Porcine Hepatic Response to Fumonisin B1 in a Short Exposure Period: Fatty Acid Profile and Clinical Investigations
Abstract
:1. Introduction
2. Results
2.1. Body, Organ Weight, Feed Intake, and Its Utilization Efficiency
2.2. Fatty Acid Profile of the Hepatic Phospholipids
2.3. Fatty Acid Profile of the Triglycerides from Hepatic Tissue
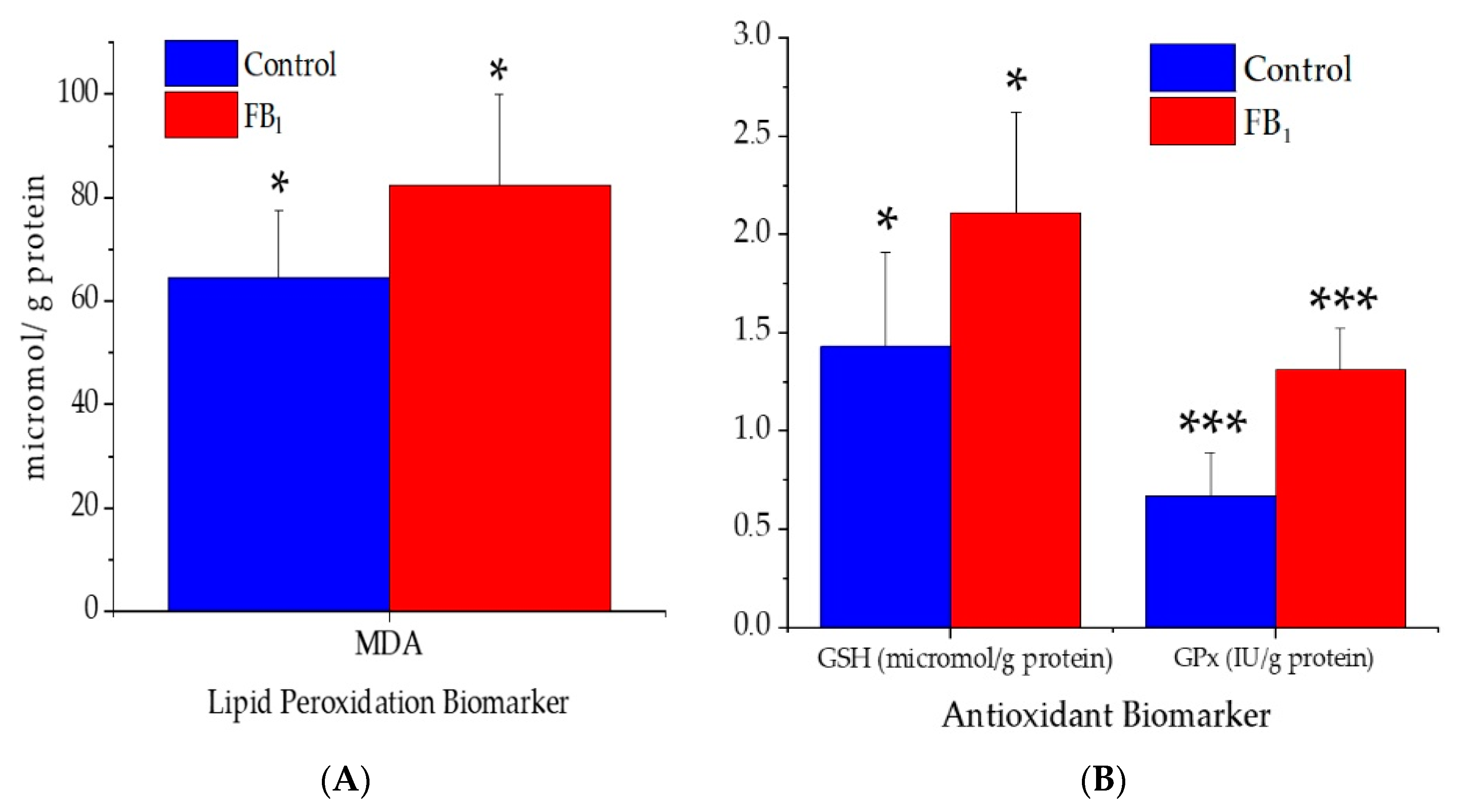
2.4. Lipid Peroxidation and Antioxidant Parameters
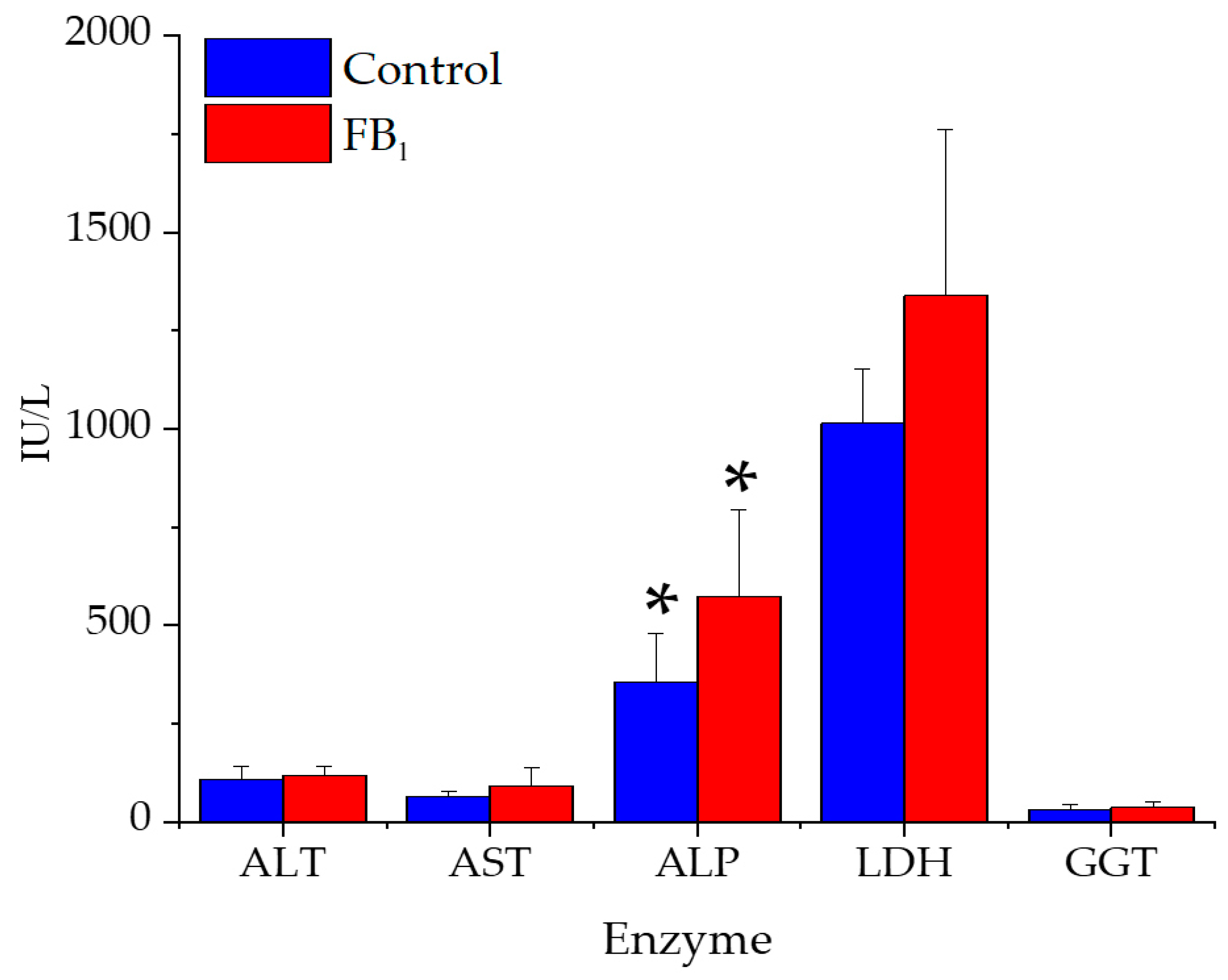
2.5. Serum Biochemical Parameters
2.6. Histopathological Results
3. Discussion
3.1. Growth, Feed Intake, and Organ Weights
3.2. Fatty Acid Profile of the Hepatic Phospholipid FA Profile
3.3. Fatty Acid Profile of the Hepatic Triglycerides
3.4. Lipid Peroxidation and Antioxidant Parameters
3.5. Serum Clinical Chemistry
3.6. Histopathological Investigation
4. Conclusions
5. Material and Methods
5.1. Ethical Allowance
5.2. Experimental Design and Nutrition
5.3. Lipid Analysis of the Hepatic Tissue
5.4. Determination of Lipid Peroxidation and Antioxidant Capacity
5.5. Serum Clinical Chemistry Analysis
5.6. Histopathological Analysis
5.7. Statistical Evaluation
Author Contributions
Funding
Conflicts of Interest
References
- Anttila, A.; Bhat, R.V.; Bond, J.A.; Borghoff, S.J.; Bosch, F.X.; Carlson, G.P.; Castegnaro, M.; Cruzan, G.; Wentzel, C.A.; Hass, U.; et al. IARC Monographs on the evaluation of carcinogenic risks to humans: some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 301–366. [Google Scholar]
- Voss, K.A.; Riley, R.T. Fumonisin Toxicity and Mechanism of Action: Overview and Current Perspectives. Food Saf. 2013, 1, 2013006. [Google Scholar] [CrossRef]
- Gelderblom, W.C.; Kriek, N.P.; Marasas, W.F.; Thiel, P.G. Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 1991, 12, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Haschek, W.M.; Gunnprecht, L.A.; Snnith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin Toxicosis in Swine: An Overview of Porcine Pulmonary Edema and Current Perspectives. Environ. Health Perspect. 2001, 109, 251–257. [Google Scholar]
- Riedel, S.; Abel, S.; Swanevelder, S.; Gelderblom, W.C.A. Induction of an altered lipid phenotype by two cancer promoting treatments in rat liver. Food Chem. Toxicol. 2015, 78, 96–104. [Google Scholar] [CrossRef]
- Riedel, S.; Abel, S.; Burger, H.M.; van der Westhuizen, L.; Swanevelder, S.; Gelderblom, W.C.A. Differential modulation of the lipid metabolism as a model for cellular resistance to fumonisin B1—Induced cytotoxic effects in vitro. Prostaglandins Leukot. Essent. Fat. Acids 2016, 109, 39–51. [Google Scholar] [CrossRef]
