Varanid Lizard Venoms Disrupt the Clotting Ability of Human Fibrinogen through Destructive Cleavage
Abstract
1. Introduction
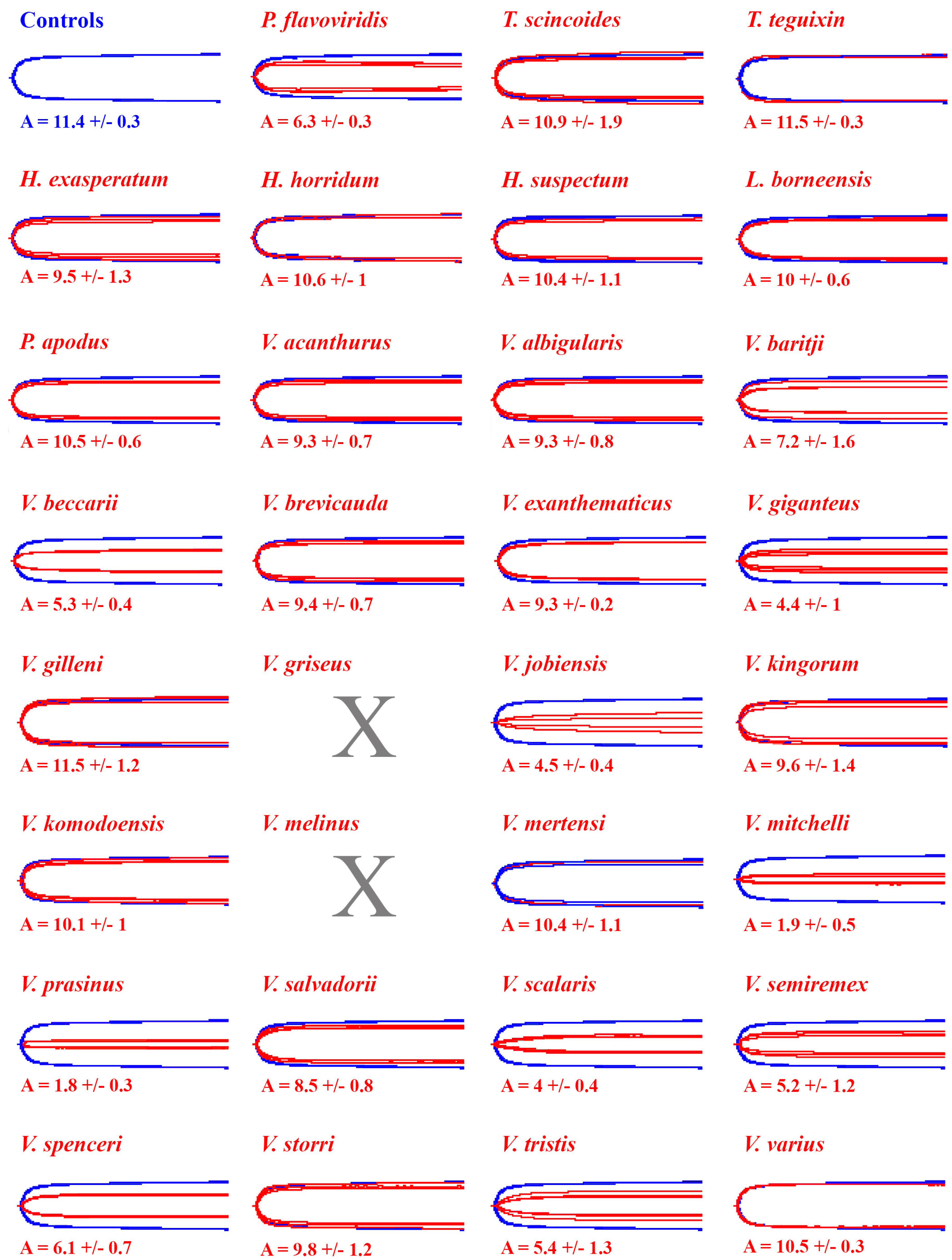
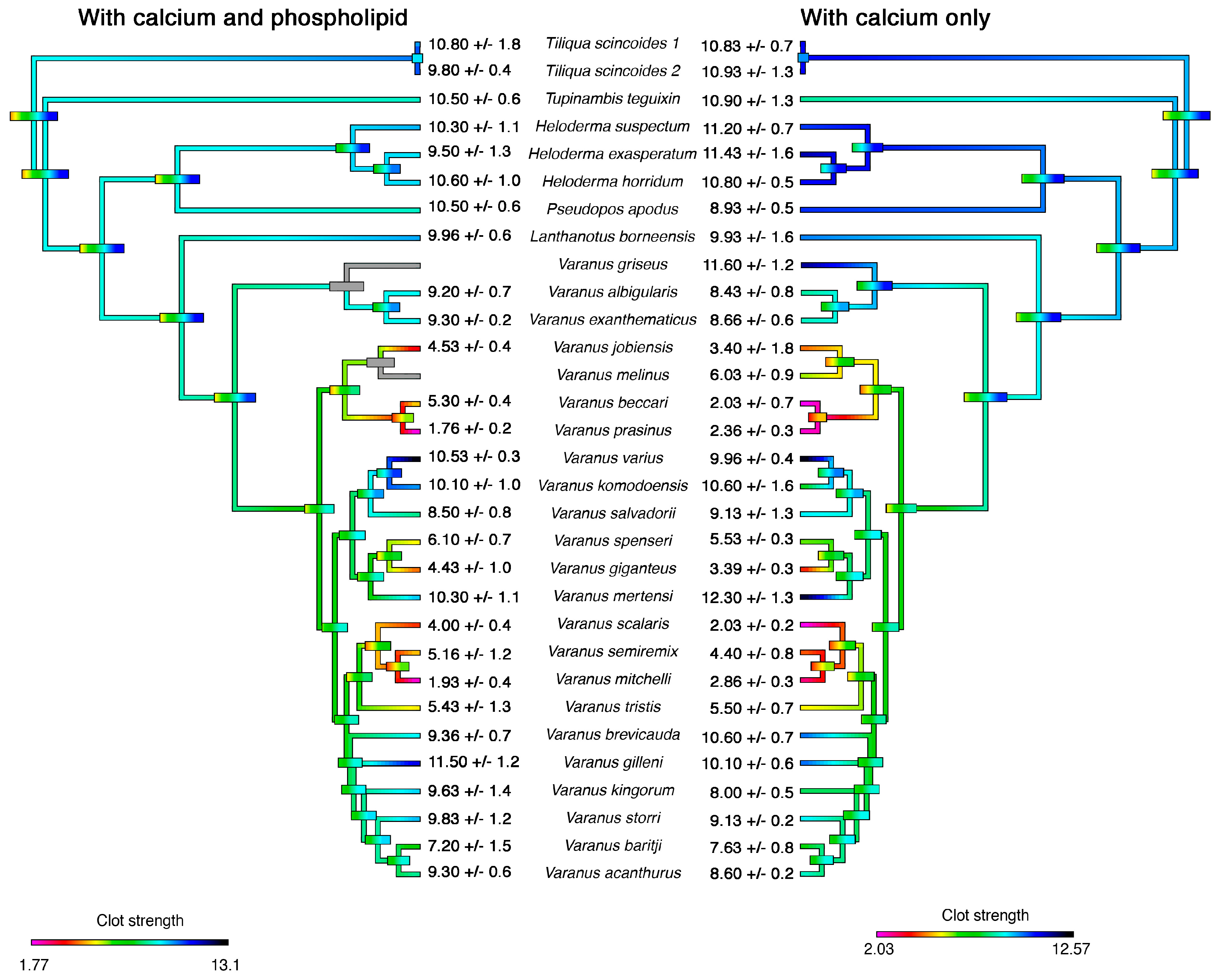
2. Results
3. Discussion
4. Materials and Methods
4.1. Venom Samples
4.2. Thromboelastography
4.3. Figures and Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Hedges, S.B. The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. Comptes Rendus Biol. 2005, 328, 1000–1008. [Google Scholar] [CrossRef]
- Vidal, N.; Hedges, S.B. The molecular evolutionary tree of lizards, snakes, and amphisbaenians. Comptes Rendus Biol. 2009, 332, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Pyron, R.; Burbrink, F.; Wiens, J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef]
- Pyron, R.A. Novel approaches for phylogenetic inference from morphological data and total-evidence dating in squamate reptiles (Lizards, Snakes, and Amphisbaenians). Syst. Biol. 2017, 66, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.B.; Vidal, N. Lizards, snakes, and amphisbaenians (Squamata). In The Timetree of Life; Hedges, S.B., Kumar, S., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 383–389. [Google Scholar]
- Reeder, T.W.; Townsend, T.M.; Mulcahy, D.G.; Noonan, B.P.; Wood, P.L.; Sites, J.W.; Wiens, J.J. Integrated analyses resolve conflicts over squamate Reptile Phylogeny and Reveal Unexpected Placements for Fossil Taxa. PLoS ONE 2015, 10, 1–22. [Google Scholar] [CrossRef]
- Wiens, J.J.; Kuczynski, C.A.; Townsend, T.; Reeder, T.W.; Mulcahy, D.G.; Sites, J.W. Combining phylogenomics and fossils in higher-level squamate reptile phylogeny: Molecular data change the placement of fossil taxa. Syst. Biol. 2010, 59, 674–688. [Google Scholar] [CrossRef]
- Zheng, Y.; Wiens, J.J. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 2016, 94, 537–547. [Google Scholar] [CrossRef]
- Losos, J.B.; Hillis, D.M.; Greene, H.W. Who speaks with a forked tongue? Science 2012, 338, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Sweet, S.S.; Pianka, E.R. Monitors, Mammals, and Wallace’s Line. In Advances in Monitor Research III; Horn, H.G., Boehme, W., Krebs, U., Eds.; Mertensiella: Rheinbach, Germany, 2007; pp. 79–99. [Google Scholar]
- Vitt, L.J. Walking the Natural-History Trail. Herpetologica 2013, 69, 105–117. [Google Scholar] [CrossRef]
