Occurrence and Risk Assessment of Mycotoxins through Polish Beer Consumption
Abstract
1. Introduction
2. Results and Discussion
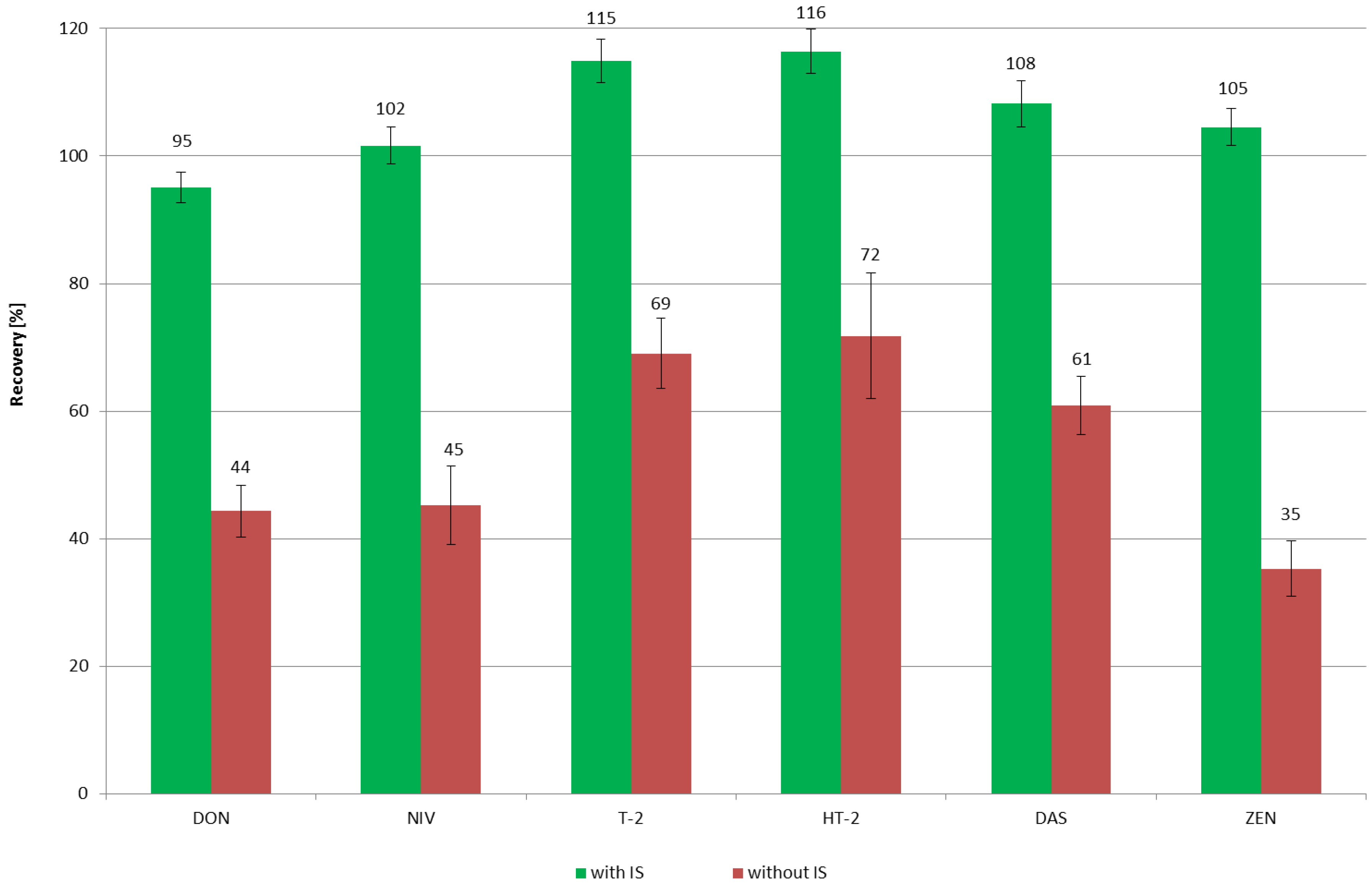
2.1. Method Validation
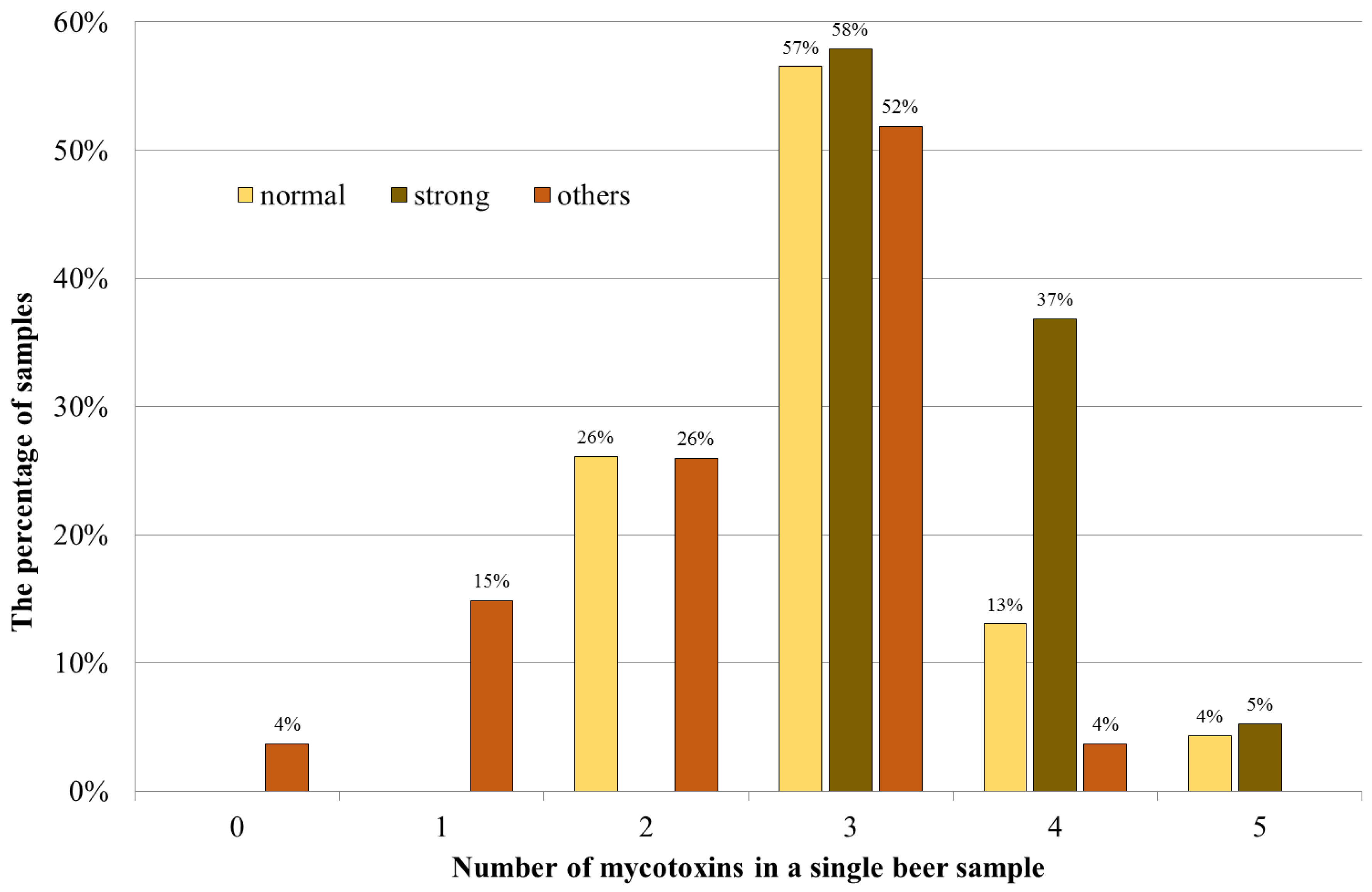
2.2. Mycotoxins Occurrence
2.3. Dietary Exposure Assessment
3. Conclusions
4. Materials and Methods
4.1. Sampling
4.2. Standards and Chemicals
4.3. Sample Preparation
4.4. Instrumental Analysis
4.5. Statistical Analysis
4.6. Dietary Exposure Assessment
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abv | alcohol by volume |
| ACN | acetonitrile |
| b.w. | body-weight |
| DAS | diacetoxyscirpenol |
| DON | deoxynivalenol |
| EDI | estimated daily intake |
| HPLC | high performance liquid chromatography |
| ISs | internal standards |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| NIV | nivalenol |
| OTA | ochratoxin A |
| TDI | tolerable daily intake |
| ZEN | zearalenone |
References
- Hornsey, I. A History of Beer and Brewing; The Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- European Commission. Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- European Commission. Commission regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- European Commission. Commission regulation (EU) No 105/2010 of 5 February 2010 amending regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A. Off. J. Eur. Union 2010, L35, 7–8. [Google Scholar]
- European Commission. Commission regulation (EU) No 165/2010 of 26 February 2010 amending Regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, L50, 8–12. [Google Scholar]
- European Commission. Commission regulation (EU) No 594/2012 of 5 July 2012 amending Regulation (EC) 1881/2006 as regards the maximum levels of the contaminants ochratoxin A, non dioxin-like PCBs and melamine in foodstuffs. Off. J. Eur. Union 2012, L176, 43–45. [Google Scholar]