- Burger, H.M.; Abel, S.; Gelderblom, W.C.A. Modulation of key lipid raft constituents in primary rat hepatocytes by fumonisin B1—Implications for cancer promotion in the liver. Food Chem. Toxicol. 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Riley, R.T.; Voss, K.A.; Yoo, H.-S.; Gelderblom, W.C.A.; Merrill, A.H. Mechanism of Fumonisin Toxicity and Carcinogenesis. J. Food Prot. 1994, 57, 638–644. [Google Scholar] [CrossRef]
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Terciolo, C.; Bracarense, A.P.; Souto, P.C.; Cossalter, A.-M.; Dopavogui, L.; Loiseau, N.; Oliveira, C.A.F.; Pinton, P.; Oswald, I.P. Fumonisins at Doses below EU Regulatory Limits Induce Histological Alterations in Piglets. Toxins 2019, 11, 548. [Google Scholar] [CrossRef]
- Riley, R.T.; An, N.H.; Showker, J.L.; Yoo, H.S.; Norred, W.P.; Chamberlain, W.J.; Wang, E.; Merrill, A.H.L.; Motelin, G.; Beasley, V.R.; et al. Alteration of tissue and serum sphinganine to sphingosine ratio: An early biomarker of exposure to Fumonisin-containing feeds in pig. Toxicol. Appl. Pharmacol. 1993, 118, 105–112. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to fumonisins as undesirable substances in animal feed. EFSA J. 2005, 235, 1–32. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2005.235 (accessed on 6 September 2019).
- Guilloteau, P.; Zabielski, R.; Hammon, H.M.; Metges, C.C. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr. Res. Rev. 2010, 23, 4–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fodor, J.; Balogh, K.; Weber, M.; Mézes, M.; Kametler, L.; Pósa, R.; Mamet, R.; Bauer, J.; Horn, P.; Kovács, F.; et al. Absorption, distribution and elimination of fumonisin B1 metabolites in weaned piglets. Food Addit. Contam. Part A 2008, 25, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Zomborszkyné-Kovács, M.; Vetés, I.F.; Kovács, F.; Bata, Á.; Repa, I.; Horn, P. A fusarium moniliforme fumonizin-B1 toxinjának tolerálható határértékére és perinatalis toxikózist eloîdézo hatására vonatkozó vizsgálatok sertésben (Investigations on the tolerable limit values and the perinatal toxic effect of mycotoxins produced by Fus. Magy. Allatorvosok Lapja 2000, 122, 168–175. [Google Scholar]
- Haschek, W.M.; Motelin, G.; Ness, D.K.; Harlin, K.S.; Hall, W.F.; Vesonder, R.F.; Peterson, R.E.; Beasley, V.R. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia 1992, 117, 83–96. [Google Scholar] [CrossRef]
- Casteel, S.W.; Turk, J.R.; Cowart, R.P.; Rottinghaus, G.E. Chronic toxicity of fumonisin in weanling pigs. J. Vet. Diagn. Investig. 1993, 5, 413–417. [Google Scholar] [CrossRef]
- Gumprecht, L.A.; Beasley, V.R.; Weigel, R.M.; Parker, H.M.; Tumbleson, M.E.; Bacon, C.W.; Meredith, F.I.; Haschek, W.M. Development of fumonisin-induced hepatotoxicity and pulmonary edema in orally dosed swine: Morphological and biochemical alterations. Toxicol. Pathol. 1998, 26, 777–788. [Google Scholar] [CrossRef]