- Sweet, S.S. Chasing Flamingos: Toxicofera and the Misinterpretation of Venom in Varanid Lizards. Proceedings of the 2015 Interdisciplinary World Conference on Monitor Lizards; Cota, M., Ed.; Suan Sunandha Rajabhat University: Bangkok, Thailand, 2016; pp. 123–149. [Google Scholar]
- Russell, F.E.; Bogert, C.M. Gila Monster: Its Biology, Venom and Bite-A Review. Toxicon 1981, 19, 341–359. [Google Scholar] [CrossRef]
- Hargreaves, A.; Tucker, A.S.; Mulley, J.A.; Tucker, A.S.; Mulley, J. A Critique of the Toxicoferan Hypothesis. In Evolution of Venomous Animals and Their Toxins; Gopalakrishnakone, P., Malhotra, A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–15. [Google Scholar]
- Montgomery, J.M.; Gillespie, D.; Sastrawan, P.; Fredeking, T.M.; Stewart, G.L. Aerobic salivary bacteria in wild and captive Komodo dragons. J. Wildl. Dis. 2002, 38, 545–551. [Google Scholar] [CrossRef]
- Pianka, E.R.; Sweet, S.S. Field Observations by Two American Varanophiles. Proceedings of the 2015 Interdisciplinary World Conference on Monitor Lizards; Cota, M., Ed.; Suan Sunandha Rajabhat University: Bangkok, Thailand, 2016; pp. 1–68. [Google Scholar]
- Weinstein, S.A.; Smith, T.L.; Kardong, K.V. Reptile venom glands: Form., function, and future. In CRC Handbook of Reptile Venoms and Toxins; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Goldstein, E.J.C.; Tyrrell, K.L.; Citron, D.M.; Cox, C.R.; Recchio, I.M.; Okimoto, B.; Bryja, J.; Fry, B.G. Anaerobic and aerobic bacteriology of the saliva and gingiva from 16 captive komodo dragons (varanus komodoensis): New implications for the “bacteria as venom” model. J. Zoo Wildl. Med. 2013, 44, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chiang, H.-S. Effect on human platelet aggregation of phospholipase A. Biochim. Biophys. Acta 1994, 1211, 61–68. [Google Scholar] [CrossRef]
- Preston, C.A. Hypotension, Myocardial Infarction, and Coagulopathy Following Gila Monster Bite. J. Emerg. Med. 1989, 7, 37–40. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Winter, K.; Hodgson, W.C.; Griesman, L.; Kwok, H.F.; Scanlon, D.; Karas, J.; Shaw, C.; Wong, L.; et al. Novel venom proteins produced by differential domain-expression strategies in beaded lizards and gila monsters (genus Heloderma). Mol. Biol. Evol. 2010, 27, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Grundemar, L.; Hogestatt, D. Vascular effects of helodermin, helospectin I and helospectin II: A comparison with vasoactive intestinal peptide (VIP). Br. J. Pharmacol. 1990, 99, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Tsueshita, T.; Önyükusel, H.; Sethi, V.; Gandhi, S.; Rubinstein, I. Helospectin I and II evoke vasodilation in the intact peripheral microcirculation. Peptides 2004, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hendon, R.A.; Tu, A.T. Biochemical characterization of the lizard toxin gilatoxin. Biochemistry 1981, 20, 3517–3522. [Google Scholar] [CrossRef]
