Co-Occurrence of DON and Emerging Mycotoxins in Worldwide Finished Pig Feed and Their Combined Toxicity in Intestinal Cells
Abstract
1. Introduction
2. Results
2.1. Occcurrence and Abundance of Emerging Mycotoxins and DON in Finished Pig Feed
2.2. Intestinal Toxicity of Emerging Mycotoxins Found in Pig Feed, alone or Combined with DON
2.2.1. Individual Toxicity of DON and Emerging Mycotoxins
2.2.2. Combined Toxicity of DON and Emerging Mycotoxins
2.2.3. Combined Toxicity of DON and the Non-Toxic Secondary Metabolites (BRV-F, Cyclo and TRPT)
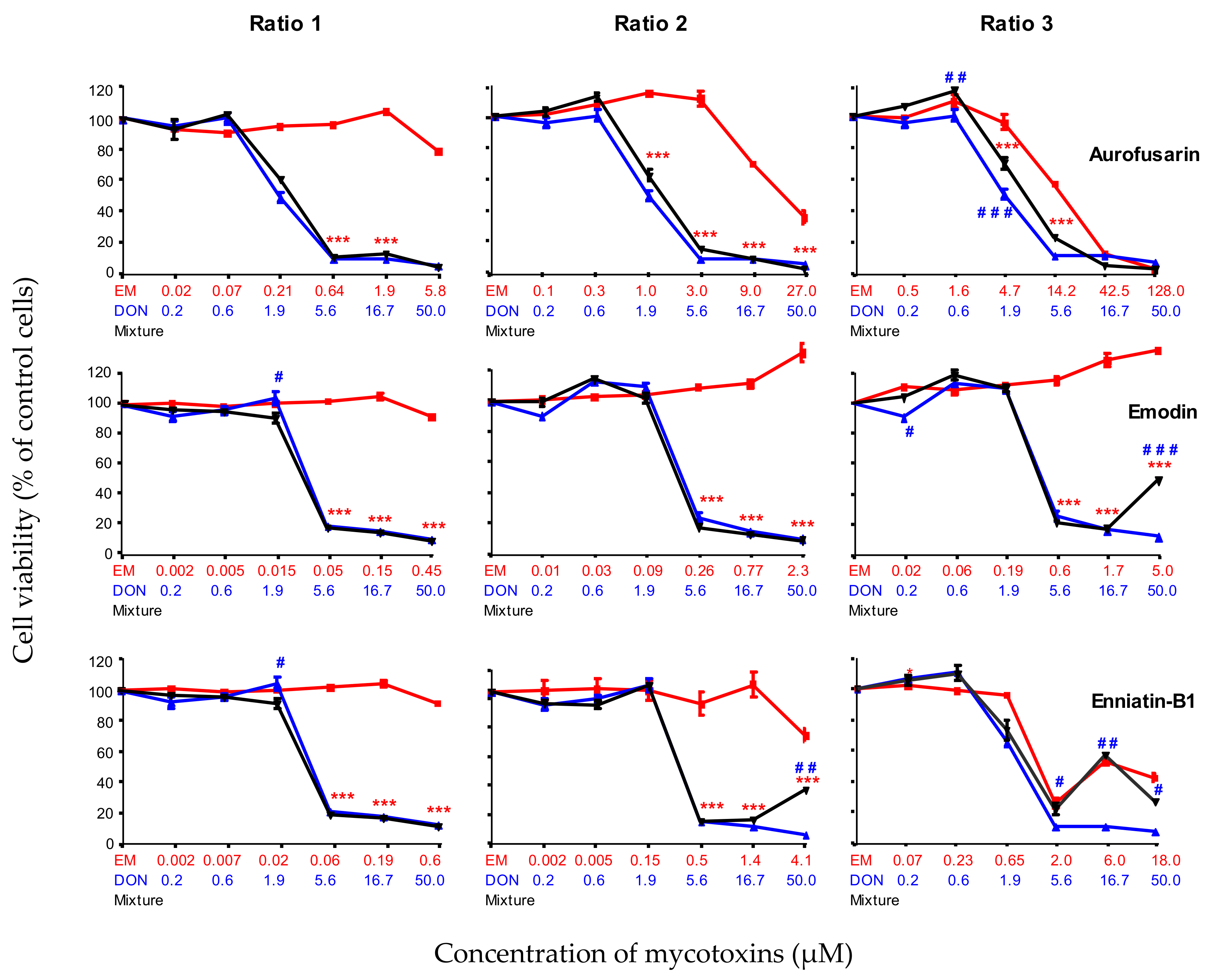
2.2.4. Combined Toxicity of DON and the Moderately Toxic Secondary Metabolites (AFN, EMO and ENN-B1)
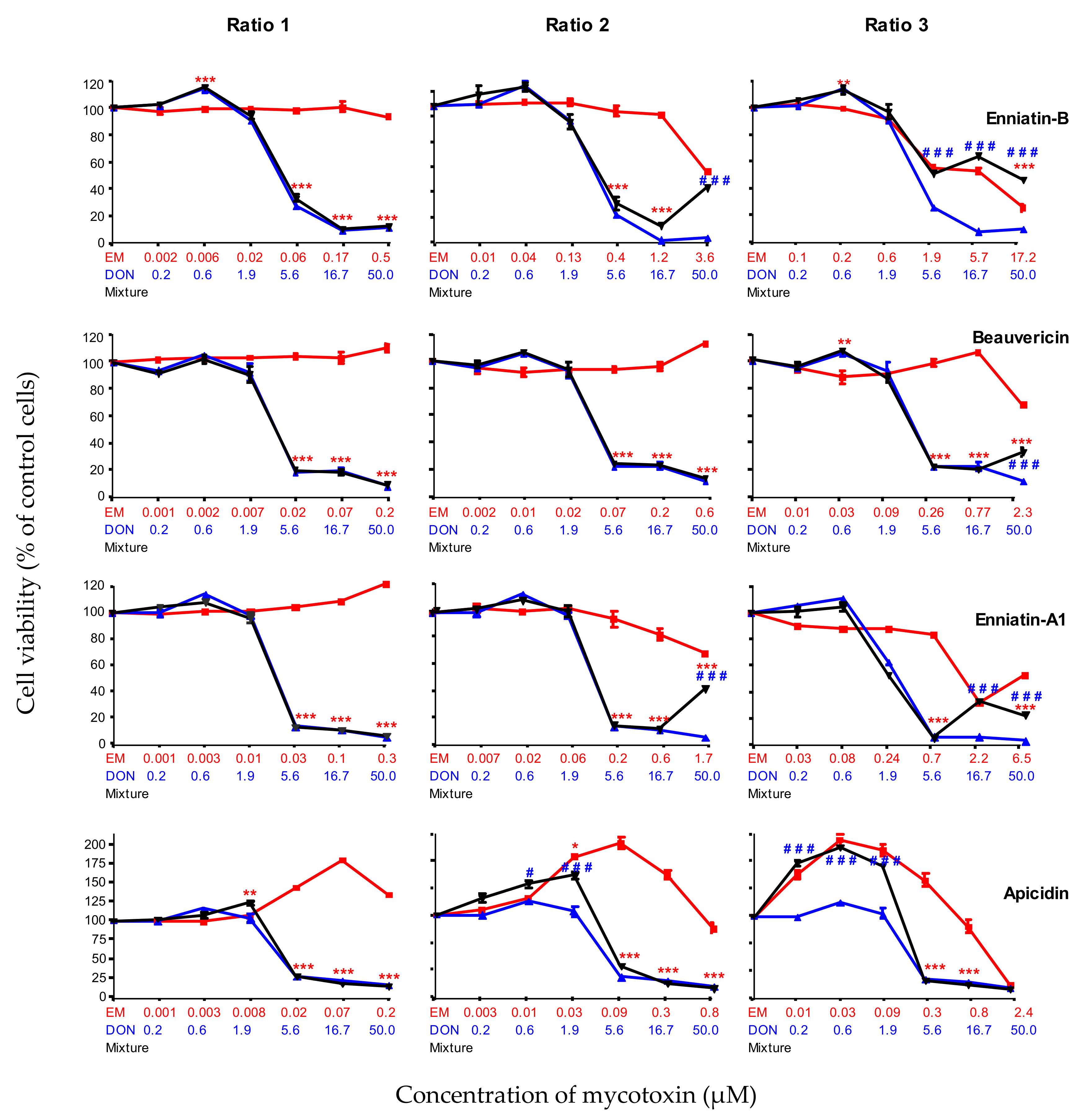
2.2.5. Combined Toxicity of DON and The Highly Toxic Secondary Metabolites (ENN-B, BEA, ENN-A1and API)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Extraction and Analysis of Metabolites
5.2. Toxins
5.3. Cell Culture and Cytotxicity Assay
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal productsText with EEA relevance. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2016, 65, 7052–7070. [Google Scholar] [CrossRef]
- Rychlik, M.; Humpf, H.-U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. in press. [CrossRef]
- Schatzmayr, G.; Streit, E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar]
- IARC. Toxins derived from Fusarium graminearum, F. culmorum and F. crookwellense: Zearalenone, deoxynivalenol, nivalenol and fusarenone X. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 56, 397–444. [Google Scholar]
- Charmley, L.; Trenholm, H. RG-8 Regulatory Guidance:Contaminants in Feed (Formerly RG-1, Chapter 7). Available online: https://www.inspection.gc.ca/animals/feeds/regulatory-guidance/rg-8/eng/134738394320 3/1347384015909?chap=1#s1c1 (accessed on 21 November 2019).
