The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens
Abstract
1. Introduction
The Biological Activities of Alkaloids
2. Natural Alkaloid Used to Control Agricultural Pests (Herbivores)
3. Natural Alkaloid Used as Anticancer Agents
4. Antibacterial Activities
4.1. Antibacterial Indole Alkaloids
4.2. Antibacterial Mechanism of Action of Alkaloids
5. Antiviral Activity
6. Antifungal Activity
Antifungal Activity of Alkaloids
7. Toxicity of Plant Secondary Metabolites
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.T.H.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Hassan, E.; Ibrahim, N. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia Grandiflora L. Nat. Prod. Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.L.; Chao, C.F.; Wang, X.; Zhang, Z.; Huong, S.M.; Huang, E.S. Human cytomegalovirus-inhibitory flavonoids: Studies on antiviral activity and mechanism of action. Antivir. Res. 2005, 68, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lin, T.; Wang, W.; Xin, Z.; Zhu, T.; Gu, Q.; Li, D. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J. Nat. Prod. 2013, 76, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Kainsa, S.; Kumar, P.; Rani, P. Medicinal plants of Asian origin having anticancer potential: Short review. Asian J. Biomed. Pharm. Sci. 2012, 2, 1–7. [Google Scholar]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [PubMed]
- Marella, A.; Tanwar, O.P.; Saha, R.; Ali, M.R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Frick, S.; Kramell, R.; Schmidt, J.; Fist, A.J.; Kutchan, T.M. Comparative qualitative and quantitative determination of alkaloids in narcotic and condiment Papaver s omniferum cultivars. J. Nat. Prod. 2005, 68, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, M. Simple, one-pot, and three-component coupling reactions of azaarenes (phenanthridine, isoquinoline, and quinoline), with acetylenic esters involving methyl propiolate or ethyl propiolate in the presence of nh-heterocyclic or 1, 3-dicarbonyl compounds. Synth. Commun. 2013, 43, 157–168. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A. Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chawla, R.; Rai, A.; Yadav, L.D.S. NHC-catalysed diastereoselective synthesis of multifunctionalised piperidines via cascade reaction of enals with azalactones. Chem. Commun. 2012, 48, 3766–3768. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.A.; Hilário, F.F.; Carvalho, L.O.; Silveira, M.L.; Alves, R.B.; Freitas, R.P.; Coimbra, E.S. Effect of 3-Alkylpyridine Marine Alkaloid Analogues in Leishmania Species Related to American Cutaneous Leishmaniasis. Chem. Biol. Drug Des. 2012, 80, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Cronemberger, S.; Calixto, N.; Moraes, M.N.; Castro, I.D.; Lana, P.C.; Loredo, A.F. Efficacy of one drop of 2% pilocarpine to reverse the intraocular pressure peak at 6: 00 am in early glaucoma. Vision Pan-Am. Pan-Am. J. Ophthalmol. 2012, 11, 14–16. [Google Scholar]
- Parmar, N.J.; Pansuriya, B.R.; Barad, H.A.; Kant, R.; Gupta, V.K. An improved microwave assisted one-pot synthesis, and biological investigations of some novel aryldiazenyl chromeno fused pyrrolidines. Bioorg. Med. Chem. Lett. 2012, 22, 4075–4079. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.S.; Das, B.; Naik, C.G. Quinolizidines alkaloids: Petrosin and xestospongins from the sponge Oceanapia sp. J. Chem. Sci. 2011, 123, 601–607. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, B.; Bakshi, N. Biological activity of alkaloids from Solanum dulcamara L. Nat. Prod. Res. 2009, 23, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Zoraghi, R.; Worrall, L.; See, R.H.; Strangman, W.; Popplewell, W.L.; Gong, H.; Samaai, T.; Swayze, R.D.; Kaur, S.; Vuckovic, M. Methicillin-resistant Staphylococcus aureus (MRSA) pyruvate kinase as a target for bis-indole alkaloids with antibacterial activities. J. Biol. Chem. 2011, 286, 44716–44725. [Google Scholar] [CrossRef] [PubMed]
- Villinski, J.; Dumas, E.; Chai, H.B.; Pezzuto, J.; Angerhofer, C.; Gafner, S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm. Biol. 2003, 41, 551–557. [Google Scholar] [CrossRef]