- Alagon, B.Y.A.; Possani, L.D.; Smart, J.; Schleuning, W. Helodermatine, A Kallikrein-like, Hypotensive Enzyme from the Venom of Heloderma horridum horridum (Mexican Beaded Lizard). J. Exp. Med. 1986, 164, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Utaisincharoenso, P.; Mackessyn, S.P.; Miller, R.A.; Tusii, A.T. Complete Primary Structure and Biochemical Properties of Gilatoxin, a Serine Protease with Kallikrein-like and Activities. J. Biol. Chem. 1993, 268, 21975–21983. [Google Scholar]
- Datta, G.; Tu, A.T. Structure and other chemical characterizations of gila toxin, a lethal toxin from lizard venom. J. Pept. Res. 1997, 50, 443–450. [Google Scholar] [CrossRef]
- Fry, B.G.; Winter, K.; Norman, J.A.; Roelants, K.; Nabuurs, R.J.A.; van Osch, M.J.P.; Teeuwisse, W.M.; van der Weerd, L.; Mcnaughtan, J.E.; Kwok, H.F.; et al. Functional and Structural Diversification of the Anguimorpha Lizard Venom System. Mol. Cell. Proteom. 2010, 9, 2369–2390. [Google Scholar] [CrossRef] [PubMed]
- Kwok, F.H.; Chen, T.; Rourke, M.O.; Ivanyi, C.; Hirst, D.; Shaw, C. Helokinestatin: A new bradykinin B 2 receptor antagonist decapeptide from lizard venom. J. Proteomics 2008, 29, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, M.; Zhou, M.; Wu, Y.; Wang, L.; Chen, T.; Ding, A.; Shaw, C. Peptides The natriuretic peptide/helokinestatin precursor from Mexican beaded lizard (Heloderma horridum) venom: Amino acid sequence deduced from cloned cDNA and identification of two novel encoded helokinestatins. Peptides 2011, 32, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, H.; Wu, Y.; Zhou, M.; Lowe, G.; Wang, L.; Zhang, Y.; Chen, T.; Shaw, C. Peptides Helokinestatin-7 peptides from the venoms of Heloderma lizards. Peptides 2012, 35, 300–305. [Google Scholar] [CrossRef]
- Koludarov, I.; Sunagar, K.; Undheim, E.A.B.; Jackson, T.N.W.; Ruder, T.; Whitehead, D.; Saucedo, A.C.; Mora, G.R.; Alagon, A.C.; King, G.; et al. Structural and molecular diversification of the anguimorpha lizard mandibular venom gland system in the arboreal species abronia graminea. J. Mol. Evol. 2012, 75, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wickramaratana, J.C.; Lemme, S.; Beuve, A.; Garbers, D.; Hodgson, W.C.; Alewood, P. Novel natriuretic peptides from the venom of the inland taipan (Oxyuranus microlepidotus): Isolation, chemical and biological characterisation. Biochem. Biophys. Res. Commun. 