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.-P.F.L.; Schatzmayr, G.; He, J.W.; Zhou, T.; Moll, W.-D.; et al. Microbial biotransformation of DON: Molecular basis for reduced toxicity. Sci. Rep. 2016, 6, 29105. [Google Scholar] [CrossRef]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed: Beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Novak, B.; Rainer, V.; Sulyok, M.; Haltrich, D.; Schatzmayr, G.; Mayer, E. Twenty-Eight Fungal Secondary Metabolites Detected in Pig Feed Samples: Their Occurrence, Relevance and Cytotoxic Effects In Vitro. Toxins 2019, 11, 537. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Girgin, G.; Baydar, T.; Krska, R.; Sulyok, M. Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS: Toxic fungal and bacterial metabolites in feed and maize from Egypt. J. Sci. Food Agric. 2017, 97, 4419–4428. [Google Scholar] [CrossRef]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Anjorin, S.T.; Fapohunda, S.; Sulyok, M.; Krska, R. Natural Co-occurrence of Emerging and Minor Mycotoxins on Maize Grains from Abuja, Nigeria. Ann. Agric. Environ. Sci. 2016, 1, 21–29. [Google Scholar]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Fremy, J.-M.; Alassane-Kpembi, I.; Oswald, I.P.; Cottrill, B.; Van Egmond, H.P. A review on combined effects of moniliformin and co-occurring Fusarium toxins in farm animals. World Mycotoxin J. 2019, 12, 281–291. [Google Scholar] [CrossRef]
- Assunção, R.; Silva, M.J.; Alvito, P. Challenges in risk assessment of multiple mycotoxins in food. World Mycotoxin J. 2016, 9, 791–811. [Google Scholar] [CrossRef]
- More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Herna, A.F.; Koutsoumanis, K.; Naegeli, H.; Schlatter, J.R.; et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, 5634. [Google Scholar]
