pure and mixed culture | Antibacterial potency | [2] | |||
| Group A Streptococcus bacterium | 16–48 µg/mL | Antibiofilm activity | [3] | ||
| Antiviral Activity | 2,4-DTBP | Coxsackievirus B-3 (CVB-3) and herpes virus type 2 (HSV-2) | 6.32 ± 0.67 and 5.24 ± 0.82 | Antiviral activity | [117] |
| Antifungal Activities | 2,4-DTBP | Spore and hyphae growth of Fusarium oxysporum | 1–500 µg/mL | β-tubulin in microtubules | [10] |
| Phytophthora capsici | 100 µg/mL | Mycelial growth | [8] | ||
| Pepper seed infected by P. capsici | 1–100 g/mL | Radicle infection | [8] | ||
| Cladosporium fulvum | 0.1 mmol/L | Mycelium growth | [118] | ||
| Verticillium dahliae | 0.50 to 2.00 mmol/L | Mycelium growth | [119] | ||
| Aspergillus niger, F. oxysporum and Penicillium chrysogenum on wheat grains | 2 mg/25 mL | Fungal mycelial growth | [6] | ||
| Aspergillus | 5–200 µg/L | Mycelial growth and conidial germination ROS production | [11,120] | ||
| Biofilms of Candida albicans | 2.5–100 µg/mL | Hemolysins, phospholipases, and aspartyl proteinase | [121] | ||
| Allelopathy | 2,4-DTBP | Seed and seedling of Lactuca sativa var. ramosa Hort. and L. sativa L. | 0–0.10 mmol/L | Seed germination and seedling growth | [63] |
| Seed and seedling of of Bidens pilosa L. and Leucaena leucocaphala L. de Wit | 0.1 mg/mL | Root and shoot growth | [123] | ||
| Root and leaf tissues of Leptochloa chinensis (L.) Nees and Hedyotis verticillata (L.) Lam | 50 and 200 µg/mL | Lamina wilting and necrosis, and root and shoot growth | [122,126] | ||
| L. chinensis in soil | 0.60 kg a.i. ha−1 | Root growth | [127] | ||
| Leaf of weed plant | 2.5–100 µg/mL | Reactive oxygen species and chloroplasts | [121,128] | ||
| Seed and seedling Atractylodes macrocephala | 0.1, 1, and 10 mmol/L | Plant immune system | [132] | ||
| Rhizosphere soil of Litchi chinensis Sonn. | Abundance | [90] | |||
| Autotoxicity | 2,4-DTBP | Seed and seedling of of Imperata cylindrical (L.) | 0.1 mg/mL | Seed germination and growth | [123] |
| Seed and seedling of Masson′s pine | 0.25–1.0 mg/mL | Seed germination, seed viability, hypocotyl and radicle growth, and seedling growth | [33] | ||
| Microorganism in the rhizosphere soil of Hamulus lupulus L. | 7.5 and 15 mmol/m2 | Photosynthesis and growth of hop seedlings | [130,131] | ||
| Seed and seedling of of Brassica napus L., Echinochloa crus-galli (L.) Beauv | 0.1 mg/mL | Root and shoot growth | [123] | ||
| Seed and seedling of of Brassica napus L. | 0–0.10 mmol/L | Seed germination and seedling growth | [63] | ||
| Seed and seedling chilli pepper | More than 2 mmol/L | Seed germination and seedling growth | [133] | ||
| Seedling of eggplant | 0.10–1.00 mmol/L | Seedling growth | [104] | ||
| Bulb of Fusarium | Fusarium wilt in the lily | [134] | |||
| 2,5-DTBP | Boehmeria nivea | Soil sickness in the field | [77] |
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, D.H. Volatiles analysis of several nitrogen-fixing Cyanobacteria isolated from rice fields using gas chromatography-mass spectrometry. J. Anhui Agric. Sci. 2018, 46, 145–148. [Google Scholar]
- Aissaoui, N.; Mahjoubi, M.; Nas, F.; Mghirbi, O.; Arab, M.; Souissi, Y.; Hoceini, A.; Masmoudi, A.S.; Mosbah, A.; Cherif, A.; et al. Antibacterial potential of 2, 4-di-tert-butylphenol and calixarene-based prodrugs from thermophilic Bacillus licheniformis isolated in Algerian hot spring. Geomicrobiology 2019, 36, 53–62. [Google Scholar] [CrossRef]
- Viszwapriya, D.; Prithika, U.; Deebika, S.; Balamurugan, K.; Pandian, S.K. In vitro and in vivo antibiofilm potential of 2,4-di-tert-butylphenol from seaweed surface associated bacterium Bacillus subtilis against group A streptococcus. Microbiol. Res. 2016, 191, 19–31. [Google Scholar] [CrossRef]
- Akshatha, J.V.; Prakash, H.S.; Nalini, M.S. Actinomycete endophytes from the ethno medicinal plants of Southern India: Antioxidant activity and characterization studies. J. Biol. Act. Prod. Nat. 2016, 6, 166–172. [Google Scholar] [CrossRef]
- Zhang, D.J.; Gong, C.Y.; Wei, H.G.; Li, S.I.; Li, Y.G.; Shen, G.M. Chemical constituents of the culture broth of Paenibacillus polymyxa HY96-2. J. East China Univ. Sci. Technol. (Nat. Sci. Ed.) 2008, 34, 71–73. [Google Scholar]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef]
- Belghit, S.; Driche, E.H.; Bijani, C.; Zitouni, A.; Sabaou, N.; Badji, B.; Mathieu, F. Activity of 2,4-Di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J. Mycol. Med. 2016, 26, 160–169. [Google Scholar] [CrossRef]
- Sang, M.K.; Kim, K.D. The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper. J. Appl. Microb. 2012, 113, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.; Burher, E.M. Aphelenchoides xylophilus, n. sp, a nematode associated with blue stain and other fungi in timber. J. Agric. Res. 1934, 48, 949–951. [Google Scholar]
- Dharni, S.; Maurya, A.; Samad, A.; Srivastava, S.K.; Sharma, A.; Patra, D.D. Purification, characterization, and in vitro activity of 2,4-Di-tert-butylphenol from Pseudomonas monteilii PsF84: Conformational and molecular docking studies. J. Agric. Food Chem. 2014, 62, 6138–6146. [Google Scholar] [CrossRef]
- Gong, A.; Li, H.P.; Shen, L.; Zhang, J.B.; Wu, A.B.; He, W.J.; Yuan, Q.S.; He, J.D.; Liao, Y.C. The Shewanella algae strain YM8 produces volatiles with strong inhibition activity against Aspergillus pathogens and aflatoxins. Front. Microbiol. 2015, 6, 1091. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, S.B.; Xu, Z.X.; Han, X.; Yang, M.Z.; Chen, X.L. Response of the toxic cyanobacterium Microcystis aeruginosa to bacteria signaling molecules AHLs—Change of volatile components production. Adv. Mater. Res. 2013, 718, 255–260. [Google Scholar] [CrossRef]
- Padmavathi, A.R.; Abinaya, B.; Pandian, S.K. Phenol, 2,4-bis(1,1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen Serratia marcescens. Biofouling 2014, 30, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Comparative application of SDE and SPME for analysis of aroma-active compounds in button mushroom (Agaricus bisporus). Shipin Kexue 2015, 36, 185–188. [Google Scholar]
- Li, S.; Wang, A.; Liu, L.; Tian, G.; Wei, S.; Xu, F. Evaluation of nutritional values of shiitake mushroom (Lentinus edodes) stipes. J. Food Meas. Charact. 2018, 12, 2012–2019. [Google Scholar] [CrossRef]
- Zhao, Y. Study on the Biological Characteristics and Pharmacological Activities of Trametes Suavelens. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2018. [Google Scholar]
- Zhao, J.; Shan, T.; Huang, Y.; Liu, X.; Gao, X.; Wang, M.; Jiang, W.; Zhou, L. Chemical composition and in vitro antimicrobial activity of the volatile oils from Gliomastix murorum and Pichia guilliermondii, two endophytic fungi in Paris polyphylla var. yunnanensis. Nat. Prod. Commun. 2009, 4, 1491–1496. [Google Scholar]