- European Commission. Commission recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- Beer Production 2010–2016. In Beer Statistics 2017; The Brewers of Europe: Brussels, Belgium, 2017.
- Milićević, D.R.; Škrinjar, M.; Baltić, T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins 2010, 2, 572–592. [Google Scholar] [CrossRef]
- Bayman, P.; Baker, J.L. Ochratoxins: A global perspective. Mycopathologia 2006, 162, 215–223. [Google Scholar] [CrossRef]
- Anli, E.; Mert Alkis, I. Ochratoxin A and brewing technology: A review. J. Inst. Brew. 2010, 116, 23–32. [Google Scholar] [CrossRef]
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostalek, P.; Sachambula, L. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. 2008, 25, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Kostelanska, M.; Zachariasova, M.; Lacina, O.; Fenclova, M.; Kollos, A.L.; Hajslova, J. The study of deoxynivalenol and its masked metabolites fate during the brewing process realised by UPLC-TOFMS method. Food Chem. 2011, 126, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Hall, C.E. Mold and mycotoxin problems encountered during malting and brewing. Int. J. Food Microbiol. 2007, 119, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Habschied, K.; Šarkanj, B.; Klapec, T.; Krstanović, V. Distribution of zearalenone in malted barley fractions dependent on Fusarium graminearum growing conditions. Food Chem. 2011, 129, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Bĕláková, S.; Benešová, K.; Čáslavský, J.; Svoboda, Z. The occurrence of the selected Fusarium mycotoxins in Czech malting barley. Food Control. 2014, 37, 93–99. [Google Scholar] [CrossRef]
- Jørgensen, K. Survey of pork, poultry, coffee, beer and pulses for ochratoxin A. Food Addit. Contam. 1998, 15, 550–554. [Google Scholar] [CrossRef]