- Otshudi, A.L.; Apers, S.; Pieters, L.; Claeys, M.; Pannecouque, C.; De Clercq, E.; Van Zeebroeck, A.; Lauwers, S.; Frederich, M.; Foriers, A. Biologically active bisbenzylisoquinoline alkaloids from the root bark of Epinetrum villosum. J. Eethnopharmacol. 2005, 102, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Meepagala, K.M.; Wedge, D.E.; Harries, D.; Hale, A.L.; Aliotta, G.; Duke, S.O. Natural fungicides from Ruta graveolens L. leaves, including a new quinolone alkaloid. J. Agric. Food Chem. 2003, 51, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Simons, V.; Morrissey, J.P.; Latijnhouwers, M.; Csukai, M.; Cleaver, A.; Yarrow, C.; Osbourn, A. Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2006, 50, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Cretton, S.; Dorsaz, S.; Azzollini, A.; Favre-Godal, Q.; Marcourt, L.; Ebrahimi, S.N.; Voinesco, F.; Michellod, E.; Sanglard, D.; Gindro, K. Antifungal quinoline alkaloids from Waltheria indica. J. Nat. Prod. 2016, 79, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Yokoyama, S.; Saiki, I.; Hayakawa, Y. Selective anticancer activity of hirsutine against HER2-positive breast cancer cells by inducing DNA damage. Oncol. Rep. 2015, 33, 2072–2076. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Su, B.S.; Chang, L.H.; Gao, Q.; Chen, K.L.; An, P.; Huang, C.; Yang, J.; Li, Z.F. Oxymatrine induces apoptosis in human cervical cancer cells through guanine nucleotide depletion. Anti-Cancer Drugs 2014, 25, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, P.; Adamski, Z.; Bednarz, P.; Slocinska, M.; Ziemnicki, K.; Lelario, F.; Scrano, L.; Bufo, S.A. Cardioinhibitory properties of potato glycoalkaloids in beetles. Bull. Environ. Contam. Toxicol. 2010, 84, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, P.; Kolińska, A.; Spochacz, M.; Chowański, S.; Adamski, Z.; Scrano, L.; Falabella, P.; Bufo, S.A.; Rosiński, G. Differentiated Effects of Secondary Metabolites from Solanaceae and Brassicaceae Plant Families on the Heartbeat of Tenebrio molitor Pupae. Toxins 2019, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Büyükgüzel, E.; Büyükgüzel, K.; Erdem, M.; Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Ventrella, E.; Scrano, L.; Bufo, S.A. The influence of dietary α-solanine on the waxmoth Galleria mellonella L. Arch. Insect Biochem. Physiol. 2013, 83, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, E.; Adamski, Z.; Chudzińska, E.; Miądowicz-Kobielska, M.; Marciniak, P.; Büyükgüzel, E.; Büyükgüzel, K.; Erdem, M.; Falabella, P.; Scrano, L. Solanum tuberosum and Lycopersicon esculentum leaf extracts and single metabolites affect development and reproduction of Drosophila melanogaster. PLoS ONE 2016, 11, e0155958. [Google Scholar] [CrossRef] [PubMed]
- Spochacz, M.; Chowański, S.; Szymczak, M.; Lelario, F.; Bufo, S.; Adamski, Z. Sublethal Effects of Solanum nigrum Fruit Extract and Its Pure Glycoalkaloids on the Physiology of Tenebrio molitor (Mealworm). Toxins 2018, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Yim, M.J.; Kim, B.H.; Kang, K.R.; Lee, S.Y.; Oh, J.S.; You, J.S.; Kim, S.G.; Yu, S.J.; Lee, G.J. Berberine-induced anticancer activities in FaDu head and neck squamous cell carcinoma cells. Oncol. Rep. 2015, 34, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Rahmat, A.; Ismail, P.; Ling, K.H. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J Pharmacol. 2014, 740, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Letašiová, S.; Jantová, S.; Čipák, L.U.; Múčková, M. Berberine—Antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006, 239, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.; Castro, F.; Alves, A.; Pessoa, C.; Moraes, M.; Silveira, E.; Lima, M.; Elmiro, F.; Costa-Lotufo, L. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Sunila, E.; Kuttan, G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J. Eethnopharmacol. 2004, 90, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wirasathien, L.; Boonarkart, C.; Pengsuparp, T.; Suttisri, R. Biological Activities of Alkaloids from Pseuduvaria setosa. Pharm. Biol. 2006, 44, 274–278. [Google Scholar] [CrossRef]
- Chopra, I. The 2012 Garrod lecture: Discovery of antibacterial drugs in the 21st century. J. Antimicrob. Chemother. 2012, 68, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Oleksiewicz, M.B.; Nagy, G.; Nagy, E. Anti-bacterial monoclonal antibodies: Back to the future? Arch. Insect Biochem. 2012, 526, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Henein, A. What are the limitations on the wider therapeutic use of phage? Bacteriophage 2013, 3, e24872. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Kao, C.L.; Wu, H.M.; Li, W.J.; Huang, C.T.; Li, H.T.; Chen, C.Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef] [PubMed]