- Meek, B.; Roobis, A.R.; Crofton, K.M.; Heinemeyer, G.; Raaij, M.V.; Vickers, C. Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regul. Toxicol. Pharmacol. 2011, 60, S1–S14. [Google Scholar]
- Kortenkamp, A.; Faust, M. Regulate to reduce chemical mixture risk. Science 2018, 361, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Puel, O.; Oswald, I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015, 89, 1337–1346. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Puel, O.; Pinton, P.; Cossalter, A.-M.; Chou, T.-C.; Oswald, I. Co-exposure to low doses of the food contaminants deoxynivalenol and nivalenol has a synergistic inflammatory effect on intestinal explants. Arch. Toxicol. 2017, 91, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Kolf-Clauw, M.; Sassahara, M.; Lucioli, J.; Rubira-Gerez, J.; Alassane-Kpembi, I.; Lyazhri, F.; Borin, C.; Oswald, I.P. The emerging mycotoxin, enniatin B1, down-modulates the gastrointestinal toxicity of T-2 toxin in vitro on intestinal epithelial cells and ex vivo on intestinal explants. Arch. Toxicol. 2013, 87, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Jennen, D.; Caiment, F.; Manyes, L. Transcriptomic study of the toxic mechanism triggered by beauvericin in Jurkat cells. Toxicol. Lett. 2018, 284, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Braicu, C.; Nougayrede, J.-P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol Impairs Porcine Intestinal Barrier Function and Decreases the Protein Expression of Claudin-4 through a Mitogen-Activated Protein Kinase-Dependent Mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Council for Agricultural Science and Technology. Mycotoxins: Risks in Plant, Animal, and Human Systems; Task Force report; Council for Agricultural Science and Technology: Ames, IA, USA, 2003; ISBN 978-1-887383-22-6. [Google Scholar]