2005, 327, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins Into Animal Venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Koludarov, I.; Jackson, T.N.W.; Sunagar, K.; Nouwens, A.; Hendrikx, I.; Fry, B.G. Fossilized venom: The unusually conserved venom profiles of Heloderma species (beaded lizards and gila monsters). Toxins 2014, 6, 3582–3595. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Fasoli, E.; Sanz, L.; Boschetti, E.; Righetti, P.G. Exploring the venom proteome of the western diamondback rattlesnake, Crotalus atrox, via snake venomics and combinatorial peptide ligand library approaches. J. Proteome Res. 2009, 8, 3055–3067. [Google Scholar] [CrossRef] [PubMed]
- López-Lozano, J.L.; Valle, M.; Sousa, D.; Andre, C.; Cha, C.; Bu, P.F. Ontogenetic variation of metalloproteinases and plasma coagulant activity in venoms of wild Bothrops atrox specimens from Amazonian rain forest. Toxicon 2002, 40, 997–1006. [Google Scholar] [CrossRef]
- Mackessy, S.P.; Leroy, J.; Mociño-Deloya, E.; Setser, K.; Bryson, R.W.; Saviola, A.J. Venom ontogeny in the mexican lance-headed rattlesnake (Crotalus polystictus). Toxins 2018, 10, 271. [Google Scholar] [CrossRef]
- Cipriani, V.; Debono, J.; Goldenberg, J.; Jackson, T.N.W.; Arbuckle, K.; Dobson, J.; Koludarov, I.; Li, B.; Hay, C.; Dunstan, N.; et al. Correlation between ontogenetic dietary shifts and venom variation in Australian brown snakes (Pseudonaja). Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2017, 197, 53–60. [Google Scholar] [CrossRef]
- Jackson, T.N.W.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; Op Den Brouw, B.; Masci, P.P.; Nouwens, A.; Josh, P.; et al. Rapid radiations and the race to redundancy: An investigation of the evolution of Australian elapid snake venoms. Toxins 2016, 8, 309. [Google Scholar] [CrossRef]
- Sunagar, K.; Undheim, E.A.B.; Scheib, H.; Gren, E.C.K.; Cochran, C.; Person, C.E.; Koludarov, I.; Kelln, W.; Hayes, W.K.; King, G.F.; et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri: Biodiscovery, clinical and evolutionary implications. J. Proteomics 2014, 99, 68–83. [Google Scholar] [CrossRef]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; de M. Chalkidis, H.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef]