- Harvey, R.B.; Edrington, T.S.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Genovese, K.J.; Ziprin, R.L.; Nisbet, D.J. Toxicity of fumonisin from Fusarium verticillioides culture material and moniliformin from Fusarium fujikuroi culture material when fed singly and in combination to growing barrows. J. Food Prot. 2002, 65, 373–377. [Google Scholar] [CrossRef]
- Dilkin, P.; Zorzete, P.; Mallmann, C.A.; Gomes, J.D.F.; Utiyama, C.E.; Oetting, L.L.; Corrêa, B. Toxicological effects of chronic low doses of aflatoxin B1 and fumonisin B1-containing Fusarium moniliforme culture material in weaned piglets. Food Chem. Toxicol. 2003, 41, 1345–1353. [Google Scholar] [CrossRef]
- Kovács, M.; Pósa, R.; Tuboly, T.; Donkó, T.; Repa, I.; Tossenberger, J.; Szabó-Fodor, J.; Stoev, S.; Magyar, T. Feed exposure to FB1 can aggravate pneumonic damages in pigs provoked by P. multocida. Res. Vet. Sci. 2016, 108, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, N.; Polizzi, A.; Dupuy, A.; Therville, N.; Rakotonirainy, M.; Loy, J.; Viadere, J.L.; Cossalter, A.M.; Bailly, J.D.; Puel, O.; et al. New insights into the organ-specific adverse effects of fumonisin B1: Comparison between lung and liver. Arch. Toxicol. 2015, 89, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- US-Food and Drug Administration (FDA). Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds; Washington, DC, USA, November 2001. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-fumonisin-levels-human-foods-and-animal-feeds (accessed on 24 September 2019).
- European Union. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- Gelderblom, W.C.A.; Smuts, C.M.; Abel, S.; Snyman, S.D.; Van Der Westhuizen, L.; Huber, W.W.; Swanevelder, S. Effect of fumonisin B1 on the levels and fatty acid composition of selected lipids in rat liver in vivo. Food Chem. Toxicol. 1997, 35, 647–656. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Lebepe-Mazur, S.; Snijman, P.W.; Abel, S.; Swanevelder, S.; Kriek, N.P.J.; Marasas, W.F.O. Toxicological effects in rats chronically fed low dietary levels of fumonisin B1. Toxicology 2001, 161, 39–51. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Moritz, W.; Swanevelder, S.; Smuts, C.M.; Abel, S. Lipids and Δ6-desaturase activity alterations in rat liver microsomal membranes induced by fumonisin B1. Lipids 2002, 37, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.M.; Abel, S.; Snijman, P.W.; Swanevelder, S.; Gelderblom, W.C.A. Altered lipid parameters in hepatic subcellular membrane fractions induced by fumonisin B1. Lipids 2007, 42, 249–261. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Repa, I.; Kovács, M. Acute hepatic effects of low-dose fumonisin B1 in rats. Acta Vet. Hung. 2016, 64, 436–448. [Google Scholar]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Bázár, G.; Kocsó, D.; Ali, O.; Kovács, M. Individual and combined effects of fumonisin B1, deoxynivalenol and zearalenone on the hepatic and renal membrane lipid integrity of rats. Toxins 2018, 10, 4. [Google Scholar]