- Medina, A.; Valle-Algarra, F.M.; Gimeno-Adelantado, J.V.; Mateo, R.; Mateo, F.; Jiménez, M. New method for determination of ochratoxin A in beer using zinc acetate and solid-phase extraction silica cartridges. J. Chromatogr. A 2006, 1121, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Odhav, B.; Naicker, V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, P.; Berner, D.; Jodlbauer, J.; Lindner, W. Determination of zearalenone and its metabolites alpha- and beta-zearalenol in beer samples by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000, 738, 233–241. [Google Scholar] [CrossRef]
- Schothorst, R.C.; Jekel, A.A. Determination of trichothecenes in beer by capillary gas chromatography with flame ionisation detection. Food Chem. 2003, 82, 475–479. [Google Scholar]
- Rodríguez-Carrasco, Y.; Fattore, M.; Albrizio, S.; Berrada, H.; Mañes, J. Occurrence of Fusarium mycotoxins and their dietary intake through beer consumption by the European population. Food Chem. 2015, 178, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, K.C.; Savi, G.D.; Olivo, G.; Scussel, V.M. Quality and occurrence of deoxynivalenol and fumonisins in craft beer. Food Control 2015, 50, 925–929. [Google Scholar] [CrossRef]
- European Commission. Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- European Commission. Commission regulation (EU) No 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union 2014, L147, 29–43. [Google Scholar]
- Habler, K.; Gotthardt, M.; Schüler, J.; Rychlik, M. Multi-mycotoxin stable isotope dilution LC-MS/MS method for Fusarium toxins in beer. Food Chem. 2017, 218, 447–454. [Google Scholar] [CrossRef]
- Bresch, H.; Urbanek, M.; Hell, K. Ochratoxin A in coffee, tea and beer. Arch. Fur Lebensm. 2000, 51, 89–94. [Google Scholar]
- Tangni, E.K.; Ponchaut, S.; Maudoux, M.; Rozenberg, R.; Larondelle, Y. Ochratoxin A in domestic and imported beers in Belgium: Occurrence and exposure assessment. Food Addit. Contam. 2002, 19, 1169–1179. [Google Scholar] [CrossRef]