- Lenartowicz, P.; Kafarski, P.; Lipok, J. The overproduction of 2,4-DTBP accompanying to the lack of available form of phosphorus during the biodegradative utilization of aminophosphonates by Aspergillus terreus. Biodegradation 2015, 26, 65–76. [Google Scholar] [CrossRef]
- Jiang, S.C.; Zhang, B.; Li, Y.S.; Li, D.H.; Li, Y. An analysis of volatile components in Didymium iridis by GLME/GC-MS. J. Fungal Res. 2017, 15, 255–258. [Google Scholar]
- Canturk, Z.; Kocabiyik, E.; Ozturk, N.; Ilhan, S. Evaluation of antioxidant and antiproliferative metabolites of Penicillium flavigenum isolated from hypersaline environment: Tuz (Salt) Lake by Xcelligence technology. Microbiology (Mosc. Russ. Fed.) 2017, 86, 346–354. [Google Scholar] [CrossRef]
- Kumar, K.M.; Poojari, C.C.; Ryavalad, C.; Lakshmikantha, R.Y.; Satwadi, P.R.; Vittal, R.R.; Melappa, G. Anti-diabetic activity of endophytic fungi, Penicillium species of Tabebuia argentea; in silico and experimental analysis. Res. J. Phytochem. 2017, 11, 90–110. [Google Scholar]
- Nameirakpam, N.D.; Singh, M.S. GC-MS analysis of metabolites from endophytic fungus Collectotrichum gloeosporiodes isolated from Phlogacanthus thyrsiflorus Nees. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 392–395. [Google Scholar]
- Shaukat, K.; Sffrasayab, S.; Hasnain, S. Growth responses of Triticum aestivum to plant growth promoting rhizobacteria used as a biofertilizer. Res. J. Microbiol. 2006, 1, 330–338. [Google Scholar]
- Hashem, M.; Alamri, S.A.; Hesham, A.E.; Alqahtani, F.M.H.; Kilany, M. Biocontrol of apple blue mould by new yeast strains: Cryptococcus albidus KKUY0017 and Wickerhamomyces anomalus KKUY0051 and their mode of action. Biocontrol. Sci. Technol. 2014, 24, 1137–1152. [Google Scholar] [CrossRef]
- Prestegard, S.K.; Erga, S.R.; Steinrucken, P.; Mjas, S.A.; Knutsen, G.; Rohloff, J. Specific metabolites in a Phaeodactylum tricornutum strain isolated from Western Norwegian fjord water. Mar. Drugs 2016, 14, 9. [Google Scholar] [CrossRef]
- Krishnan, R.; Murugan, K. Comparison of GC-MS analysis of phytochemicals in the ethanolic extracts of Marchantia linearis LEHM & Lindenb. and Marchantia polymorpha L. (Bryophyta). Int. J. Pharm. Sci. Res. 2014, 5, 1981–1987. [Google Scholar]
- Bouazzi, S.; Jmii, H.; Mokni, R.E.; Faidi, K.; Falconieri, D.; Piras, A.; Jaidane, H.; Porcedda, S.; Hammami, S. Cytotoxic and antiviral activities of the essential oils from Tunisian Fern, Osmunda regalis. S. Afr. J. Bot. 2018, 118, 52–57. [Google Scholar] [CrossRef]
- Hamid, J.; Ahmed, D.; Waheed, A. Evaluation of anti-oxidative, antimicrobial and anti-diabetic potential of Adiantum venustum and identification of its phytochemicals through GC-MS. Pak. J. Pharm. Sci. 2017, 30, 705–712. [Google Scholar]
- Kang, X.L.; Liu, X.; Luo, J.R. Analysis of fat-soluble constituents in pine cone of Pinus yunnanensis from various habitats by GC-MS. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 106–109. [Google Scholar]
- Zhang, X.L.; Zhang, J.Y.; Wang, Y.Y.; Bao, C.Y.; Deng, G.B. Analysis of the voilatile components of the bark of Pinus yunnanensis French by GC-MS. Fine Chem. 2008, 25, 45–48. [Google Scholar]
- Luo, J.R.; Li, D.M.; Zhang, Z.; Liu, G.M. GC-MS analysis on the constituents of n-hexane extracts from the pinecone of Pinus kesiya var. langbianensis. J. Anhui Agric. Sci. 2011, 39, 18647–18648. [Google Scholar]
- Tian, K.; Wang, X.Y. GC-MS analysis of water extracts of pine needles of Pinus tabulaeformis. Tianjin Agric. For. Sci. Technol. 2018, 2018, 1–10. [Google Scholar]
- Wei, W. Study on the Chemical Constitutions and Allelopathy in Rhizosphere Soil Extracts of the 1st and 2nd Pinus Massoniana Plantation. Master’s Thesis, Guizhou University, Guiyang, China, 2017. [Google Scholar]
- Kong, D.X.; Li, Y.Q.; Zhou, R.; Shi, Y.C.; Wei, X. GC-MS and FTIR identification and analysis of chemical component in Artemisia annua and its closely related species. Guihaia 2017, 37, 234–241. [Google Scholar] [CrossRef]
- Sun, F.L.; Lin, W.J.; Xu, X.M.; Zhang, Y.M.; Xu, R.Q. GC-MS Analysis of Essential Oils from Seeds of Alisma orientalis. Strait Pharm. J. 2018, 30, 24–26. [Google Scholar]
- Han, Z.H.; Cao, W.H.; Li, X.B.; Luo, T.L.; Liu, G.J. Analysis of chemical composition of essential oil in Cornus officinalis Sieb. Et Zucc by GC-MS. Fine Chem. 2006, 23, 130–132. [Google Scholar]
- Qu, B.L.; Wu, H.Y.; Ren, F.L.; Yu, W.F.; Wang, J.Z. Analysis of essential oil composition of Paeionia lactiflora Pall. by GC/MS. Food Sci. Technol. 2009, 34, 92–95. [Google Scholar] [CrossRef]
- Feng, L.; Ji, H.W.; Wang, D.C.; Wang, J.M.; Liu, R.M. Analysis of Volatile Constituents from Different Parts of Salvia miltiorrhiza by GC-MS. China Pharm. 2010, 21, 3706–3709. [Google Scholar]
- Zhang, H.; Chen, Z.E.; Fu, S.H. Analysis of volatile components from Rubia cordifolia L. by GC-MS. Guangzhou Chem. Ind. 2016, 44, 68–70. [Google Scholar]
- Zhang, C.; Liu, S.J.; Yang, L.; Hu, J.M. Determination of volatile components from flowers of Dendrobium moniliforme (L.) Sw. in Yunnan by GC-MS. J. Yunnan Agric. Univ. (Nat. Sci.) 2017, 32, 174–178. [Google Scholar]
- Fan, M.Y.; Cao, F.F.; Xu, M.; Zhang, X.F. Volatile component analysis of five species of genus Chimonanthus leaves by HS-SPME/GC-MS. Mol. Plant Breed. 2017, 15, 2381–2388. [Google Scholar]
- Huang, M.Z.; Li, X. Kind and content of volatile components in Gastrodia elata by SDE-GC-MS analysis. Guizhou Agric. Sci. 2018, 46, 110–113. [Google Scholar]
- Huang, M.Z.; Li, X. Volatile compounds of Perilla frutescens analysis by SDE-GC-MS. J. Anhui Agric. Sci. 2018, 46, 182–185. [Google Scholar]
- Zhu, Y.; Shao, C.Y.; Zhang, Y.; Lin, Z.; Lv, H.P. Comparison of differences in aroma constituents of longjing tea produced from different tea germplasms. Sci. Technol. Food Ind. 2018, 39, 241–246. [Google Scholar]
- Meiw, E.L.; Zeng, Y.B.; Liu, J.; Dai, H.F. GC-MS analysis of volatile constituents from five different kinds of Chinese eaglewood. J. Chin. Med. Mater. 2007, 30, 551–555. [Google Scholar]
- Xu, H.Y.; Li, Y.L.; Peng, X.J.; XChen, Y.B.; Yang, X.J. Study on composition and antioxidant activity of volatile oil from Gentiana apiata N. E. Br. Tradit. Chin. Drug Res. Clin. Pharmacol. 2019, 30, 106–109. [Google Scholar]
- Yu, A.X.; Lin, F.; Xu, H.H. Effect of extracts from Pennisetum orientale on Chinese sprangletop (Leptochloa chinensis (L.) Nees). World Pestic. 2015, 37, 55–58. [Google Scholar]
- Lee, C.Y.; Whitaker, J.R. (Eds.) Enzymatic Browing and Its Prevention; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Pirttilä, A.M.; Podolich, O.; Koskimäki, J.J.; Hohtola, E.; Hohtola, A. Role of origin and endophyte infection in browning of bud-derived tissue cultures of scots pine (Pinus sylvestris L.). Plant Cell Tissue Organ 2008, 95, 47–55. [Google Scholar] [CrossRef]