- Bauden, M.; Tassidis, H.; Ansari, D. In vitro cytotoxicity evaluation of HDAC inhibitor Apicidin in pancreatic carcinoma cells subsequent time and dose dependent treatment. Toxicol. Lett. 2015, 236, 8–15. [Google Scholar] [CrossRef]
- Glenn, A.E.; Bacon, C.W.; Price, R.; Hanlin, R.T. Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 1996, 88, 369–383. [Google Scholar] [CrossRef]
- Kosalec, I.; Ramić, S.; Jelić, D.; Antolović, R.; Pepeljnjak, S.; Kopjar, N. Assessment of Tryptophol Genotoxicity in Four Cell Lines In Vitro: A Pilot Study with Alkaline Comet Assay. Arch. Ind. Hyg. Toxicol. 2011, 62, 41–49. [Google Scholar] [CrossRef]
- Muto, A.; Hori, M.; Sasaki, Y.; Saitoh, A.; Yasuda, I.; Maekawa, T.; Uchida, T.; Asakura, K.; Nakazato, T.; Kaneda, T.; et al. Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol. Cancer Ther. 2007, 6, 987–994. [Google Scholar] [CrossRef]
- Meca, G.; Font, G.; Ruiz, M.J. Comparative cytotoxicity study of enniatins A, A1, A2, B, B1, B4 and J3 on Caco-2 cells, Hep-G2 and HT-29. Food Chem. Toxicol. 2011, 49, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Fraeyman, S.; Meyer, E.; Devreese, M.; Antonissen, G.; Demeyere, K.; Haesebrouck, F.; Croubels, S. Comparative in vitro cytotoxicity of the emerging Fusarium mycotoxins beauvericin and enniatins to porcine intestinal epithelial cells. Food Chem. Toxicol. 2018, 121, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.E.; Burlacu, R.; Pinton, P.; Damian, V.; Oswald, I.P. Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins. Arch. Anim. Nutr. 2010, 64, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. submitted.