- Lister, C.; Arbuckle, K.; Jackson, T.N.W.; Debono, J.; Zdenek, C.N.; Dashevsky, D.; Dunstan, N.; Allen, L.; Hay, C.; Bush, B.; et al. Catch a tiger snake by its tail: Differential toxicity, co-factor dependence and antivenom efficacy in a procoagulant clade of Australian venomous snakes. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2017, 202, 39–54. [Google Scholar] [CrossRef]
- Pianka, E.R.; King, D.; King, R. Varanoid Lizards of the World; Indiana University Press: Indianapolis, IN, USA, 2004. [Google Scholar]
- Arbuckle, K. Ecological Function of Venom in Varanus, with a Compilation of Dietary Records from the Literature. Biawak 2009, 3, 46–56. [Google Scholar]
- Fry, B.G.; Wroe, S.; Teeuwisse, W.; van Osch, M.J.P.; Moreno, K.; Ingle, J.; McHenry, C.; Ferrara, T.; Clausen, P.; Scheib, H.; et al. A central role for venom in predation by Varanus komodoensis (Komodo Dragon) and the extinct giant Varanus (Megalania) priscus. Proc. Natl. Acad. Sci. USA 2009, 106, 8969–8974. [Google Scholar] [CrossRef]
- Auffenberg, W. The Behavioral Ecology of the Komodo Monitor; University Press of Florida: Gainesville, FL, USA, 1981. [Google Scholar]
- Greene, H.W. Diet and arboreality in the emerald monitor, Varanus prasinus, with comments on the study of adaptation. Fieldiana Zool. 1986, 31, 1–12. [Google Scholar]
- Vikrant, S.; Verma, B.S. Monitor lizard bite-induced acute kidney injury—A case report. Ren. Fail. 2014, 6049, 444–446. [Google Scholar] [CrossRef]
- Ducey, S.D.; Cooper, J.S.; Wadman, M.C. Bitten by a Dragon. Wilderness Environ. Med. 2016, 27, 291–293. [Google Scholar] [CrossRef]
- Tehrani, H.; Tejero-Trujeque, R.; Dhital, S.K. Re: Septic arthritis due to a Savannah Monitor lizard bite: A case report. J. Hand Surg. Am. 2008, 33, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Koludarov, I.; Jackson, T.N.W.; op den Brouw, B.; Dobson, J.; Dashevsky, D.; Arbuckle, K.; Clemente, C.J.; Stockdale, E.J.; Cochran, C.; Debono, J.; et al. Enter the dragon: The dynamic and multifunctional evolution of anguimorpha lizard venoms. Toxins 2017, 9, 242. [Google Scholar] [CrossRef]
- Mosesson, M.W. Fibrinogen functions and fibrin assembly. Fibrinolysis Proteolysis 2000, 14, 182–186. [Google Scholar] [CrossRef]