- Liu, J.X.; Luo, Y.Y.; Liu, X.H.; Song, J.P. Comparison of volatile components in Xanthii Herba and Xanthii Fructus by GC-MS. Nat. Prod. Res. Dev. 2016, 28, 1929–1935. [Google Scholar]
- Yao, H.J.; Yao, H.M.; Bu, S.H.; Lu, X.T.; Zhang, J. Analysis of the volatile oil constituent of Atractylodes Coreana (Nakai) Kitam by GC-MS. Chin. J. Pharmacovigil. 2013, 10, 148–151. [Google Scholar]
- Wu, Y.X.; Jiang, H.T.; Cui, P.; Fang, J.X.; Wang, W.D.; Hu, C.Y. Study on bioactive components and antioxidant, antibacterial activities of Atractylodes macrocephala Koidz grown in Qimen. Food Mach. 2018, 34, 154–158. [Google Scholar] [CrossRef]
- Chen, Y.X.; Shi, H.F. Analysis of volatile components in Lindera rubronervia and Lindera angustifolia xylem by GC-MS. J. Mianyang Teachers’ Coll. 2018, 37, 19–23. [Google Scholar]
- Chen, Y.; Shi, X.Y.; Li, H.L.; Luo, G.H. Analysis of volatile oil of infructescence of Sibiraea angustata (Rehd.) Hand.-Mazz. J. Chin. Med. Mater. 2016, 39, 2261–2263. [Google Scholar]
- Wei, J.; Mo, H.; Meng, Q.; Zhao, H.; Lu, R. Chemical constituents from Zhuang medicine Sauropus rostratus (II). Chin. Tradit. Herb. Drugs 2016, 47, 3560–3564. [Google Scholar]
- Pirttilä, A.M.; Joensuu, P.; Pospiech, H.; Jalonen, J.; Hohtola, A. Bud endophytes of Scots pine produce adenine derivatives and other compounds that affect morphology and mitigate browning of callus cultures. Physiol. Plant. 2004, 121, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Z.; Peng, X.T.; Xia, H.; Zhou, Y.T.; Peng, L.J.; Hu, D.J. GC-MS analysis of volatile oil components by steam distillation and ethanol extraction from water chestnut. J. Changjiang Veg. 2017, 54–60. [Google Scholar] [CrossRef]
- Hu, X.Z.; Zheng, D.; Peng, X.T.; Xia, H.; Zhou, Y.X.; Peng, L.J.; Sun, D.L. GC-MS analysis of volatile oil components by steam distillation and ethanol extraction from lotus. Jiangsu Agric. Sci. 2018, 46, 226–230. [Google Scholar]
- Hu, R. Analysis of Volatile Compounds of Lysimachia Foenum-graecum Powder by HS-SPME-GC/MS. Shandong Chem. Ind. 2018, 47, 94–98. [Google Scholar]
- Hu, P.; Cai, J.; Zhang, Y.J.; Li, X.; Chen, J.W. Analysis of volatile oil and fatty oil in seed of Metaplexis japonica. China Pharm. 2017, 28, 2532–2535. [Google Scholar]
- Zhou, K.; Jiang, P.; Liang, W.Y.; Liang, L.J.; Cui, Y.P.; Ye, T.; Zhang, L.Z. Research process of chemical constituents and pharmacological effects of Tibetan medicine triphala. World Sci. Technol./Mod. Tradit. Chin. Med. Mater. Med. 2018, 20, 1608–1614. [Google Scholar]
- Huang, Z.F.; Lu, W.J.; Tan, X.; Lu, G.S.; Huang, J.Y.; Hu, X.X. Study on the components and antioxidant activites of the liposoluble constituents from Murdannia nudiflora (Linn.) Brenan. Chin. J. Mod. Appl. Pharm. 2018, 35, 674–677. [Google Scholar]
- Huang, X.J.; Li, Y.C.; Jiang, P.; Zhang, X.; Zhang, X.Y.; Tan, P.Y.; Tian, W. Identification of chrysanthemum root exudates and allelopathic effects of the three plants. Hubei Agric. Sci. 2017, 56, 1061–1071. [Google Scholar]
- Gao, X.Y.; Wang, H.Y.; Liu, Z.M. GC-MS analysis of fresh fruits extracts of Lonicera maackii. Chin. Wild Plant Res. 2018, 37, 25–28. [Google Scholar] [CrossRef]
- Li, G.; Bai, W.B.; Ren, A.X. Effects of continuous cropping duration of sorghum on components of root exudates and contents of allelochemicals. Chin. J. Ecol. 2017, 36, 3535–3544. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiong, Y.; Yu, X.; Ren, M.; Xiang, B.; He, Q. Analysis of antibacterial activity and chemical constituents of ethanol extracts from Houttuynia cordata roots and aerial part. J. Chongqi Norm. Univ. (Nat. Sci.) 2019, 36, 103–108. [Google Scholar]
- Zhu, P.H. Study on the Chemical Components and Bioactivity of Eomecon Chionantha Hance. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2017. [Google Scholar]
- Zhang, R.X.; Zhao, D.G. GC-MS study on the essential oil of Aconitum carmichaeli produced in Guizhou. Guizhou Agric. Sci. 2011, 39, 55–58. [Google Scholar]
- Wang, L.F.; Hu, K.; He, H.L.; Ding, W.B.; Li, Y.Z. Southern rice black-streaked dwarf virus-induced volatiles from rice plants and behavioral responses of adult Sogatella furcifera (Hemiptera: Delphacidae) to the components of these volatiles. Acta Entomol. Sin. 2017, 60, 412–420. [Google Scholar]
- Li, T.T.; Li, G.J.; Li, J.N. Comparison of the components in different parts of Dalbergia odorifera wood planted in Hainan by GC-MS. J. Northwest. For. Univ. 2018, 33, 172–178. [Google Scholar] [CrossRef]
- Johnson, J.A.; Citarasu, T.; Manjusha, W.A. Antimicrobial screening and identification of bioactive compounds present in marine sponge Zygomycale sp. collected from Kanyakumari coast. J. Chem. Biol. Phys. Sci. 2012, 2, 1842–1848. [Google Scholar]
- Yoon, M.A.; Jeong, T.S.; Park, D.S.; Xu, M.Z.; Oh, H.W.; Song, K.B.; Lee, W.S.; Park, H.Y. Antioxidant effects of quinoline alkaloids and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol. Pharm. Bull. 2006, 29, 735–739. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G. Acaricidal, repellent, and oviposition-deterrent activities of 2,4-ditert-butylphenol and ethyl oleate against the carmine spider mite Tetranychus cinnabarinus. J. Pest. Sci. 2015, 88, 645–655. [Google Scholar] [CrossRef]
- Chu, C.; Gu, Q.; Fang, Y. Isolation and identification of a phenolic derivative from the ascidian. Qingdao Haiyang Daxue Xuebao 1999, 29, 53–56. [Google Scholar]
- Xu, M.; Piao, G. Anti-atherosclerotic activities of two compounds from mantidis ootheca. Anhui Nongye Kexue 2012, 4, 15722–15723. [Google Scholar]
- Zhu, X.Y.; Zhang, Y.Q.; Chen, Y.Q.; Ma, B.L.; Yan, X.Y. Advances in chemical constituents and pharmacological activities of plants of genus Salix. Chin. Tradit. Herbal Drugs 2018, 49, 5952–5960. [Google Scholar]
- Liu, N.N.; Bai, Y.C.; Li, X.L.; Yang, R.F.; Guo, T.; Cui, G.X. Allelopathic potential evaluation of water extracts from ramie rhizosphere soil. J. Plant Nutr. Fertil. 2017, 23, 834–842. [Google Scholar] [CrossRef]
- Yang, T.; Yang, G.Y.; Zhang, Y.P.; Sun, J.P.; Chen, W.Z.; Fang, H.; Hong, Z. Study on chemical constituents of Grateloupia filicina. Chin. J. Mar. Drugs 2019, 38, 37–41. [Google Scholar]
- Ma, H.F.; Lang, N.J.; He, L.P.; Yu, Z.; Zheng, K.; Peng, M.J.; Xiang, Z.Y.; Kong, J.J.; Yuab, R.L. Volatile components from plant parts of Jatropha curcas in Jianshui, Yunnan Province. J. Zhejiang A F Univ. 2011, 28, 674–679. [Google Scholar]