- Lamchouri, F.; Zemzami, M.; Jossang, A.; Abdellatif, A.; Israili, Z.H.; Lyoussi, B. Cytotoxicity of alkaloids isolated from Peganum harmala seeds. Pak. J. Pharm. Sci. 2013, 26, 699–706. [Google Scholar] [PubMed]
- Kobayashi, Y.; Nakano, Y.; Kizaki, M.; Hoshikuma, K.; Yokoo, Y.; Kamiya, T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med. 2001, 67, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y. The nociceptive and anti-nociceptive effects of evodiamine from fruits of Evodia rutaecarpa in mice. Planta. Med. 2003, 69, 425–428. [Google Scholar] [PubMed]
- Shin, Y.W.; Bae, E.A.; Cai, X.F.; Lee, J.J.; Kim, D.H. In vitro and in vivo antiallergic effect of the fructus of Evodia rutaecarpa and its constituents. Biol. Pharm. Bull. 2007, 30, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Wang, Y.H.; Liou, K.T.; Chen, C.M.; Chen, C.H.; Wang, W.Y.; Chang, S.; Hou, Y.C.; Chen, K.T.; Chen, C.F. Anti-inflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur. J. Pjarmacol. 2007, 555, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.F.; Zhou, Q.M.; Zhang, T.L.; Lu, Y.Y.; Zhang, H.; Su, S.B. Evodiamine induces apoptosis and inhibits metastasis in MDA-MB-231 human breast cancer cells in vitro and in vivo. Oncol. Rep. 2013, 30, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Matsunaga, T.; Takahashi, S.; Saiki, I.; Suzuki, H. Anti-invasive and metastatic activities of evodiamine. Biol. Pharm. Bull. 2002, 25, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.F.; Wang, B.X.; Li, T.J.; Tashiro, S. i.; Minami, M.; Xing, D.J.; Ikejima, T. Evodiamine, a constituent of Evodiae Fructus, induces anti-proliferating effects in tumor cells. Cancer Sci. 2003, 94, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.J.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Intracellular regulation of evodiamine-induced A375-S2 cell death. Biol. Pharm. Bull. 2003, 26, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.G.; Lin, S.; Lee, C.C.; Chen, E.; Lin, L.C.; Wang, B.W.; Tsai, S.C. Evodiamine inhibits in vitro angiogenesis: Implication for antitumorgenicity. Life Sci. 2006, 78, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Kim, E.J.; Kim, S.; Jung, E.M.; Park, J.W.; Jeong, S.H.; Park, S.E.; Yoo, Y.H.; Kwon, T.K. Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human leukemic U937 cells. Mol. Cancer Ther. 2006, 5, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, S.; Wang, M.W. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-κB signaling pathways and augmented by ubiquitin—Proteasome inhibition. Toxicol. Vitr. 2010, 24, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.I.; Onodera, S.; Ikejima, T. Reactive oxygen species and nitric oxide regulate mitochondria-dependent apoptosis and autophagy in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic. Res. 2008, 42, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.P.; Lin, L.W.; Lai, Z.Y.; Wu, J.Y.; Chen, C.E.; Hwang, J.; Chen, C.S.; Lin, C.M. Immobilizing topoisomerase I on a surface plasmon resonance biosensor chip to screen for inhibitors. J. Biomed. Sci. 2010, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Chang, W.S.; Chen, L.M.; Lee, C.M.; Chen, C.E.; Lin, C.M.; Hwang, J.L. Evodiamine stabilizes topoisomerase I-DNA cleavable complex to inhibit topoisomerase I activity. Molecules 2009, 14, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Sheng, C.; Wang, S.; Miao, Z.; Yao, J.; Zhang, W. Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J. Med. Chem. 2010, 53, 7521–7531. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Guh, J.H.; Teng, C.M. Induction of mitotic arrest and apoptosis by evodiamine in human leukemic T-lymphocytes. Life Sci. 2004, 75, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.I.; Onodera, S.; Ikejima, T. Critical roles of reactive oxygen species in mitochondrial permeability transition in mediating evodiamine-induced human melanoma A375-S2 cell apoptosis. Free Radic. Res. 2007, 41, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; He, X.W.; Jiang, Y.; Chen, F. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 2003, 375, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, R.; Zheng, P.; Yan, L.; Wu, Y.; Xiao, X.; Dai, G. Cardioprotective effect of matrine on isoproterenol-induced cardiotoxicity in rats. J. Pharm. Pharmacol. 2010, 62, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yan, F.; Liu, Y.; Liu, Y.; Zhao, Y. Matrine inhibits 3T3-L1 preadipocyte differentiation associated with suppression of ERK1/2 phosphorylation. Bio. Chem. Bioph Res. Commun. 2010, 396, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zheng, P.; Zhou, X.; Yan, L.; Zhou, R.; Fu, X.Y.; Dai, G.D. Relaxant effects of matrine on aortic smooth muscles of guinea pigs. Biomed. Environ. Sci. 2009, 22, 327–332. [Google Scholar] [CrossRef]
- Long, Y.; Lin, X.; Zeng, K.; Zhang, L. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat. Dis. Int. 2004, 3, 69–72. [Google Scholar] [PubMed]
- Yadav, V.; Krishnan, A.; Vohora, D. A systematic review on Piper longum L.: Bridging traditional knowledge and pharmacological evidence for future translational research. J. Ethnopharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yixiang, H.; Shenghui, Z.; Jianbo, W.; Kang, Y.; Yu, Z.; Lihui, Y.; Laixi, B. Matrine induces apoptosis of human multiple myeloma cells via activation of the mitochondrial pathway. Leuk. Lymphoma 2010, 51, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Wen, X.; Wei, Y.; Peng, X.; Li, H.; Wei, L. Effect of matrine on HeLa cell adhesion and migration. Eur. J. Pharmacol. 2007, 563, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.J.; Gao, J.; Ji, Z.Z.; Wang, X.J.; Ren, H.T.; Liu, X.X.; Wu, W.Y.; Kang, H.F.; Guan, H.T. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3. J. Ethnopharmacol. 2009, 123, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Z.; Chong, T.; Ji, Z. Matrine inhibits proliferation and induces apoptosis of the androgen-independent prostate cancer cell line PC-3. Mol. Med. Rep. 2012, 5, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Z.; Zhang, J.K.; Shi, Z.; Liu, B.; Shen, C.Q.; Tao, H.M. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother. Pharmacol. 2012, 69, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Song, Y.; Chen, H.; Pan, S.; Sun, X. Matrine inhibits proliferation and induces apoptosis of pancreatic cancer cells in vitro and in vivo. Biol. Pharm. Bull. 2010, 33, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wu, W.; Wang, J.; Wang, Y.; Wu, X.; Fei, X.; Li, S.; Zhang, J.; Dong, P. Effects of Matrine on Proliferation and Apoptosis in Gallbladder Carcinoma Cells (GBC-SD). Phytother. Res. 2012, 26, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hou, C.; Zhang, S.; Xie, H.; Zhou, W.; Jin, Q.; Cheng, X.; Qian, R.; Zhang, X. Matrine upregulates the cell cycle protein E2F-1 and triggers apoptosis via the mitochondrial pathway in K562 cells. Eur. J. Pharmacol. 2007, 559, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, G.; Jiang, X.; Qiao, H.; Pan, S.; Jiang, H.; Kanwar, J.R.; Sun, X. Therapeutic effects of matrine on primary and metastatic breast cancer. Am. J. Chin. Med. 2010, 38, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wen, S.; Zhan, Y.; He, Y.; Liu, X.; Jiang, J. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med. 2008, 74, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, B.T.; Xu, Y.H. Effects of matrine on invasion and metastasis of leukemia cell line Jurkat. Chin. J. Integr. Tradit. West. Med. 2008, 28, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Q.; Liu, K.; Yagasaki, K.; Wu, E.; Zhang, G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology 2009, 59, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Zhang, H.F.; Li, D.Y.; Zhang, X.; Xue, H.Z.; Zhao, S.H. Matrine inhibits matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. J. Asian Nat. Prod. Res. 2011, 13, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhong, H.J.; Zhu, L.M.; Wu, X.G.; Ying, J.E.; Wang, X.H.; Lü, W.X.; Xu, Q.; Zhu, Y.L.; Huang, J. Inhibition of matrine against gastric cancer cell line MNK45 growth and its anti-tumor mechanism. Mol. Biol. Rep. 2012, 39, 5459–5464. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Piperine: Researchers discover new flavor in an ancient spice. Trends Pharmacol. Sci. 2005, 26, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Hong, S.S.; Han, X.H.; Hwang, J.S.; Oh, G.J.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. 2005, 53, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Wang, M.; Li, W.; Matsumoto, K.; Tang, Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007, 80, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012, 33, 523. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Qu, Y.; Zheng, H.; Zhang, G.; Lin, H.; Yang, J. Differentiation of erythroleukemia K562 cells induced by piperine. Chin. J. Cancer 2008, 27, 571–574. [Google Scholar]