- Szabó, A.; Fébel, H.; Ali, O.; Mézes, M.; Balogh, K.; Kovács, M. Fumonisin B1 induced compositional modifications of the renal and hepatic membrane lipids in rats—Dose and exposure time dependence. Food Addit. Contam. Part A 2019, 36, 1722–1739. [Google Scholar]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Romvári, R.; Kovács, M. Individual and combined haematotoxic effects of fumonisin B1 and T-2 mycotoxins in rabbits. Food Chem. Toxicol. 2014, 72, 257–264. [Google Scholar]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Bajzik, G.; Kovács, M. Oral administration of fumonisin B1 and T-2 individually and in combination affects hepatic total and mitochondrial membrane lipid profile of rabbits. Physiol. Int. 2016, 103, 321–333. [Google Scholar]
- FAO/WHO. Safety evaluation of certain mycotoxins in food. Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Int. Program. Chem. Saf. World Health Organ. 2001, 47, 420–555. [Google Scholar]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, e05242. [Google Scholar]
- Marasas, W.F.O. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109, 239–243. [Google Scholar]
- Souto, P.C.M.C.; Ramalho, L.N.Z.; Ramalho, F.S.; Gregorio, M.C.; Bordin, K.; Cossalter, A.M.; Oswald, I.P.; Oliveira, C.A.F. Ganho de peso, consumo de ração e histologia de órgãos de leitões alimentados com rações contendo baixos níveis de fumonisina B1. Pesqui. Vet. Bras. 2015, 35, 451–455. [Google Scholar] [CrossRef]
- Gbore, F.A. Reproductive organ weights and semen quality of pubertal boars fed dietary fumonisin B1. Animal 2009, 3, 1133–1137. [Google Scholar] [CrossRef]
- Akande, K.E.; Abubakar, M.M.; Adegbola, T.A.; Bogoro, S.E. Nutritional and Health Implications of Mycotoxins in Animal Feeds: A Review. Pakistan J. Nutr. 2006, 5, 398–403. [Google Scholar] [Green Version]
- Andretta, I.; Kipper, M.; Lehnen, C.R.; Hauschild, L.; Vale, M.M.; Lovatto, P.A. Meta-analytical study of productive and nutritional interactions of mycotoxins in broilers. Poult. Sci. 2011, 90, 1934–1940. [Google Scholar] [CrossRef]
- Dobrzyń, A.; Górski, J. Ceramides and sphingomyelins in skeletal muscles of the rat: Content and composition. Effect of prolonged exercise. Am. J. Physiol. Metab. 2015, 282, E277–E285. [Google Scholar] [CrossRef]
- Müller, S.; Dekant, W.; Mally, A. Fumonisin B1 and the kidney: Modes of action for renal tumor formation by fumonisin B1 in rodents. Food Chem. Toxicol. 2012, 50, 3833–3846. [Google Scholar]