- Wang, J.; Li, X.H.; Yin, H.F.; Fan, Z.Q.; Li, J.Y. Volatile components in different floral organs and flowering stages of Camellia sasanqua ‘Dongxing’. J. Yunnan Agric. Univ. (Nat. Sci.) 2018, 33, 904–910. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Zhang, H.J.; Li, B.; Lu, R.M.; Zhu, X.Y. GC-MS analysis of volatile oil of Taxillus chinensis (DC.) Danser on oleander. Guangxi J. Tradit. Chin. Med. 2013, 36, 57–60. [Google Scholar]
- Liu, H.T. Study on the Chemical Composition Analysis and ISSR Molecular Marker of Chimonanthus. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2013. [Google Scholar]
- Gao, Y.Q.; Guo, L.Y.; Meng, X.X.; Zhang, L.N.; Yang, G.D. The optimal GC-MS analysis of essential oil (fresh, dried and bud) and aroma enhanced by β-glucosidase on Aesculus chinensis flowers. For. Prod. Spec. China 2018, 5, 1–4. [Google Scholar]
- Yuan, S.J.; He, X.S.; Yuan, X.H.; Huang, Y. Antioxidant activity evaluation and low-polarity components analysis of Coriolus versicolor and Trametes robiniophila. Sci. Technol. Food Ind. 2019, 40, 1–5. [Google Scholar]
- Pan, W.G.; Li, Y.; Zhu, X.Y.; Zhu, Y.L.; Li, Y.H.; Luo, P. GC-MS analysis of volatile oil from Hedyotis lancea. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 130–134. [Google Scholar]
- Chen, H.P.; Peng, Y.; Liu, G.; Li, H.; Gao, L.Q.; Zhan, N.; Xie, Y. Dynamic changes of volatile components from developing seeds of Plukenetia volubilis. Sci. Silvae Sin. 2018, 54, 157–168. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.Y.; Hui, Y.; Pi, W.B.; Chen, W.H.; Huang, G.H. GC-MS analysis of essential oil from the whole plant of Eupatorium catarium. Guangzhou Chem. Ind. 2015, 43, 115–117. [Google Scholar]
- Li, H.L.; Yang, X.H.; Li, G.; Guan, E.J. GC-MS analysis of volatile oil of Geum aleppicum Jacp. from Changbai Mountain. Acad. Period. Changchun Coll. Tradit. Chin. Med. 2005, 21, 31–32. [Google Scholar] [CrossRef]
- Zhao, K.; Yu, Y.F.; Xie, J.P.; Hu, C.H.; Huang, Y.M. Components of volatile metabolites of Nectria analyzed by GC-MS. J. Southwest Univ. (Nat. Sci. Ed.) 2007, 29, 150–153. [Google Scholar]
- Jiang, G.; Lin, S.; Wen, L.; Jiang, Y.; Zhao, M.; Chen, F.; Prasad, K.N.; Duan, X.; Yang, B. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013, 136, 563–568. [Google Scholar] [CrossRef]
- Babu, B.; Wu, J.T. Production of natural butylated hydroxytoluene as an antioxidant by freshwater phytoplankton. J. Phycol. 2008, 44, 1447–1454. [Google Scholar] [CrossRef]
- Li, S.Q.; Zhang, Z.N. EAG responses of Monochamus alternatus Hope (Coleoptera: Cerambycidae) to volatiles from larval frass and the repellency tests in fields. Acta Entomol. Sin. 2008, 51, 284–289. [Google Scholar]
- Li, S.Q.; Zhang, Z.N. Influence of larval frass extracts on the oviposition behaviour of Monochamus alternatus (Col., Cerambycidae). J. Appl. Entomol. 2006, 130, 177–182. [Google Scholar] [CrossRef]
- Wu, S.P.; Chen, H.; Wu, Q. Volatile compounds in the frass of adult Chinese white pine beetle. J. Northwest For. Univ. 2012, 27, 111–116. [Google Scholar]
- Zhao, L.L.; Wei, W.; Kang, L.; Sun, J.H. Chemotaxis of the pinewood nematode, Bursaphelenchus xylophilus, to volatiles associated with host pine, Pinus massoniana, and its vector Monochamus alternatus. J. Chem. Ecol. 2007, 33, 1207–1216. [Google Scholar] [CrossRef]
- Zhou, W.M.; Zheng, H.Y.; Zeng, Q.Q.; Wang, R.Y.; Chen, L.S. Distribution of volatile components and inorganic elements from Vernonia amygdalina. Chin. Tradit. Pat. Med. 2018, 40, 1345–1360. [Google Scholar]
- Choi, S.J.; Kim, J.K.; Kim, H.K.; Harris, K.; Kim, C.J.; Park, G.G.; Park, C.S.; Shin, D.H. 2,4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. J. Med. Food 2013, 16, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Abo-Shady, A.; Eldeen, H.A.S.; Soror, H.A.; Shousha, W.G.; Abdel-Barry, O.; Saleh, A.M. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem. Cent. J. 2013, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Code of Federal Regulations, Title 21: Food and Drugs; Office of the Federal Register: Washington, DC, USA, 2001; Chapter I. [Google Scholar]
- The Commission of the European Communities. European Parliament and Council Directive No. 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners. Off. J. Eur. Union 1995, 61, 1–40. [Google Scholar]
- Hou, C.S. Studies on Butylated Hydroxytoluene Antisenile of the Active Molecular in Roval Jelly. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, April 2007. [Google Scholar]
- Murakami, Y.; Kawata, A.; Katayama, T.; Fujisawa, S. Anti-inflammatory activity of the artificial antioxidants 2-tert-butyl-4-methoxyphenol (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT) and 2,4,6-tri-tert-butylphenol (TBP), and their various combinations. In Vivo 2015, 29, 197–206. [Google Scholar]
- Nair, R.V.R.; Jayasree, D.V.; Biju, P.G.; Baby, S. Anti-inflammatory and anticancer activities of erythrodiol-3-acetate and 2,4-di-tert-butylphenol isolated from Humboldtiaunijuga. Nat. Prod. Res. 2018, 26, 1–4. [Google Scholar] [CrossRef]
- Hirata-Koizumi, M.; Hamamura, M.; Furukawa, H.; Fukuda, N.; Ito, Y.; Wako, Y.; Yamashita, K.; Takahashi, M.; Kamata, E.; Ema, M.; et al. Elevated susceptibility of newborn as compared with young rats to 2-tert-butylphenol and 2,4-di-tert-butylphenol toxicity. Congenit. Anom (Kyoto) 2005, 45, 146–153. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Satoh, K.; Ando, H.; Kubo, Y.; Nagasawa, A.; Yano, N.; Yuzawa, K.; Takahashi, H.; Ogat, A. Uterotrophic effect of 2,4-di-tert-butylphenol on ovariectomized CD1 mice. Ann. Rep. Tokyo Metr. Inst. PH. 2005, 56, 343–346. [Google Scholar]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Oikawa, S.; Nishino, K.; Oikawa, S.; Inoue, S.; Mizutani, T.; Kawanishi, S. Oxidative DNA damage and apoptosis induced by metabolites of butylated hydroxytoluene. Biochem. Pharmacol. 1998, 56, 361–370. [Google Scholar] [CrossRef]
- Dubinskaia, N.I.; Burlakova, E.B. Effect of the antioxidant, 4-methyl-2,6 di-tert-butylphenol on the mitotic division of epithelial cells of the small-intestinal crypts of mice depending on the degree of differentiation. Tsitologiia 1977, 19, 763–767. [Google Scholar] [PubMed]
- Saito, M.; Sakagami, H.; Fujisawa, S. Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). Anticancer Res. 2003, 23, 4693–4701. [Google Scholar] [PubMed]
- Wang, Z.Q.; Wu, Y.X.; Zhou, H.; He, Y.Q. Nematicidal Activity of 2,4-Di-tert-butylphenol against Caenorhabditis elegans. Agrochemicals 2014, 53, 298–300. [Google Scholar]
- Burkholder, W.E.; Schwalbe, C.P.; Boush, G.M. Antimicrobial food additives and their effects on Trogoderma variabile and Attagenus megatoma (Coleoptera, Dermestidae). J. Stored Prod. Res. 1973, 9, 205–211. [Google Scholar] [CrossRef]