- Blandino, M.; Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A. Effect of agronomic programmes with different susceptibility to deoxynivalenol risk on emerging contamination in winter wheat. Eur. J. Agron. 2017, 85, 12–24. [Google Scholar] [CrossRef]
- Prosperini, A.; Font, G.; Ruiz, M.J. Interaction effects of Fusarium enniatins (A, A1, B and B1) combinations on in vitro cytotoxicity of Caco-2 cells. Toxicol. Vitr. 2014, 28, 88–94. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.-J. Interaction effects of enniatin B, deoxinivalenol and alternariol in Caco-2 cells. Toxicol. Lett. 2016, 241, 38–48. [Google Scholar] [CrossRef]
- Ireland, J.J.; Roberts, R.M.; Palmer, G.H.; Bauman, D.E.; Bazer, F.W. A commentary on domestic animals as dual-purpose models that benefit agricultural and biomedical research. Am. Soc. Anim. Sci. 2008, 86, 2797–2805. [Google Scholar]
- García, G.R.; Payros, D.; Pinton, P.; Dogi, C.A.; Laffitte, J.; Neves, M.; González Pereyra, M.L.; Cavaglieri, L.R.; Oswald, I.P. Intestinal toxicity of deoxynivalenol is limited by Lactobacillus rhamnosus RC007 in pig jejunum explants. Arch. Toxicol. 2018, 92, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrède, J.-P.; Del Rio, J.-C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.-P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed]
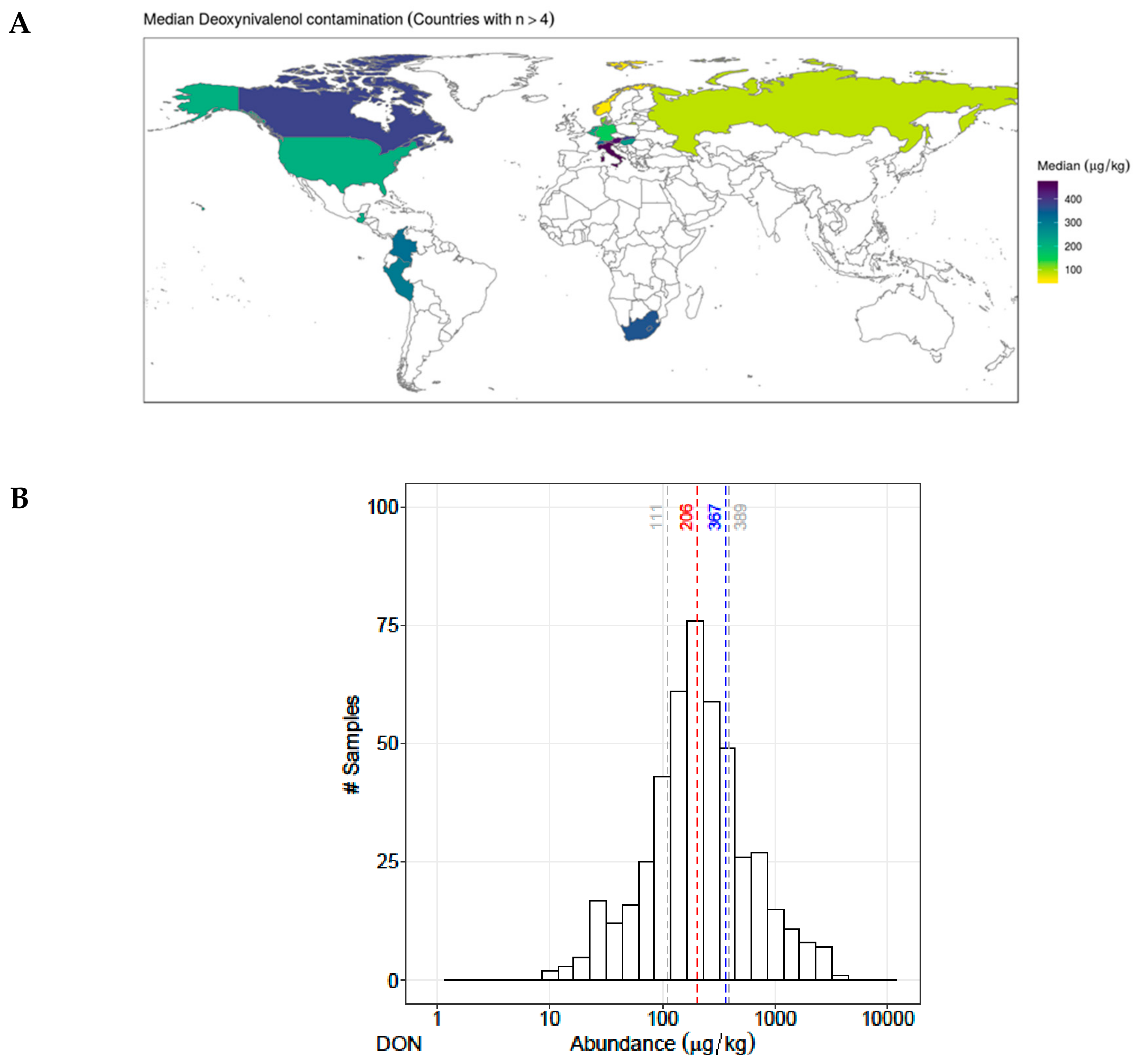


| Metabolites | Occurrence n (%) | Co-occurrence with DON n (%) | Contamination Level (µg/kg Feed) | Correlation (DON and Other Mycotoxins) | ||||
|---|---|---|---|---|---|---|---|---|
| P25 | P50 | P75 | Coefficient | p-Value | ||||
| 1 | Deoxynivalenol | 463 (88%) | 463 (100%) | 111 | 206 | 389 | 1.00 | NA |
| 2 | Culmorin | 492 (94%) | 458 (99%) | 38 | 107 | 247 | 0.50 | 0.00 |
| 3 | Zearalenone | 502 (96%) | 449 (97%) | 9 | 18 | 46 | 0.64 | 0.00 |
| 4 | Brevianamide F | 500 (95%) | 446 (96%) | 17 | 28 | 45 | 0.17 | 0.00 |
| 5 | Cyclo-(L-Pro-L-Tyr) | 494 (94%) | 434 (94%) | 117 | 196 | 371 | 0.14 | 0.00 |