- Hulbert, A.J. On the importance of fatty acid composition of membranes for aging. J. Theor. Biol. 2005, 234, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n−3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, K.I.; Duelund, L.; Qvortrup, K.; Pedersen, J.S.; Ipsen, J.H. Mechanics and dynamics of triglyceride-phospholipid model membranes: Implications for cellular properties and function. Biochim. Biophys. Acta Biomembr. 2011, 1808, 1947–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.C.M.; Simonyi, A.; Sun, A.Y.; Sun, G.Y. Phospholipases A2 and neural membrane dynamics: Implications for Alzheimer’s disease. J. Neurochem. 2011, 116, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Klapisz, E.; Masliah, J.; Béréziat, G.L.; Wolf, C.; Koumanov, K.S. Sphingolipids and cholesterol modulate membrane susceptibility to cytosolic phospholipase A2. J. Lipid. Res. 2000, 41, 1680–1688. [Google Scholar] [PubMed]
- Piccirella, S.; Czegle, I.; Lizák, B.; Margittai, É.; Senesi, S.; Papp, E.; Csala, M.; Fulceri, R.; Csermely, P.; Mandl, J.; et al. Uncoupled redox systems in the lumen of the endoplasmic reticulum: Pyridine nucleotides stay reduced in an oxidative environment. J. Biol. Chem. 2006, 281, 4671–4677. [Google Scholar] [CrossRef]
- Bánhegyi, G.; Margittai, I.; Szarka, A.; Mandl, J.; Csala, M. Crosstalk and barriers between the electron carriers of the endoplasmic reticulum. Antioxid. Redox Signal. 2012, 16, 772–780. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Grotto, D.; Santa Maria, L.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, F.; Yu, D.F.; Wu, P.F.; Chen, J.G. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur. J. Pharmacol. 2011, 650, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouadio, J.; Moukha, S.; Brou, K.; Gnakri, D. Lipid metabolism disorders, lymphocytes cells death, and renal toxicity induced by very low levels of deoxynivalenol and fumonisin B1 alone or in combination following 7 days oral administration to mice. Toxicol. Int. 2013, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Polizzi, A.; Lukowicz, C.; Smati, S.; Lasserre, F.; Lippi, Y.; Naylies, C.; Latte, J.; Bétoulières, C.; Montagner, A.; et al. The protective role of liver X receptor (LXR) during fumonisin B1-induced hepatotoxicity. Arch. Toxicol. 2019, 93, 505–517. [Google Scholar]
- Oswald, I.P.; Desautels, C.; Laffitte, J.; Fournout, S.; Peres, S.Y.; Odin, M.; Le Bars, P.; Le Bars, J.; Fairbrother, J.M. Mycotoxin Fumonisin B1 Increases Intestinal Colonization by Pathogenic Escherichia coli in Pigs. Appl. Environ. Microbiol. 2003, 69, 5870–5874. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.W.; Constable, P.D.; Smith, A.R.; Bacon, C.W.; Meredith, F.I.; Wollenberg, G.K.; Haschek, W.M. Effects of fumonisin-containing culture material on pulmonary clearance in swine. Am. J. Vet. Res. 1996, 57, 1233–1248. [Google Scholar]