- García, D.; Girardi, N.S.; Passone, M.A.; Nesci, A.V.; Etcheverry, M.G. Harmful Effects on Oryzaephilus surinamensis (L.) and Tribolium castaneum by food grade antioxidants and their formulations in peanut kernel. J. Food Chem. Nanotechnol. 2017, 3, 86–92. [Google Scholar] [CrossRef]
- Pullen, E.M. Controlling Insects and Parasites on Plants Using Surfactants and High Terpene Oil. U.S. Patent US6582712B2, 24 June 2003. [Google Scholar]
- Collignon, R.M.; Halloran, S.; Serrano, J.M.; McElfresh, J.S.; Millar, J.G. An unstable monoterpene alcohol as a pheromone component of the longhorned beetle Paranoplium gracile (Coleoptera: Cerambycidae). J. Chem. Ecol. 2019, 45, 339–347. [Google Scholar] [CrossRef]
- Lin, H.P.; Fan, J.T.; Su, X. A Composition to Repel Sawyer Beetles (Monochamus alternatus). China Patent CN102726463A, 29 June 2012. [Google Scholar]
- Padmavathi, A.R.; Periyasamy, M.; Pandian, S.K. Assessment of 2,4di-tert-butylphenol induced modifications in extracellular polymeric substances of Serratia marcescens. Bioresour. Technol. 2015, 188, 185–189. [Google Scholar] [CrossRef]
- Leila, A.; Lamjed, B.; Roudaina, B.; Najla, T.; Taamalli, A.; Jellouli, S.; Mokhtar, Z. Isolation of an antiviral compound from Tunisian olive twig cultivars. Microb. Pathog. 2019, 128, 245–249. [Google Scholar] [CrossRef]
- Zhou, B.; Li, N.; Liu, S.; Fu, R.; Li, G. Effects of 2,4-di-tert-butylphenol on tomato leaf mould and seedling growth. Shengtaixue Zazhi 2013, 32, 1203–1207. [Google Scholar]
- Chen, Z.X.; Zhou, B.L.; Du, L.; Ye, X.L. Effects of phenol 2,4-bis(1,1-dimethylethyl) on seedling growth of eggplant and Verticillium dahlia. Allelopath. J. 2012, 30, 81–92. [Google Scholar]
- María Teresa, R.; Rosaura, V.; Elda, C.; Ernesto, G.T. The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol. 2014, 87, 32–41. [Google Scholar] [CrossRef]
- Padmavathi, A.R.; Bakkiyaraj, D.; Thajuddin, N.; Pandian, S.K. Effect of 2, 4-di-tert-butylphenol on growth and biofilm formation by an opportunistic fungus Candida albicans. Biofouling 2015, 31, 565–574. [Google Scholar] [CrossRef]
- Chuah, T.S.; Norhafizah, M.Z.; Ismail, B.S. Evaluation of the biochemical and physiological activity of the natural compound, 2,4-ditert-butylphenol on weeds. Crop. Pasture Sci. 2015, 66, 214–223. [Google Scholar] [CrossRef]
- Xuan, T.D.; Toyama, T.; Fukuta, M.; Khanh, T.D.; Tawata, S. Chemical interaction in the invasiveness of Cogongrass (Imperata cylindrica (L.) Beauv.). J. Agric. Food Chem. 2009, 57, 9448–9453. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Lee, J.H.; Ravichandran, V.; Kim, J.C.; Park, J.G.; Lee, J. Nematicidal and insecticidal activities of halogenated indoles. Sci. Rep. 2019, 9, 2010. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, S.O.; Lee, D.H.; Choi, W.S.; Jung, C.S.; Jeon, J.H.; Kim, J.E.; Park, I.K. Identification of the aggregation-sex pheromone produced by male Monochamus saltuarius, a major insect vector of the pine wood nematode. J. Chem. Ecol. 2017, 43, 670–678. [Google Scholar] [CrossRef]
- Chuah, T.S.; Zain, N.M.; Naimah, A.H.; Ismail, B.S. Phytotoxic activity of the allelochemical, 2,4-di-tert-butylphenol on two selected weed species. Sains Malays. 2016, 45, 963–967. [Google Scholar]
- Chuah, T.S.; Norhafizah, M.Z.; Ismail, S. Phytotoxic effects of the extracts and compounds isolated from Napiergrass (Pennisetum purpureum) on Chinese sprangletop (Leptochloa chinensis) germination and seedling. Weed Sci. 2014, 62, 457–467. [Google Scholar] [CrossRef]
- Halim, N.A.; Razak, S.B.A.; Simbak, N.; Seng, C.T. 2,4-Di-tert-butylphenol-induced leaf physiological and ultrastructural changes in chloroplasts of weedy plants. S. Afr. J. Bot. 2017, 112, 89–94. [Google Scholar] [CrossRef]
- Norhafizah, Z.M. Characterization and Mode of Action of Phytotoxic Compounds Isolated from Pennisetum Purpureum (Napier grass). Ph.D Thesis, University Malaysia Terengganu, Kuala Terengganu, Malaysia, 2014. [Google Scholar]
- Zhang, X.H.; Zhang, E.H.; Chai, Q.; He, Q.X.; Ren, B.C. Effects of phenol, 2,4-bis(1,1-dimethy lethyl) on photosynthetic characters of hops seedling. J. Gansu Agric. Univ. 2006, 41, 50–54. [Google Scholar]
- Zhang, X.; Zhang, E.; Lang, D. Autotoxic compounds from rhizosphere soil of Humulus lupulus L. extracts: Identification and biological activity. Agron. J. 2011, 103, 695–701. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Gao, J.; Niu, F.; Duan, X.; Yin, L.; Tian, W. Identification of autotoxic compounds from Atractylodes macrocephala Koidz and preliminary investigations of their influences on immune system. J. Plant Physiol. 2018, 230, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhao, Z.L.; Zang, S.S.; Xie, Z.H.; Shi, L.L.; Guo, J.W. Influence of several allelochemicals from root exudates on own seed germination and seedling growth in chilli pepper. Acta Agric. Bor.-Occid. Sin. 2013, 22, 137–143. [Google Scholar]
- Huang, W. Study on the Synergetic Effect of 2,4-DTBP and Fusarium on the Occurence of Fusarium Wilt in Lanzhou Lily. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2018. [Google Scholar]
- Li, S.; Wang, P.; Yuan, W.; Su, Z.; Bullard, S.H. Endocidal regulation of secondary metabolites in the producing organisms. Sci. Rep. 2016, 6, 29315. [Google Scholar] [CrossRef]
- Suga, T.; Ohta, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M. Endogenous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Zheng, Y.N.; Yang, Z.Q.; Wang, X.Y. Mechanism of chemical ecology of Bursaphelenchus xylophilus vectored by Monochamus alternatus. Plant Prot. 2014, 40, 12–15. [Google Scholar]
- Gumiere, T.; Ribeiro, C.M.; Vasconcellos, R.L.F.; Cardoso, E.J.B.N. Indole-3-acetic acid producing root-associated bacteria on growth of Brazil pine (Araucaria angustifolia) and Slash pine (Pinus elliottii). Antonie Leeuwenhoek 2014, 105, 663. [Google Scholar] [CrossRef]
- Barriuso, J.; Ramos Solano, B.; Santamaría, C.; Daza, A.; Gutiérrez Mañero, F.J. Effect of inoculation with putative plant growth-promoting rhizobacteria isolated from Pinus spp. on Pinus pinea growth, mycorrhization and rhizosphere microbial communities. J. Appl. Microbiol. 2008, 105, 1298–1309. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Wang, Y.; Bao, C.; Deng, G. Analysis of the volatile components of the bark of Pinus yunnanensis Franch by GC-MS. Jingxi Huagong 2008, 25, 45–48. [Google Scholar]
- Xu, N.; Wang, C.; Wei, M.; Shi, W.; Wang, X. Allelopathy of welsh onion root exudates on cucumber seed germination and Fusarium oxysporum f. sp. cucumerinum and GC-MS analysis. Yuanyi Xuebao 2012, 39, 1511–1520. [Google Scholar]
- Jianu, C.; Misca, C.; Pop, G.; Rusu, L.C.; Ardelean, L.; Gruia, A.T. Chemical composition and antimicrobial activity of essential oils obtained from dill (Anethum graveolens L.) grown in western Romania. Rev. Chim. 2012, 63, 641–645. [Google Scholar]