| 6 | Enniatin B | 479 (91%) | 430 (93%) | 9 | 32 | 83 | 0.02 | 0.61 |
| 7 | Enniatin B1 | 481 (92%) | 430 (93%) | 10 | 37 | 87 | 0.04 | 0.39 |
| 8 | Asperglaucide | 470 (90%) | 419 (90%) | 18 | 38 | 94 | -0.11 | 0.02 |
| 9 | Emodin | 472 (90%) | 418 (90%) | 3 | 5 | 10 | -0.01 | 0.91 |
| 10 | Aurofusarin | 464 (89%) | 417 (90%) | 87 | 211 | 548 | 0.59 | 0.00 |
| 11 | Moniliformin | 469 (90%) | 416 (90%) | 7 | 17 | 45 | 0.11 | 0.03 |
| 12 | Beauvericin | 464 (89%) | 411 (89%) | 4 | 7 | 13 | 0.32 | 0.00 |
| 13 | Enniatin A1 | 459 (88%) | 411 (89%) | 5 | 16 | 33 | 0.09 | 0.08 |
| 14 | 3-Nitropropion acid | 455 (87%) | 407 (88%) | 3 | 6 | 10 | -0.03 | 0.57 |
| 15 | Tryptophol | 454 (87%) | 407 (88%) | 119 | 197 | 319 | 0.10 | 0.04 |
| 16 | 15-Hydroxyculmorin | 429 (82%) | 391 (84%) | 76 | 142 | 277 | 0.79 | 0.00 |
| 17 | Equisetin | 424 (81%) | 386 (83%) | 5 | 10 | 23 | 0.01 | 0.80 |
| 18 | Infectopyron | 409 (78%) | 366 (79%) | 108 | 263 | 449 | -0.16 | 0.00 |
| 19 | DON-3 Glucoside | 380 (73%) | 362 (78%) | 10 | 21 | 47 | 0.79 | 0.00 |
| 20 | Neoechinulin A | 407 (78%) | 357 (77%) | 10 | 19 | 42 | 0.06 | 0.22 |
| 21 | Tenuazonic-acid | 384 (73%) | 347 (75%) | 53 | 90 | 182 | 0.04 | 0.49 |
| 22 | Alternariol | 366 (70%) | 333 (72%) | 2 | 4 | 9 | 0.01 | 0.79 |
| 23 | Rugulusovin | 373 (71%) | 332 (72%) | 4 | 7 | 14 | 0.15 | 0.01 |
| 24 | Tentoxin | 342 (65%) | 319 (69%) | 2 | 3 | 6 | -0.03 | 0.56 |
| 25 | Apicidin | 341 (65%) | 310 (67%) | 3 | 7 | 11 | -0.13 | 0.02 |
| 26 | Fumonisin B1 | 332 (63%) | 304 (66%) | 26 | 70 | 254 | 0.14 | 0.02 |
| 27 | Nivalenol | 315 (60%) | 296 (64%) | 10 | 24 | 57 | 0.14 | 0.02 |
| 28 | Cyclo-(L-Pro-L-Val) | 337 (64%) | 286 (62%) | 85 | 137 | 246 | 0.15 | 0.01 |
| 29 | Epiequisetin | 307 (59%) | 285 (62%) | 2 | 4 | 7 | 0.05 | 0.42 |
| 30 | Citreorosein | 317 (60%) | 283 (61%) | 3 | 5 | 8 | 0.12 | 0.05 |
| 31 | Enniatin A | 306 (58%) | 282 (61%) | 2 | 3 | 5 | -0.01 | 0.81 |