- Medina, A.; Jiménez, M.; Gimeno-Adelantado, J.V.; Valle-Algarra, F.M.; Mateo, R. Determination of ochratoxin A in beer marketed in Spain by liquid chromatography with fluorescence detection using lead hydroxyacetate as a clean-up agent. J. Chromatogr. A 2005, 1083, 7–13. [Google Scholar] [CrossRef]
- Varga, E.; Malachowa, A.; Schwartz, H.; Krska, R.; Berthiller, F. Survey of deoxynivalenol and its conjugates deoxynivalenol-3-glucoside and 3-acetyl-deoxynivalenol on 374 beer samples. Food Addit. Contam. 2013, 30, 137–146. [Google Scholar] [CrossRef]
- Bauer, J.I.; Gross, M.; Gottschalk, C.; Usleber, E. Investigations on the occurrence of mycotoxins in beer. Food Control 2016, 63, 135–139. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Co-occurrence of nivalenol, deoxynivalenol and deoxynivalenol-3-glucoside in beer samples. Food Control. 2018, 92, 319–324. [Google Scholar] [CrossRef]
- Pascari, X.; Ortiz-Solá, J.; Marín, S.; Ramos, A.J.; Sanchis, V. Survey of mycotoxins in beer and exposure assessment through the consumption of commercially available beer in Lleida, Spain. LWT Food Sci. Technol. 2018, 92, 87–91. [Google Scholar] [CrossRef]
- Peters, J.; van Dam, R.; van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- The Yearbook of Foreign Trade Statistics of the Polish Central Statistical Office, Warsaw. 2017. Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-statystyczny-handlu-zagranicznego-2017,9,11.html (accessed on 4 April 2019).
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Statement on recent scientific information on the toxicity of Ochratoxin A. EFSA J. 2010, 8, 1626. [Google Scholar] [CrossRef]
- European Commission Task 3.2.7. Assessment of dietary intake of Ochratoxin A by the population of EU Member States. 2002. Available online: https://ec.europa.eu/food/safety/chemical_safety/contaminants/catalogue/ochratoxin_en (accessed on 4 April 2019).