- Sugunabai, J.; Jeyaraj, M.; Karpagam, T.; Varalakshmi, B.; Senthil Rani, S.; Shanmugapriya, A.; Kalaiyarasi, G.; Renuga, R.; Gomathi, S. Outlining of phytochemicals and GC-MS profile of Centella asiatica. Int. J. Pharm. Drug. Anal. 2018, 6, 252–256. [Google Scholar]
- Kołodziej, B.; Kowalski, R.; Hołderna-Kedzia, E. Chemical composition and chosen bioactive properties of Panax quinquefolius extracts. Chemija 2013, 24, 151–159. [Google Scholar]
- Nadaf, M.; Asrabadi, M.N.; Halimi, M.; Yazadani, Z. Identification of non-polar chemical compounds Acroptilon repens growing in Iran by GC-MS. Middle East J. Sci. Res. 2013, 17, 590–592. [Google Scholar]
- Hourie, K.; Shandiz, A.S.S.; Baghbani-Arani, F. Anticancer properties of phyto-synthesized silver nanoparticles from medicinal plant Artemisia tschernieviana Besser aerial parts extract toward HT29 human colon adenocarcinoma cells. J. Clust. Sci. 2017, 28, 1617–1636. [Google Scholar]
- Rincón, A.; Ruiz-Diez, B.; Garcia-Fraile, S.; Garcia, J.A.L.; Fernandez-Pascual, M.; Pueyo, J.J.; de Felipe, M.R. Colonisation of Pinus halepensis roots by Pseudomonas fluorescens and interaction with the ectomycorrhizal fungus Suillus granulatus. FEMS Microbiol. Ecol. 2005, 51, 303–311. [Google Scholar] [CrossRef]
- Villalobos-Amador, E.; Rodríguez-Hernández, G.; Pérez-Molphe-Balch, E. Organogenesis and Agrobacterium rhizogenes-induced rooting in Pinus maximartinezii Rzedowsky and P. pinceana Gordon. Plant Cell Rep. 2002, 20, 779–785. [Google Scholar] [CrossRef]
- Pokojska-Burdziej, A.; Strzelczyk, E.; Dahm, H.; Li, C.Y. Effect of endophytic bacterium Pseudomonas fulva on growth of pine seedlings (Pinus sylvestris), formation of mycorrhizae and protection against pathogens. Phytopathol. Pol. 2004, 32, 33–47. [Google Scholar]
- Aswathy, J.M.; Preetha, T.S.; Murugan, K. Comparison of bioactive anthocyanin components from Begonia malabarica Lam. and Begonia rex-Cultorum ‘Baby Rainbow’ L.H.Bailey by GC-MS analysis. Int. J. Appl. Biol. Pharm. Technol. 2015, 6, 217–222. [Google Scholar]
- Vonderwell, J.D.; Enebak, S.A.; Samuelson, L.J. Influence of two plant growth-promoting rhizobacteria on loblolly pine root respiration and IAA activity. For. Sci. 2001, 47, 197–202. [Google Scholar] [CrossRef]
- Logesh, R. Phytochemical and GC-MS analysis of Brassica oleracea var. Capitata, F. Rubra. World J. Pharm. Res. 2018, 7, 1392–1400. [Google Scholar]
- He, S.; Ding, C.; Yang, G.; Zhu, J.; Zhao, J. Effect of microwave drying on quality and volatile profiles of rapeseeds. Zhongguo Liangyou Xuebao 2013, 28, 48–54. [Google Scholar]
- Li, M.; Leung, D.W.M. Root induction in radiata pine using Agrobacterium rhizogenes. Electron. J. Biotechnol. 2003, 6, 254–261. [Google Scholar]
- Gayathri, G.; Vijayalakshmi, K.; Saraswathy, A. GC-MS & HPTLC fingerprinting of Bauhinia variegata leaves for anti-cancer activity. World J. Pharm. Res. 2014, 3, 907–915. [Google Scholar]
- Mamiya, Y.; Endo, N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica 1972, 18, 159–162. [Google Scholar] [CrossRef]
- Wagay, N.A.; Khan, N.A.; Rothe, S.P. Profiling of secondary metabolites and antimicrobial activity of Crateva religiosa G. Forst. bark-A rare medicinal plant of Maharashtra India. Int. J. Biosci. 2017, 10, 343–354. [Google Scholar]
- Ghosh, S.; Saha, M.; Bandyopadhyay, P.K.; Jana, M. Extraction, isolation and characterization of bioactive compounds from chloroform extract of Carica papaya seed and it’s in vivo antibacterial potentiality in Channa punctatus against Klebsiella PKBSG14. Microb. Pathog. 2017, 111, 508–518. [Google Scholar] [CrossRef]
- Cho, J.; Kim, M.S.; Lee, Y.G.; Jeong, H.Y.; Lee, H.J.; Ham, K.S.; Moon, J.H. A phenyl lipid alkaloid and flavone C-diglucosides from Spergularia marina. Food Sci. Biotechnol. 2016, 25, 63–69. [Google Scholar] [CrossRef]
- Lakshmi, M.; Nair, B.R. GC-MS analysis of the chloroform extract of bark of Terminalia travancorensis Wight & Arn. (Combretaceae). Int. J. Pharm. Sci. Res. 2017, 8, 794–798. [Google Scholar]
- Morimoto, K.; Iwasaki, A. Role of Monochamus alternatus (Coleoptera: Cerambycidae) as a vector of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae). J. Jpn. For. Soc. 1972, 54, 177–183. [Google Scholar]
- Tayad, A.B.; Dhar, P.; Kumar, J.; Sharma, M.; Chauhan, R.S.; Chaurasia, O.P.; Srivastava, R.B. Chemometric profile of root extracts of Rhodiola imbricata Edgew. with hyphenated gas chromatography mass spectrometric technique. PLoS ONE 2013, 8, e52797. [Google Scholar] [CrossRef] [PubMed]
- Kour, J.; Ali, M.N.; Ganaie, H.A.; Tabassum, N. Amelioration of the cyclophosphamide induced genotoxic damage in mice by the ethanolic extract of Equisetum arvense. Toxicol. Rep. 2017, 4, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, G.; Kalaivani, M.K.; Sumathy, A. In-vitro alpha amylase inhibitory and anti-oxidant activities of ethanolic leaf extract of Croton bonplandianum. Asian J. Pharm. Clin. Res. 2013, 6, 32–36. [Google Scholar]
- Malayaman, V.; Sheik Mohamed, S.; Senthilkumar, R.P.; Ghouse Basha, M. Analysis of phytochemical constituents in leaves of Bhumyamalaki (Phyllanthus debilis Klein ex Willd.) from Servaroy hills, Tamil Nadu, India. J. Pharm. Phytochem. 2019, 8, 2678–2683. [Google Scholar]
- Zhao, L.L.; Zhang, S.; Wei, W.; Hao, H.; Zhang, B.; Butcher, R.A.; Sun, J. Chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Curr. Biol. 2013, 23, 2038–2043. [Google Scholar] [CrossRef]
- Li, Y.; Ma, F.; Wu, L.; Liu, C.; Wang, A.; Wang, C. Difference effects of rotation and continuous soybean root secretion on seedling growth of soybean. J. NE Agric. Univ. 2010, 41, 1–6. [Google Scholar]
- Duangnin, N.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. In vitro and in vivo investigation of natural compounds from seed extract of Mucuna pruriens lacking L-DOPA for the treatment of erectile dysfunction. Asian Pac. J. Trop. Med. 2017, 10, 238–252. [Google Scholar] [CrossRef]
- Ishan, U.K.; Suresh, M.; Suresh, M.; Bagh, R. Volatile phyto-chemical compounds screening in alcoholic extract of western Rajasthan growing Mung-Bean (Vigna radiata) by Gas Chromatography-Mass Spectroscopy. Trends Biosci. 2017, 10, 1588–1595. [Google Scholar]
- Necibi, S.; Linit, M.J. Effect of Monochamus carolinensis on Bursaphelenchus xylophilus dispersal stage formation. J. Nematol. 1998, 30, 246–254. [Google Scholar]
- Gao, H.; Song, Y.; Lv, C.; Chen, X.; Yu, H.; Peng, J.; Wang, M. The possible allelopathic effect of Hydrilla verticillata on phytoplankton in nutrient-rich water. Environ. Earth Sci. 2015, 73, 5141–5151. [Google Scholar] [CrossRef]
- Cui, C.; Cai, J.; Zhang, S. Isolation and identification of the allelochemicals in walnut (Juglans regia) root exudates. Linye Kexue 2013, 49, 54–60. [Google Scholar]