- Voss, K.A.; Chamberlain, W.J.; Bacon, C.W.; Norred, W.P. A preliminary investigation on renal and hepatic toxicity in rats fed purified fumonisin B1. Nat. Toxins 1993, 1, 222–228. [Google Scholar] [CrossRef]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone homeostasis in experimental fumonisins intoxication of rats. Ann. Anim. Sci. 2019, 19, 403–419. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Crawford, J.M. The Pathology of Cholestasis. Semin. Liver Dis. 2004, 24, 21–42. [Google Scholar] [PubMed]
- Abado-Becognee, K.; Mobio, T.A.; Ennamany, R.; Fleurat-Lessard, F.; Shier, W.T.; Badria, F.; Creppy, E.E. Cytotoxicity of fumonisin B1: Implication of lipid peroxidation and inhibition of protein and DNA syntheses. Arch. Toxicol. 1998, 72, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Bondy, G.; Barker, M.; Mueller, R.; Fernie, S.; Miller, J.D.; Armstrong, C.; Hierlihy, S.L.; Rowsell, P.; Suzuki, C. Fumonisin B1 Toxicity in Male Sprague-Dawley Rats. In Fumonisins in Food; Springer: Boston, MA, USA, 1996; pp. 251–264. [Google Scholar]
- Fodor, J.; Kametier, L.; Kovács, M. Practical aspects of fumonisin production under laboratory conditions. Mycotoxin Res. 2006, 22, 211–216. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Leray, C.; Andriamampandry, M.; Gutbier, G.; Cavadenti, J.; Klein-Soyer, C.; Gachet, C.; Cazenave, J.P. Quantitative analysis of vitamin E, cholesterol and phospholipid fatty acids in a single aliquot of human platelets and cultured endothelial cells. J. Chromatogr. B Biomed. Appl. 1997, 696, 33–42. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar]
- Buttemer, W.A.; Battam, H.; Hulbert, A.J. Fowl play and the price of petrel: Long-living Procellariiformes have peroxidation-resistant membrane composition compared with short-living Galliformes. Biol. Lett. 2008, 4, 351–354. [Google Scholar] [CrossRef]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Species, Tissue and Subcellular Distribution of Non Se-Dependent Glutathione Peroxidase Activity. J. Nutr. 1978, 108, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]


| Somatic Traits | Control | FB1 | ||||
|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | |
| BW initial (kg) | 13.3 | ± | 1.90 | 13.1 | ± | 1.60 |
| BW final (kg) | 15.9 | ± | 2.40 | 15.8 | ± | 1.80 |
| DBWG (g) | 266 | ± | 66.3 | 269 | ± | 33.3 |
| FC (kg/10 days) | 4458 | ± | 1022 | 4650 | ± | 443 |
| FCR (g diet/g BWG) | 1.69 | ± | 0.13 | 1.74 | ± | 0.20 |
| Liver (g) | 359 | ± | 78.9 | 358 | ± | 56.2 |
| Kidney (g) | 85.2 | ± | 9.99 | 81.3 | ± | 11.4 |
| Spleen (g) | 38.3 | ± | 4.29 | 36.1 | ± | 5.16 |
| Lung (g) | 198 | ± | 34.4 | 189 | ± | 37.7 |
| Heart (g) | 99.2 | ± | 14.9 | 106 | ± | 12.5 |
| Fatty Acids | Hepatic Total PL (%) | Hepatic Total TG (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | FB1 | Control | FB1 | |||||||||||
| Mean | ± | SD | Mean | ± | SD | Sig. | Mean | ± | SD | Mean | ± | SD | Sig. | |