- Coronel, M.B.; Marín, S.; Cano-Sancho, G.; Ramos, A.J.; Sanchis, V. Exposure assessment to ochratoxin A in Catalonia (Spain) based on the consumption of cereals, nuts, coffee, wine, and beer. Food Addit. Contam. Part A 2012, 29, 979–993. [Google Scholar] [CrossRef]
- GEMS/Food Consumption Cluster Diets. Global Environment Monitoring System Food Contamination Monitoring and Assessment Programme. Department of Food Safety and Zoonoses, World Health Organization: Geneva, 2012. Available online: https://extranet.who.int/sree/Reports?op=vs&path=/WHO_HQ_Reports/G7/PROD/EXT/GEMS_cluster_diets_2012&userid=G7_ro&password=inetsoft123 (accessed on 4 April 2019).
| Beer Production (105 L) | |||||
|---|---|---|---|---|---|
| Country | 2012 | 2013 | 2014 | 2015 | 2016 |
| Germany | 94.6 | 94.4 | 95.3 | 95.6 | 95.0 |
| United Kingdom | 44.2 | 44.2 | 44.3 | 44.0 | 43.7 |
| Poland | 39.3 | 40.0 | 40.1 | 40.9 | 41.4 |
| Spain | 33.0 | 32.7 | 33.6 | 35.0 | 36.5 |
| The Netherlands | 24.3 | 23.6 | 23.7 | 24.0 | 24.6 |
| France | 17.6 | 18.3 | 19.9 | 20.3 | 20.7 |
| Belgium | 18.8 | 18.1 | 18.2 | 19.8 | 20.7 |
| Czech Republic | 18.7 | 18.7 | 19.1 | 19.5 | 20.5 |
| Romania | 18.0 | 16.1 | 14.8 | 16.0 | 15.8 |
| Mycotoxin | Spiking Level [ng/mL] | Calculated Concentration [ng/mL] | Recovery [%] | Precision [%] | LOD [ng/mL] | LOQ [ng/mL] |
|---|---|---|---|---|---|---|
| OTA | 0.05 | 0.0408 | 81.6 | 2.5 | 0.003 | 0.011 |
| 0.25 | 0.199 | 79.6 | 6.1 | |||
| 1.0 | 0.858 | 85.8 | 4.2 | |||
| DON | 40 | 38.16 | 95.4 | 2.9 | 3.50 | 11.6 |
| 200 | 190.6 | 95.3 | 1.2 | |||
| 400 | 378.2 | 94.5 | 3.9 | |||
| NIV | 40 | 40.84 | 102.1 | 5.2 | 4.30 | 14.3 |
| 200 | 202.2 | 101.1 | 1.7 | |||
| 400 | 407.2 | 101.8 | 1.3 | |||
| T-2 | 8 | 9.36 | 117.0 | 3.9 | 0.31 | 1.03 |
| 40 | 44.64 | 111.6 | 3.0 | |||
| 80 | 92.8 | 116.0 | 1.3 | |||
| HT-2 | 8 | 9.10 | 113.8 | 5.2 | 0.36 | 1.21 |
| 40 | 47.64 | 119.1 | 3.0 | |||
| 80 | 93.04 | 116.3 | 1.2 | |||
| DAS | 8 | 8.72 | 109.0 | 4.0 | 0.28 | 0.92 |
| 40 | 43.92 | 109.8 | 2.6 | |||
| 80 | 84.64 | 105.8 | 4.1 | |||
| ZEN | 4 | 4.14 | 103.5 | 3.4 | 0.07 | 0.23 |
| 20 | 20.74 | 103.7 | 2.5 | |||
| 40 | 42.56 | 106.4 | 3.0 |
| Toxin | Positive/Samples | Mean [ng/mL] | Median [ng/mL] | Positive Samples [ng/mL] | ||
|---|---|---|---|---|---|---|
| Min | Max | Mean; SD | ||||
| OTA | 64/69 | 0.053 | 0.032 | <LOQ | 0.347 | 0.057; 0.065 |
| DON | 66/69 | 16.3 | <LOQ | <LOQ | 56.2 | 17.1; 9.0 |