- Sreeja, P.S.; Arunachalam, K.; Saikumar, S.; Kasipandi, M.; Dhivya, S.; Murugan, R.; Parimelazhagan, T. Gastroprotective effect and mode of action of methanol extract of Sphenodesme involucrata var. paniculata (C.B. Clarke) Munir (Lamiaceae) leaves on experimental gastric ulcer models. Biomed. Pharmacother. 2018, 97, 1109–1118. [Google Scholar]
- Warren, J.E.; Linit, M.J. Effect of Monochamus carolinensis on the Life history of the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 1993, 25, 703–709. [Google Scholar] [PubMed]
- Bolla, R.I.; Nosser, C.; Tamura, H. Chemistry of response of pines to Bursaphelenchus xylophilus: Resin acids. Jpn. J. Nematol. 1989, 19, 1–6. [Google Scholar]
- Chen, F.; Xu, M.; Yang, X.; Liu, J.; Xiao, Y.; Yang, L. An improved approach for the isolation of essential oil from the leaves of Cinnamomum longepaniculatum using microwave-assisted hydrodistillation concatenated double-column liquid-liquid extraction. Sep. Purif. Technol. 2018, 195, 110–120. [Google Scholar] [CrossRef]
- Kim, C.R.; Xu, M.; Yang, X.; Liu, J.; Xiao, Y.; Yang, L. Cinnamomum loureirii extract inhibits acetylcholinesterase activity and ameliorates trimethyltin-induced cognitive dysfunction in mice. Biol. Pharm. Bull. 2016, 39, 113–1136. [Google Scholar] [CrossRef]
- Zhu, M.; Luk, C.T.; Lew, T.H. Cytoprotective effect of Lindera aggregate roots against ethanol-induced acute gastric injury. Pharm. Biol. 1998, 36, 222–226. [Google Scholar] [CrossRef]
- Gnanendra Shanmugam, G.; Lee, S.K.; Jeon, J. Identification of potential nematicidal compounds against the pine wood nematode, Bursaphelenchus xylophilus through an in silico approach. Molecules 2018, 23, 1828. [Google Scholar] [CrossRef]
- Channabasava Govindappa, M.; Chandrashekhar, N. Determination of phytochemicals by GC-MS in two fractions (17 and 21) of methanol extract of Loranthus Micranthus and their antioxidant and anti-Inflammatory activity. Nat. Prod. J. 2015, 5, 1–18. [Google Scholar] [CrossRef]
- Chen, R.; Huo, L.; Liao, Y.; Li, P.Y.; Lu, R. Study on the chemical constituents of essential oils from the leaves of Viscum ovalifolium and Loranthus pentapetalus roxb. parasitizing on Guaiacum spp. Asian J. Chem. 2013, 25, 1757–1758. [Google Scholar]
- Salahdeen, H.M.; Omoaghe, A.O.; Isehunwa, G.O.; Murtala, B.A.; Alada, A.R.A. Gas chromatography mass spectrometry (GC-MS) analysis of ethanolic extracts of kolanut (Cola nitida) (vent) and its toxicity studies in rats. J. Med. Plants Res. 2015, 9, 56–70. [Google Scholar] [CrossRef][Green Version]
- Subban, M.; Ramasamy, V.; Annamalai, P. Evaluation of phytochemical constituents from the leaves of Memecylon umbellatum Burm.f. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 1145–1152. [Google Scholar]
- Falaki Khalilsaraie, M.; Saima, N.; Meti, N.T.; Bhadekar, R.K.; Nerkar, D.P. Cytological study and anti-microbial activity of embryogenic callus induced from leaf cultures of Tinospora cordifolia (Willd.) Miers. J. Med. Plants Res. 2011, 5, 3002–3006. [Google Scholar]
- Khan, Y.; Ansari, H.R.; Chauhan, R.; Tamboli, E.T.; Ahmad, S. Comparative gas chromatography-mass spectroscopy, Fourier transform infrared spectroscopy, and high-performance liquid chromatography analysis of essential oils extracted using 4 methods from the leaves of Eucalyptus globulus L. Drug Dev. Ther. 2016, 7, 81–86. [Google Scholar]
- Wang, H.; Zhang, J.; Yang, W.; Wang, X.; Cheng, L. A comparative research on the allelochemicals of Eucalyptus grandis in different woodland. Hebei Shifan Daxue Xuebao Ziran Kexueban 2009, 33, 94–99. [Google Scholar]
- Silva, M.R.; Bueno, G.H.; Araujo, R.L.B.; Lacerda, I.C.A.; Freitas, L.G.; Morais, H.A.; Augusti, R.; Melo, J.O.F. Evaluation of the influence of extraction conditions on the isolation and identification of volatile compounds from cagaita (Eugenia dysenterica) using HS-SPME/GC-MS. J. Braz. Chem. Soc. 2019, 30, 379–387. [Google Scholar] [CrossRef]
- Hanawa, F.; Yamada, T.; Nakashima, T. Phytoalexins from Pinus strobus bark infected with pinewood nematode, Bursaphelenchus xylophilus. Phytochemistry 2001, 57, 223–228. [Google Scholar] [CrossRef]
- Hu, Q.; Jin, J.; Du, Y.B.; Fan, J.T. Variation of the composition of attractants in lures for Monochamus alternatus (Coleoptera: Cerambycidae) in the field and its influence on trapping efficacy. Acta Entomol. Sin. 2018, 61, 1310–1318. [Google Scholar]
- Gupta, M.; Gupta, A.; Gupta, S. In vitro antimicrobial and phytochemical analysis of dichloromethane extracts of Piper nigrum (black pepper). Orient. J. Chem. 2013, 29, 777–782. [Google Scholar] [CrossRef]
- Ajayi, G.O.; Olagunju, J.A.; Ademuyiwa, O.; Martin, O.C. Gas chromatography-mass spectrometry analysis and phytochemical screening of ethanolic root extract of Plumbago zeylanica Linn. J. Med. Plants Res. 2011, 5, 1756–1761. [Google Scholar]
- Samejo, M.Q.; Memon, S.; Bhanger, M.I.; Khan, K.M. Essential oil constituents in fruit and stem of Calligonum polygonoides. Ind. Crops Prod. 2013, 45, 293–295. [Google Scholar] [CrossRef]
- Chen, F.; Jia, J.; Zhang, Q.; Yang, L.; Gu, H. Isolation of essential oil from the leaves of Polygonum viscosum Buch-ham. using microwave-assisted enzyme pretreatment followed by microwave hydrodistillation concatenated with liquid-liquid extraction. Ind. Crops Prod. 2018, 112, 327–341. [Google Scholar] [CrossRef]
- Ikeda, T.; Enda, N.; Yamane, A.; Oda, K.; Toyoda, T. Attractants for the Japanese pine sawyer, Monochamus alternatus Hope (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1980, 15, 358–361. [Google Scholar] [CrossRef]
- Ishikawa, M.; Shuto, Y.; Watanabe, H. β-Myrcene, a potent attractant component of pine wood for the pine wood nematode, Bursaphelenchus xylophilus. Agric. Biol. Chem. 1986, 50, 1863–1866. [Google Scholar] [CrossRef]
- Ucar, E. In-vitro antioxidant and antmicrobial activities and various enzyme inhibitory activities of ethanlo extracts from different organs of Consolida regalis. Fresenius Environ. Bull. 2018, 27, 5950–5957. [Google Scholar]
- Qin, Z.; Zhang, Z.G.; Liu, H.M.; Qin, G.Y.; Wang, X.D. Acetic acid lignins from Chinese quince fruit (Chaenomeles sinensis): Effect of pretreatment on their structural features and antioxidant activities. RSC Adv. 2018, 8, 24923–24931. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Ye, J.; Li, G. Effects of phytotoxic extracts from peach root bark and benzoic acid on peach seedlings growth, photosynthesis, antioxidance and ultrastructure properties. Sci. Hortic. 2015, 215, 49–58. [Google Scholar] [CrossRef]
- Murathan, Z.T.; Zarifikhosroshahi, M.; Kafkas, E.; Sevindik, E. Chemical composition, volatiles, and antioxidant activity of Rosa iberica Stev. hips. Acta Sci. Pol. Hortorum Cultus 2016, 15, 41–54. [Google Scholar]