| C12:0 | - | ± | - | - | ± | - | - | 0.04 | ± | 0.01 | 0.03 | ± | 0.01 | NS |
| C14:0 | 0.13 | ± | 0.03 | 0.16 | ± | 0.03 | NS | 0.35 | ± | 0.14 | 0.26 | ± | 0.09 | NS |
| C15:0 | 0.13 | ± | 0.07 | 0.17 | ± | 0.11 | NS | 0.10 | ± | 0.04 | 0.12 | ± | 0.06 | NS |
| C16:0 | 15.5 | ± | 0.60 | 15.2 | ± | 0.95 | NS | 12.3 | ± | 2.25 | 11.1 | ± | 0.70 | NS |
| C16:1n-7 | 0.30 | ± | 0.07 | 0.38 | ± | 0.16 | NS | 0.56 | ± | 0.30 | 0.38 | ± | 0.14 | NS |
| C17:0 | 0.72 | ± | 0.23 | 1.09 | ± | 0.65 | NS | 0.63 | ± | 0.22 | 0.88 | ± | 0.61 | NS |
| C18:0 | 29.0 | ± | 0.93 | 29.6 | ± | 1.62 | NS | 26.6 | ± | 1.88 | 28.6 | ± | 1.11 | * |
| C18:1n-9c | 5.75 | ± | 0.54 | 6.48 | ± | 1.17 | NS | 8.17 | ± | 2.83 | 6.62 | ± | 0.92 | NS |
| C18:2n-6c | 20.6 | ± | 1.04 | 20.7 | ± | 0.75 | NS | 18.4 | ± | 1.77 | 18.0 | ± | 0.74 | NS |
| C18:3n-6 | 0.14 | ± | 0.03 | 0.18 | ± | 0.05 | NS | 0.14 | ± | 0.08 | 0.19 | ± | 0.05 | NS |
| C18:3n-3 | 0.21 | ± | 0.05 | 0.17 | ± | 0.03 | NS | 0.56 | ± | 0.28 | 0.40 | ± | 0.13 | NS |
| C20:0 | 0.07 | ± | 0.01 | 0.08 | ± | 0.02 | NS | 0.13 | ± | 0.06 | 0.11 | ± | 0.01 | NS |
| C20:1n-9 | 0.12 | ± | 0.02 | 0.12 | ± | 0.02 | NS | 0.15 | ± | 0.11 | 0.20 | ± | 0.08 | NS |
| C20:2n-7 | 0.66 | ± | 0.08 | 0.63 | ± | 0.06 | NS | 0.70 | ± | 0.10 | 0.64 | ± | 0.05 | NS |
| C20:3n-6 | 1.34 | ± | 0.35 | 1.29 | ± | 0.25 | NS | 1.29 | ± | 0.38 | 1.33 | ± | 0.25 | NS |
| C20:4n-6 | 12.1 | ± | 1.36 | 13.4 | ± | 0.41 | * | 15.5 | ± | 1.66 | 17.9 | ± | 1.12 | ** |
| C20:3n-3 | 0.14 | ± | 0.04 | 0.12 | ± | 0.02 | NS | 0.21 | ± | 0.06 | 0.20 | ± | 0.04 | NS |
| C20:5n-3 | 1.35 | ± | 0.55 | 1.37 | ± | 0.32 | NS | 1.44 | ± | 0.57 | 1.54 | ± | 0.26 | NS |
| C22:0 | 0.03 | ± | 0.00 | 0.04 | ± | 0.01 | NS | 0.10 | ± | 0.09 | 0.05 | ± | 0.02 | NS |
| C22:5n-3 | 3.04 | ± | 0.14 | 2.38 | ± | 0.29 | *** | 3.13 | ± | 0.34 | 2.89 | ± | 0.25 | NS |
| C22:6n-3 | 8.34 | ± | 0.79 | 6.28 | ± | 1.11 | * | 9.03 | ± | 1.41 | 8.14 | ± | 0.82 | NS |
| C24:0 | 0.34 | ± | 0.03 | 0.26 | ± | 0.04 | ** | 0.38 | ± | 0.07 | 0.33 | ± | 0.04 | NS |
| SFA | 46.0 | ± | 0.75 | 46.6 | ± | 0.77 | NS | 40.7 | ± | 2.73 | 41.6 | ± | 0.99 | NS |
| UFA | 54.0 | ± | 0.75 | 53.4 | ± | 0.77 | NS | 59.3 | ± | 2.73 | 58.4 | ± | 0.99 | NS |
| MUFA | 6.17 | ± | 0.59 | 6.89 | ± | 1.33 | NS | 8.88 | ± | 3.21 | 7.19 | ± | 1.10 | NS |
| PUFA | 47.8 | ± | 1.07 | 46.5 | ± | 1.39 | * | 50.4 | ± | 4.92 | 51.2 | ± | 1.32 | NS |
| Omega-6 | 34.1 | ± | 0.81 | 35.5 | ± | 0.53 | * | 35.4 | ± | 3.03 | 37.4 | ± | 0.85 | NS |
| Omega-3 | 13.0 | ± | 0.91 | 10.3 | ± | 1.35 | *** | 14.4 | ± | 2.09 | 13.2 | ± | 1.05 | NS |
| n-6:n-3 | 2.62 | ± | 0.21 | 3.49 | ± | 0.44 | *** | 2.49 | ± | 0.23 | 2.86 | ± | 0.26 | * |
| Odd Chain | 0.85 | ± | 0.29 | 1.26 | ± | 0.76 | NS | 0.73 | ± | 0.26 | 1.00 | ± | 0.66 | NS |