| NIV | 1/69 | <LOD | <LOD | - | <LOQ | - |
| T-2 | 11/69 | <LOD | <LOD | <LOQ | <LOQ | <LOQ |
| HT-2 | 51/69 | <LOQ | <LOQ | <LOQ | 1.57 | 1.23; 0.08 |
| DAS | 0/69 | - | - | - | <LOD | - |
| ZEN | 4/69 | <LOD | <LOD | <LOQ | 0.413 | 0.304; 0.073 |
| Beer Type | Positive/Samples | Mean [ng/mL] | Median [ng/mL] | Positive Samples [ng/mL] | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | |||||
| OTA | Normal lager | 23/23 | 0.051 | 0.034 | <LOQ | 0.347 | 0.051 |
| Strong lager | 19/19 | 0.062 | 0.056 | 0.012 | 0.114 | 0.062 | |
| Porter | 5/5 | 0.111 | 0.068 | <LOQ | 0.262 | 0.112 | |
| Unpasteurized | 8/9 | 0.059 | 0.023 | <LOQ | 0.269 | 0.066 | |
| Flavored | 5/8 | 0.017 | 0.014 | <LOQ | 0.046 | 0.027 | |
| Non-alcoholic | 4/5 | 0.013 | <LOQ | <LOQ | 0.030 | 0.016 | |
| DON | Normal lager | 22/23 | 17.9 | <LOQ | <LOQ | 56.2 | 18.7 |
| Strong lager | 19/19 | 19.2 | 18.8 | <LOQ | 34.7 | 19.2 | |
| Porter | 4/5 | 16.5 | <LOQ | <LOQ | 33.8 | 20.7 | |
| Unpasteurized | 9/9 | 13.4 | <LOQ | <LOQ | 16.3 | 13.4 | |
| Flavored | 8/8 | 12.3 | <LOQ | <LOQ | 16.7 | 12.3 | |
| Non-alcoholic | 4/5 | <LOQ | <LOQ | <LOQ | 12.7 | 11.9 | |
| HT-2 | Normal lager | 17/23 | <LOQ | <LOQ | <LOQ | 1.57 | 1.23 |
| Strong lager | 19/19 | 1.23 | <LOQ | <LOQ | 1.52 | 1.23 | |
| Porter | 4/5 | <LOQ | <LOQ | <LOQ | 1.52 | 1.29 | |
| Unpasteurized | 6/9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
| Flavored | 3/8 | <LOQ | <LOD | <LOQ | <LOQ | <LOQ | |
| Non-alcoholic | 2/5 | <LOQ | <LOD | <LOQ | <LOQ | <LOQ | |
| Mycotoxin | Number of Analyzed Beer Samples | Number of Positive Samples | Mycotoxin Concentration Range (ng/mL) | Reference |
|---|---|---|---|---|
| OTA | 69 | 69 | 0.008–0.498 | [18] |
| 19 | 10 | 1.5–2340 | [19] | |
| 318 | 233 | 0.01–0.293 | [27] | |
| 88 | 73 | 0.007–0.204 | [29] | |
| 1000 | 6 | <0.3–0.6 | [34] | |
| DON | 154 | 60 | 24.5–47.7 | [22] |
| 374 | 204 | 3.2–89.3 | [30] | |
| 44 | 33 | 2.2–20.0 | [31] | |
| 100 | 83 | 1.0–73.6 | [32] | |
| 64 | 4 | 20.97–46.74 | [33] | |
| 1000 | 64 | <10–412 | [34] | |
| NIV | 100 | 56 | 0.5–7.6 | [32] |
| 1000 | 4 | 8–21 | [34] | |
| T-2 | 1000 | 3 | <0.5–2.3 | [34] |
| HT-2 | 154 | 14 | 24.2–38.2 | [22] |
| 64 | 1 | 23.72 | [33] | |
| 1000 | 1 | 3.4 | [34] | |
| ZEN | 35 | 7 | 2.6–426 | [19] |
| 64 | 12 | 8.24–62.96 | [33] | |
| 44 | 44 | 0.35–2.0 | [31] | |
| 1000 | 6 | <0.3–0.3 | [34] |