- Wolberg, A.S. Thrombin generation and fibrin clot structure. Blood Rev. 2007, 21, 131–142. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Nouwens, A.; Ge, L.; Frank, N.; Kwok, H.F.; Fry, B.G. Habu coagulotoxicity: Clinical implications of the functional diversification of Protobothrops snake venoms upon blood clotting factors. Toxicol. Vitr. 2019, 55, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Oulion, B.; Dobson, J.S.; Zdenek, C.N.; Arbuckle, K.; Lister, C.; Coimbra, F.C.P.; op den Brouw, B.; Debono, J.; Rogalski, A.; Violette, A.; et al. Factor X activating Atractaspis snake venoms and the relative coagulotoxicity neutralising efficacy of African antivenoms. Toxicol. Lett. 2018, 288, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, A.; Soerensen, C.; op den Brouw, B.; Lister, C.; Dashvesky, D.; Arbuckle, K.; Gloria, A.; Zdenek, C.N.; Casewell, N.R.; Gutiérrez, J.M.; et al. Differential procoagulant effects of saw-scaled viper (Serpentes: Viperidae: Echis) snake venoms on human plasma and the narrow taxonomic ranges of antivenom efficacies. Toxicol. Lett. 2017, 280, 159–170. [Google Scholar] [CrossRef]
- Ast, J.C. Mitochondrial DNA evidence and evolution in Varanoidea (Squamata). Cladistics 2001, 17, 211–226. [Google Scholar] [CrossRef]
- Vidal, N.; Marin, J.; Sassi, J.; Battistuzzi, F.U.; Donnellan, S.; Fitch, A.J.; Fry, B.G.; Vonk, F.J.; De La Vega, R.C.R.; Couloux, A.; et al. Molecular evidence for an Asian origin of monitor lizards followed by Tertiary dispersals to Africa and Australasia. Biol. Lett. 2012, 8, 853–855. [Google Scholar] [CrossRef]
- Jackson, T.N.W.; Fry, B.G. A tricky trait: Applying the fruits of the “function debate” in the philosophy of biology to the “venom debate” in the science of toxinology. Toxins 2016, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Toxicovenomics and antivenom profiling of the Eastern green mamba snake (Dendroaspis angusticeps). J. Proteomics 2016, 136, 248–261. [Google Scholar] [CrossRef]
- Debono, J.; Dobson, J.; Casewell, N.R.; Romilio, A.; Li, B.; Kurniawan, N.; Mardon, K.; Weisbecker, V.; Nouwens, A.; Kwok, H.F.; et al. Coagulating colubrids: Evolutionary, pathophysiological and biodiscovery implications of venom variations between boomslang (Dispholidus typus) and twig snake (Thelotornis mossambicanus). Toxins 2017, 9, 171. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation-and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef]
- Green, B.; King, D.; Butler, H. Water, sodium and energy turnover in free-living perenties, Varanus giganteus. Wildl. Res. 1986, 13, 589–595. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert Ecosystems: Higher Trophic Levels. Annu. Rev. Ecol. Syst. 1974, 5, 195–214. [Google Scholar] [CrossRef]