- Pradeep, C.; Kuttan, G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin. Exp. Metastasis 2002, 19, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Yun, H.J.; Kim, H.G.; Han, E.H.; Choi, J.H.; Chung, Y.C.; Jeong, H.G. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol. Lett. 2011, 203, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, C.; Kuttan, G. Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int. Immunopharmacol. 2004, 4, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002, 302, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Y.; Jia, Y.; Li, N.; Wink, M.; Ma, Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 2011, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Golovine, K.; Canter, D.; Kutikov, A.; Simhan, J.; Corlew, M.M.; Uzzo, R.G.; Kolenko, V.M. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate 2012, 72, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Morris-Natschke, S.L.; Yang, J.; Niu, H.M.; Long, C.L.; Lee, K.H. Anticancer principles from medicinal Piper plants. J. Tradit. Complement. Med. 2014, 4, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Beecher, C. Quercetin-induced benzophenanthridine alkaloid production in suspension cell cultures of Sanguinaria canadensis. Planta Med. 1994, 60, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Vavrečková, C.; Gawlik, I.; Müller, K. Benzophenanthridine alkaloids of Chelidonium majus; I. Inhibition of 5-and 12-lipoxygenase by a non-redox mechanism. Planta. Med. 1996, 62, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Lenfeld, J.; Kroutil, M.; Maršálek, E.; Slavik, J.; Preininger, V.; Šimánek, V. Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonoum majus. Planta Med. 1981, 43, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Beuria, T.K.; Santra, M.K.; Panda, D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry 2005, 44, 16584–16593. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.H.; Wu, H.L.; Lin, B.R.; Lan, W.H.; Chang, H.H.; Ho, Y.S.; Lee, P.H.; Wang, Y.J.; Wang, J.S.; Chen, Y.J. Antiplatelet effect of sanguinarine is correlated to calcium mobilization, thromboxane and cAMP production. Atherosclerosis 2007, 191, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Reagan-Shaw, S.; Breur, J.; Ahmad, N. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett. 2007, 249, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chan, C.P.; Wang, Y.J.; Lee, P.H.; Chen, L.I.; Tsai, Y.L.; Lin, B.R.; Wang, Y.L.; Jeng, J.H. Induction of necrosis and apoptosis to KB cancer cells by sanguinarine is associated with reactive oxygen species production and mitochondrial membrane depolarization. Toxicol. Appl. Pharm. 2007, 218, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Al-Jomah, N.A.; Siraj, A.K.; Manogaran, P.; Al-Hussein, K.; Abubaker, J.; Platanias, L.C.; Al-Kuraya, K.S.; Uddin, S. Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells. Cancer. Res. 2007, 67, 3888–3897. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, T.J.; Leem, J.; Choi, K.S.; Park, J.W.; Kwon, T.K. Sanguinarine-induced apoptosis: Generation of ROS, down-regulation of Bcl-2, c-FLIP, and synergy with TRAIL. J. Cell Biochem. 2008, 104, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Aziz, M.H.; Mukhtar, H.; Ahmad, N. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin. Cancer Res. 2003, 9, 3176–3182. [Google Scholar] [PubMed]
- Adhami, V.M.; Aziz, M.H.; Reagan-Shaw, S.R.; Nihal, M.; Mukhtar, H.; Ahmad, N. Sanguinarine causes cell cycle blockade and apoptosis of human prostate carcinoma cells via modulation of cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery. Mol. Cancer Ther. 2004, 3, 933–940. [Google Scholar] [PubMed]