| Cluster | Country | Toxin Level | EDI [ng/kg b.w.] | % TDI | ||||
|---|---|---|---|---|---|---|---|---|
| OTA | DON | HT-2 | OTA | DON | HT-2 | |||
| G07 | UK | mean | 0.14 | 42.0 | 3.11 | 0.80 | 4.20 | 5.19 |
| maximum | 0.89 | 145 | 4.00 | 5.21 | 14.5 | 6.74 | ||
| G08 | Germany Poland | mean | 0.20 | 60.4 | 4.48 | 1.14 | 6.04 | 7.47 |
| maximum | 1.28 | 208 | 5.80 | 7.50 | 20.8 | 9.69 | ||
| G10 | Canada Italy USA | mean | 0.13 | 40.6 | 3.01 | 0.77 | 4.06 | 5.02 |
| maximum | 0.86 | 140 | 3.90 | 5.04 | 14.0 | 6.52 | ||
| G15 | Czech Republic Hungary Ireland Romania Slovakia | mean | 0.17 | 52.4 | 3.89 | 0.99 | 5.24 | 6.49 |
| maximum | 1.12 | 181 | 5.10 | 6.51 | 18.1 | 8.42 | ||
| Compound | Precursor Ion (m/z) | Declustering Potential (V) | Product Ions (m/z) a | Collision Energy (V) | Cell Exit Potential (V) |
|---|---|---|---|---|---|
| Nivalenol | 371.1 [M + CH3COO]− | −40 | 281.0/59.0 | −22/−40 | −14/−5 |
| Deoxyniwalenol | 355.1 [M + CH3COO]− | −35 | 264.8/58.9 | −20/−38 | −17/−1 |
| 13C-Deoxyniwalenol | 370.2 [M + CH3COO]− | −50 | 310.0 | −14 | −7 |
| Diacetoxyscirpenol | 384.1 [M + NH4]+ | 51 | 307.0/247.0 | 17/19 | 20/16 |
| T-2 toxin | 484.1 [M + NH4]+ | 61 | 215.0/185.0 | 29/25 | 12/14 |
| 13C-T-2 toxin | 508.3 [M + NH4]+ | 61 | 322.1 | 19 | 8 |
| HT-2 toxin | 442.2 [M + NH4]+ | 51 | 215.0/263.0 | 19/17 | 14/18 |
| 13C-HT-2 toxin | 464.1 [M + NH4]+ | 51 | 278.1 | 17 | 18 |
| Zearalenone | 317.1 [M − H]− | −85 | 130.8/174.9 | −40/−32 | −7/−9 |
| 13C-Zearalenone | 355.1 [M − H]− | −100 | 139.9 | −42 | −7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grajewski, J.; Kosicki, R.; Twarużek, M.; Błajet-Kosicka, A. Occurrence and Risk Assessment of Mycotoxins through Polish Beer Consumption. Toxins 2019, 11, 254. https://doi.org/10.3390/toxins11050254
Grajewski J, Kosicki R, Twarużek M, Błajet-Kosicka A. Occurrence and Risk Assessment of Mycotoxins through Polish Beer Consumption. Toxins. 2019; 11(5):254. https://doi.org/10.3390/toxins11050254
Chicago/Turabian StyleGrajewski, Jan, Robert Kosicki, Magdalena Twarużek, and Anna Błajet-Kosicka. 2019. "Occurrence and Risk Assessment of Mycotoxins through Polish Beer Consumption" Toxins 11, no. 5: 254. https://doi.org/10.3390/toxins11050254
APA StyleGrajewski, J., Kosicki, R., Twarużek, M., & Błajet-Kosicka, A. (2019). Occurrence and Risk Assessment of Mycotoxins through Polish Beer Consumption. Toxins, 11(5), 254. https://doi.org/10.3390/toxins11050254