| 32 | Alternariolmethylether | 307 (59%) | 275 (59%) | 2 | 3 | 5 | 0.09 | 0.14 |
| 33 | Altersetin | 301 (57%) | 275 (59%) | 13 | 29 | 76 | 0.18 | 0.00 |
| 34 | Asperphenamate | 311 (59%) | 269 (58%) | 5 | 11 | 27 | -0.02 | 0.75 |
| 35 | Lotaustralin | 288 (55%) | 257 (56%) | 15 | 30 | 85 | -0.10 | 0.13 |
| 36 | Butenolid | 253 (48%) | 242 (52%) | 22 | 37 | 70 | 0.32 | 0.00 |
| 37 | Kojic acid | 262 (50%) | 241 (52%) | 43 | 74 | 148 | -0.06 | 0.39 |
| 38 | Enniatin B2 | 258 (49%) | 238 (51%) | 2 | 3 | 5 | -0.01 | 0.84 |
| 39 | Fumonisin B2 | 258 (49%) | 237 (51%) | 19 | 50 | 143 | 0.16 | 0.01 |
| 40 | Zearalenone Sulfate | 236 (45%) | 222 (48%) | 10 | 25 | 53 | 0.25 | 0.00 |
| 41 | Antibiotic Y | 233 (44%) | 215 (46%) | 40 | 111 | 402 | -0.02 | 0.75 |
| 42 | T2 Toxin | 235 (45%) | 209 (45%) | 2 | 4 | 9 | 0.12 | 0.07 |
| 43 | Macrosporin | 219 (42%) | 202 (44%) | 2 | 3 | 8 | 0.02 | 0.76 |
| 44 | N-Benzoyl-Phenylalanine | 220 (42%) | 191 (41%) | 3 | 5 | 11 | -0.02 | 0.82 |
| 45 | Flavoglaucin | 206 (39%) | 175 (38%) | 7 | 16 | 34 | 0.05 | 0.51 |
| 46 | Curvularin | 196 (37%) | 171 (37%) | 2 | 4 | 8 | -0.09 | 0.27 |
| 47 | Questiomycin A | 178 (34%) | 162 (35%) | 4 | 10 | 20 | 0.22 | 0.01 |
| 48 | Rubellin D | 179 (34%) | 161 (35%) | 4 | 8 | 18 | 0.10 | 0.21 |
| 50 | Bikaverin | 171 (33%) | 153 (33%) | 10 | 25 | 56 | 0.27 | 0.00 |
| 50 | Fusarinolic-acid | 157 (30%) | 153 (33%) | 47 | 130 | 320 | 0.3 | 0.00 |
| 51 | Fumonisin B4 | 165 (31%) | 149 (32%) | 11 | 23 | 68 | 0.2 | 0.03 |
| 52 | Cytochalasin J | 170 (32%) | 146 (32%) | 13 | 29 | 63 | 0.1 | 0.46 |
| 53 | Ergometrine | 152 (29%) | 145 (31%) | 6 | 11 | 24 | 0.0 | 0.57 |
| 54 | Ergocristine | 151 (29%) | 143 (31%) | 2 | 5 | 13 | 0.2 | 0.02 |
| 55 | Fumonisin B3 | 154 (29%) | 136 (29%) | 24 | 48 | 103 | 0.1 | 0.15 |
| 56 | HT2-toxin | 149 (28%) | 134 (29%) | 13 | 20 | 30 | 0.2 | 0.01 |
| 57 | Monocerin | 144 (27%) | 133 (29%) | 1 | 2 | 3 | 0.2 | 0.02 |
| 58 | Chrysogin | 136 (26%) | 126 (27%) | 7 | 12 | 17 | 0.4 | 0.00 |