| UI | 174.3 | ± | 5.43 | 164.8 | ± | 6.50 | * | 192.8 | ± | 16.4 | 193.3 | ± | 5.36 | NS |
| ACL | 18.5 | ± | 0.05 | 18.4 | ± | 0.08 | * | 18.6 | ± | 0.15 | 18.6 | ± | 0.06 | NS |
| Parameter | Control | FB1 | ||||
|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | |
| Total protein (g/L) | 55.4 | ± | 3.64 | 52.6 | ± | 3.41 |
| Albumin (g/L) | 34.0 | ± | 1.82 | 32.7 | ± | 3.15 |
| Total cholesterol (mmol/L) | 1.98 | ± | 0.21 | 2.10 | ± | 0.18 |
| Total bilirubin (μmol/L) | 2.44 | ± | 1.50 | 1.10 | ± | 0.83 |
| Organ | Parameters | Control | FB1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal Number | |||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Liver | Vacuolar degeneration | - | - | - | - | - | - | - | 1 | 1 | - | 1 | 1 | 2 | - |
| Lung | Alveolar edema | - | - | - | - | - | - | - | 2 | - | 1 | - | 1 | - | - |
| Interstitial edema | - | - | - | - | - | - | - | - | - | - | 1 | 1 | - | - | |
| Component | Diet |
|---|---|
| DM (%) | 90.8 |
| Metabolizable energy (MJ/kg) | 14.8 |
| Digestible energy (MJ/kg) | 14.2 |
| Crude protein (% of DM) | 19.7 |
| Ether extract (% of DM) | 5.8 |
| Crude fiber (% of DM) | 3.2 |
| Ash (% of DM) | 5.1 |
| FA weight % of total FA methyl esters | |
| C10:0 | 0.02 |
| C12:0 | 0.03 |
| C14:0 | 0.4 |
| C15:0 | 0.05 |
| C16:0 | 15.2 |
| C16:1n-7 | 0.44 |
| C17:0 | 0.15 |
| C17:1n-7 | 0.07 |
| C18:0 | 4.85 |
| C18:1n-9 | 26.7 |
| C18:1n11t | 0.09 |
| C18:2n-6c | 49 |
| C18:3n-3 | 0.23 |
| C20:0 | 0.36 |
| C20:1n-9 | 2.13 |
| C20:2 n-6 | 0.1 |
| C22:0 | 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, O.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Glávits, R.; Kovács, M.; Zantomasi, A.; Szabó, A. Porcine Hepatic Response to Fumonisin B1 in a Short Exposure Period: Fatty Acid Profile and Clinical Investigations. Toxins 2019, 11, 655. https://doi.org/10.3390/toxins11110655
Ali O, Szabó-Fodor J, Fébel H, Mézes M, Balogh K, Glávits R, Kovács M, Zantomasi A, Szabó A. Porcine Hepatic Response to Fumonisin B1 in a Short Exposure Period: Fatty Acid Profile and Clinical Investigations. Toxins. 2019; 11(11):655. https://doi.org/10.3390/toxins11110655
Chicago/Turabian StyleAli, Omeralfaroug, Judit Szabó-Fodor, Hedvig Fébel, Miklós Mézes, Krisztián Balogh, Róbert Glávits, Melinda Kovács, Arianna Zantomasi, and András Szabó. 2019. "Porcine Hepatic Response to Fumonisin B1 in a Short Exposure Period: Fatty Acid Profile and Clinical Investigations" Toxins 11, no. 11: 655. https://doi.org/10.3390/toxins11110655
APA StyleAli, O., Szabó-Fodor, J., Fébel, H., Mézes, M., Balogh, K., Glávits, R., Kovács, M., Zantomasi, A., & Szabó, A. (2019). Porcine Hepatic Response to Fumonisin B1 in a Short Exposure Period: Fatty Acid Profile and Clinical Investigations. Toxins, 11(11), 655. https://doi.org/10.3390/toxins11110655