- Garrett, C.; Boyer, D.M.; Card, W.C.; Roberts, D.T.; Murphy, J.B.; Chiszar, D. Comparison of Chernosensory Behavior and Prey Trail-Following in the Varanoid Lizards Varanus gouldii and Heloderma suspectum. Zoo Biol. 1996, 15, 255–265. [Google Scholar] [CrossRef]
- Fry, B.G. From genome to “venome” Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Frank, N. The kallikrein-like activity of Heloderma venom is inhibited by carbon monoxide. J. Thromb. Thrombolysis 2019, 47, 533–539. [Google Scholar] [CrossRef]
- Marsh, N.; Williams, V. Practical applications of snake venom toxins in haemostasis. Toxicon 2005, 45, 1171–1181. [Google Scholar] [CrossRef]
- Aramadhaka, L.R.; Prorock, A.; Dragulev, B.; Bao, Y.; Fox, J.W. Connectivity maps for biosimilar drug discovery in venoms: The case of Gila Monster Venom and the anti-diabetes drug Byetta. Toxicon 2013, 69, 160–167. [Google Scholar] [CrossRef]
- Coimbra, F.C.P.; Dobson, J.; Zdenek, C.N.; op den Brouw, B.; Hamilton, B.; Debono, J.; Masci, P.; Frank, N.; Ge, L.; Kwok, H.F.; et al. Does size matter? Venom proteomic and functional comparison between night adder species (Viperidae: Causus) with short and long venom glands. Comp. Biochem. Physiol. Part.—C Toxicol. Pharmacol. 2018, 211, 7–14. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.2. Available online: http://www.mesquiteproject.org. 2018 (accessed on 6 May 2019).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobson, J.S.; Zdenek, C.N.; Hay, C.; Violette, A.; Fourmy, R.; Cochran, C.; Fry, B.G. Varanid Lizard Venoms Disrupt the Clotting Ability of Human Fibrinogen through Destructive Cleavage. Toxins 2019, 11, 255. https://doi.org/10.3390/toxins11050255
Dobson JS, Zdenek CN, Hay C, Violette A, Fourmy R, Cochran C, Fry BG. Varanid Lizard Venoms Disrupt the Clotting Ability of Human Fibrinogen through Destructive Cleavage. Toxins. 2019; 11(5):255. https://doi.org/10.3390/toxins11050255
Chicago/Turabian StyleDobson, James S., Christina N. Zdenek, Chris Hay, Aude Violette, Rudy Fourmy, Chip Cochran, and Bryan G. Fry. 2019. "Varanid Lizard Venoms Disrupt the Clotting Ability of Human Fibrinogen through Destructive Cleavage" Toxins 11, no. 5: 255. https://doi.org/10.3390/toxins11050255
APA StyleDobson, J. S., Zdenek, C. N., Hay, C., Violette, A., Fourmy, R., Cochran, C., & Fry, B. G. (2019). Varanid Lizard Venoms Disrupt the Clotting Ability of Human Fibrinogen through Destructive Cleavage. Toxins, 11(5), 255. https://doi.org/10.3390/toxins11050255