| 59 | Ergosin | 128 (24%) | 123 (27%) | 3 | 6 | 13 | -0.1 | 0.39 |
| 60 | 5-Hydroxyculmorin | 121 (23%) | 117 (25%) | 107 | 170 | 304 | 0.7 | 0.00 |
| Metabolites | Abbreviation | IC50 (µM) | Toxicity |
|---|---|---|---|
| Brevianamide F | BRV-F | Non-Toxic | Non-toxic |
| Cyclo-(L-Pro-L-Tyr) | Cyclo | Non-Toxic | |
| Tryptophol | TRPT | Non-Toxic | |
| Aurofusarin | AFN | 19.1 ± 3.4 | Moderately toxic |
| Emodin | EMO | 19.0 ± 0.7 | |
| Enniatin B1 | ENN-B1 | 13.5 ± 2.5 | |
| Enniatin B | ENN-B | 4.4 ± 0.9 | Highly toxic |
| Beauvericin | BEA | 4.3 ± 1.8 | |
| Deoxynivalenol | DON | 3.2 ± 0.7 | |
| Enniatin A1 | ENN-A1 | 1.6 ± 0.3 | |
| Apicidin | API | 1.5 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoshal, A.K.; Novak, B.; Martin, P.G.P.; Jenkins, T.; Neves, M.; Schatzmayr, G.; Oswald, I.P.; Pinton, P. Co-Occurrence of DON and Emerging Mycotoxins in Worldwide Finished Pig Feed and Their Combined Toxicity in Intestinal Cells. Toxins 2019, 11, 727. https://doi.org/10.3390/toxins11120727
Khoshal AK, Novak B, Martin PGP, Jenkins T, Neves M, Schatzmayr G, Oswald IP, Pinton P. Co-Occurrence of DON and Emerging Mycotoxins in Worldwide Finished Pig Feed and Their Combined Toxicity in Intestinal Cells. Toxins. 2019; 11(12):727. https://doi.org/10.3390/toxins11120727
Chicago/Turabian StyleKhoshal, Abdullah Khan, Barbara Novak, Pascal G. P. Martin, Timothy Jenkins, Manon Neves, Gerd Schatzmayr, Isabelle P. Oswald, and Philippe Pinton. 2019. "Co-Occurrence of DON and Emerging Mycotoxins in Worldwide Finished Pig Feed and Their Combined Toxicity in Intestinal Cells" Toxins 11, no. 12: 727. https://doi.org/10.3390/toxins11120727
APA StyleKhoshal, A. K., Novak, B., Martin, P. G. P., Jenkins, T., Neves, M., Schatzmayr, G., Oswald, I. P., & Pinton, P. (2019). Co-Occurrence of DON and Emerging Mycotoxins in Worldwide Finished Pig Feed and Their Combined Toxicity in Intestinal Cells. Toxins, 11(12), 727. https://doi.org/10.3390/toxins11120727