- Kim, J.; Lee, S.M.; Jung, Y.K.; Kwaon, Y.D.; Kim, D.S.; Lee, D.W.; Park, C.G. Field evaluation on the synergistic attractiveness of 2-(1-undecyloxy)-1-ethanol and ipsenol to Monochamus saltuarius. Entomol. Res. 2016, 46, 31–35. [Google Scholar] [CrossRef]
- Ji, G.; Yu, L.; Zhang, C.; Zou, J.; Liu, Y. Primary study on allelopathic substance in litter of Zanthoxylum planispinum. Guizhou Agric. Sci. 2011, 39, 45–47. [Google Scholar]
- Edewor, T.; Olabisi, K.N.; Oluwagbemiga, O.S. Gas chromatography-mass spectrometric analysis of methanolic leaf extracts of Lannea kerstingii and Nauclea diderrichii, two medicinal plants used for the treatment of gastrointestinal tract infections. Asian J. Pharm. Clin. Res. 2016, 9, 179–182. [Google Scholar]
- Ghahari, S.; Alinezhad, H.; Nematzadeh, G.A.; Ghahari, S. Phytochemical screening and antimicrobial activities of the constituents isolated from Koelreuteria paniculata leaves. Nat. Prod. Res. 2015, 29, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Armatu, A.; Bodirlau, R.; Nechita, C.B.; Niculaua, M.; Teaca, C.A.; Ichim, M.; Spiridon, I. Characterization of biological active compounds from Verbascum phlomoides by chromatography techniques. I. Gas chromatography. Rom. Biotechnol. Lett. 2011, 16, 6297–6304. [Google Scholar]
- Xie, Z.; Zhao, Z.; Wu, G.; Ye, X.; Shi, L.; Guo, J. Component analysis of the root exudates at different growth stages in chili pepper. Acta Agric. Bor.-Occid. Sin. 2012, 21, 175–181. [Google Scholar]
- Chang, P.; Liang, Y.; Zhang, J.; Yang, J.H.; Liu, J.Y.; Lu, J.; Zhao, J.J. Volatile components and quality characteristics of cherry tomato from five color varieties. Food Sci. 2014, 35, 215–221. [Google Scholar]
- Zhou, B.L.; Chen, Z.X.; Du, L.; Xie, Y.H.; Zhang, Q.; Ye, X.L. Allelopathy of root exudates from different resistant eggplants to Verticillium dahliae and the identification of allelochemicals. Afr. J. Biotechnol. 2011, 10, 8284–8290. [Google Scholar]
- Sharma, N.; Rautela, I.; Sharma, M.D. Mass propagation and GC-MS analysis of critically endangered plant Withania coagulans. Int. J. Appl. Biol. Pharm. Technol. 2016, 7, 62–71. [Google Scholar]
- Fu, Q.; Liu, C.; Wang, Y.; Li, C.; Hu, Q. Germination inhibitors of Sinojackia sarcocarpa L. Q. Luo drupe methanol extract. Beifang Yuanyi 2015, 2, 6–9. [Google Scholar]
- Yan, Z.M.; Zhang, Y.S.; Nan, H.J.; Wei, X.J. Medicine and Biopharmaceuticals, Proceedings of the International Conference; Guangxi Normal University: Guilin, China, 2016. [Google Scholar]
- Kim, J.; Lee, S.; Park, C.G. Bursaphelenchus xylophilus is killed by homologues of 2-(1-undecyloxy)-1-ethanol. Sci. Rep. 2016, 6, e29300. [Google Scholar] [CrossRef]
- Dar, S.A.; Yousuf, A.R.; Ganai, F.A.; Sharma, P.; Kumar, N.; Singh, R. Bioassay guided isolation and identification of anti-inflammatory and anti-microbial compounds from Urtica dioica L. (Urticaceae) leaves. Afr. J. Biotechnol. 2012, 11, 12910–12920. [Google Scholar]
- Muhammad, N.; Khan, A.; Saeed, M.; Khan, H.; Khan, S.S.; Shareef, H.; Khan, Z.; Farooq, U.; Zahoor, M. Fixed oil composition and biological screening of Viola betonicifolia in different solvents. J. Chem. Soc. Pak. 2016, 38, 115–121. [Google Scholar]
- Zhang, S.; Ao, K.; Zeng, Q.; Liu, Y. Determination of volatile oil components in Ampelopsis grossedentata by GC-MS. Zhongguo Niangzao 2014, 33, 140–143. [Google Scholar]
- Basu, S.; Choudhury, U.R.; Das, M.; Datta, G. Identification of bioactive components in ethanolic and aqueous extracts of Amorphophallus campanulatus tuber by GC-MC analysis. Int. J. Phytomedicine 2013, 5, 243–251. [Google Scholar]
- Yang, H.; Zhou, W.; Zhang, Q.; Li, W. Analysis of flavor components in the coconut juice and beverage based on GC-MS method. Xiandai Shipin Keji 2014, 30, 286–290. [Google Scholar]
- Hema, N.; Avadhani, R.; Ashwini, P.; Sunil Kumar, K.N. GC-MS characterization of n-hexane soluble compounds of Cyperus rotundus L. rhizomes. J. Appl. Pharm. Sci. 2016, 5, 96–100. [Google Scholar]
- Aneela, S.; Dey, A.; De, S. Gas chromatography-mass spectrometry analysis of Kyllinga triceps. Int. J. Pharm. Sci. Res. 2014, 5, 2999–3003. [Google Scholar]
- Seenivasan, N. Phytochemical profiling of burrowing nematode (Radopholus similis) resistant and susceptible banana (Musa spp.) genotypes for detection of marker compounds. Fruits 2018, 73, 48–59. [Google Scholar] [CrossRef]
- Kohno, T.; Togashi, K.; Fukamiya, N. The nematocidal activity and the structure-activity relationships of stilbenes. Nat. Prod. Res. 2007, 21, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Pajares, J.A.; Alvarez, G.; Ibeas, F.; Gallego, D.; Hall, D.R.; Farman, D.I. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J. Chem. Ecol. 2010, 36, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Chung, I.M.; Khanh, T.D.; Tawata, S. Identification of phytotoxic substances from early growth of barnyard grass (Echinochloa crusgalli) root exudates. J. Chem. Ecol. 2006, 32, 895–906. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, X.; Lin, R.; Jia, X.; Xiong, J.; Shen, L.; Liang, Y. Chemical components of root exudates from allelopathic rice accession PI312777 seedlings. J. App. Ecol. 2005, 16, 2383–2388. [Google Scholar]
- Hong, H.; Yu, Y.; Zhou, W.; Li, B. Analysis of extracts components of parenchyma in Phyllostachys pubescens by GC-MS. Zhongnan Linye Keji Daxue Xuebao 2015, 35, 114–117. [Google Scholar]
- Mody, N.V.; Bhattacharyya, J.; Miles, D.H.; Hedin, P.A. Constituents of marsh grass. II. Survey of the essential oil in Spartina cynosuroides. Phytochemistry 1974, 13, 1175–1178. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.; Tao, B.; Teng, C. Allelopathy and preliminary separation of allelopathic substance in the extracts of wheat seed germination. Adv. Mater. Res. 2012, 468, 565–568. [Google Scholar] [CrossRef]
- Bordoloi, A.K.; Sperkova, J.; Leclercq, P.A. Essential oils of Zingiber cassumunar Roxb. from northeast India. J. Essent. Oil Res. 1999, 11, 441–445. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. https://doi.org/10.3390/toxins12010035
Zhao F, Wang P, Lucardi RD, Su Z, Li S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins. 2020; 12(1):35. https://doi.org/10.3390/toxins12010035
Chicago/Turabian StyleZhao, Fuqiang, Ping Wang, Rima D. Lucardi, Zushang Su, and Shiyou Li. 2020. "Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs" Toxins 12, no. 1: 35. https://doi.org/10.3390/toxins12010035
APA StyleZhao, F., Wang, P., Lucardi, R. D., Su, Z., & Li, S. (2020). Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins, 12(1), 35. https://doi.org/10.3390/toxins12010035