- Weerasinghe, P.; Hallock, S.; Tang, S.C.; Trump, B.; Liepins, A. Sanguinarine overcomes P-glycoprotein-mediated multidrug-resistance via induction of apoptosis and oncosis in CEM-VLB 1000 cells. Exp. Toxicol. Pathol. 2006, 58, 21–30. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, I.; Raspaglio, G.; Zannoni, G.F.; Travaglia, D.; Prisco, M.G.; Mosca, M.; Ferlini, C.; Scambia, G.; Gallo, D. Antiproliferative and antiangiogenic effects of the benzophenanthridine alkaloid sanguinarine in melanoma. Biochem. Pharmacol. 2009, 78, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Choi, W.Y.; Hong, S.H.; Kim, S.O.; Kim, G.Y.; Lee, W.H.; Yoo, Y.H. Anti-invasive activity of sanguinarine through modulation of tight junctions and matrix metalloproteinase activities in MDA-MB-231 human breast carcinoma cells. Chem. Biol. Interact. 2009, 179, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Reagan-Shaw, S.; Eggert, D.M.; Tan, T.C.; Afaq, F.; Mukhtar, H.; Ahmad, N. Protective effect of sanguinarine on ultraviolet B-mediated damages in SKH-1 hairless mouse skin: Implications for prevention of skin cancer. Photochem. Photobiol. 2007, 83, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.C.; Park, J.G.; Song, D.K.; Baek, W.K.; Yoo, S.K.; Jung, K.H.; Park, G.Y.; Lee, T.Y.; Suh, S.I. Sanguinarine induces apoptosis in A549 human lung cancer cells primarily via cellular glutathione depletion. Toxicol. Vitr. 2009, 23, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.J.; Lu, J.J.; Zhu, H.; Xie, H.; Huang, M.; Lin, L.P.; Zhang, X.W.; Ding, J. Salvicine triggers DNA double-strand breaks and apoptosis by GSH-depletion-driven H2O2 generation and topoisomerase II inhibition. Free Radic. Bio. Med. 2008, 45, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lu, J.; Miao, Z.; Lin, L.; Ding, J. Reactive oxygen species contribute to cell killing and P-glycoprotein downregulation by salvicine in multidrug resistant K562/A02 cells. Cancer Biol. Ther. 2007, 6, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Tamewitz, A.; Skoko, J.; Sikorski, R.P.; Giuliano, K.A.; Lazo, J.S. The benzo [c] phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J. Biol. Chem. 2005, 280, 19078–19086. [Google Scholar] [CrossRef] [PubMed]
- Lopus, M.; Panda, D. The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding: A possible mechanism for its antiproliferative activity. J. FEBS 2006, 273, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.M.; Kumar, A.; Darnay, B.G.; Chainy, G.B.; Agarwal, S.; Aggarwal, B.B. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-κB activation, IκBα phosphorylation, and degradation. J. Biol. Chem. 1997, 272, 30129–30134. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, C.; Nadiminty, N.; Lou, W.; Zhu, Y.; Yang, J.; Evans, C.P.; Zhou, Q.; Gao, A.C. Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion. Prostate 2012, 72, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Li, D.G.; Wang, Z.R.; Lu, H.M. Pharmacology of tetrandrine and its therapeutic use in digestive diseases. World J. Gastroenterol. 2001, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.T.; Chiang, L.C.; Lin, Y.T.; Lin, C.C. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am. J. Chin. Med. 2006, 34, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Zhang, H.; Hayward, L.; Takemura, H.; Shao, R.G.; Pommier, Y. Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S–specific cyclin-dependent kinases and by inducing p53 and p21Cip1. Cancer Res. 2004, 64, 9086–9092. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Chen, Y.; Chen, J.C.; Lin, T.Y.; Tseng, S.H. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 2010, 287, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.C.; Tseng, S.H. Tetrandrine suppresses tumor growth and angiogenesis of gliomas in rats. Int. J. Cancer 2009, 124, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Liao, H.F.; Chang, H.H.; Chen, Y.Y.; Yu, M.C.; Chou, C.J.; Chen, Y.J. Inhibitory effect of tetrandrine on pulmonary metastases in CT26 colorectal adenocarcinoma-bearing BALB/c mice. Am. J. Chin. Med. 2004, 32, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Wang, H.; Wang, K.; Du, Y.; Zhang, J. Combination of Tetrandrine with cisplatin enhances cytotoxicity through growth suppression and apoptosis in ovarian cancer in vitro and in vivo. Cancer Lett. 2011, 304, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, B.; Wang, L.; Qian, X.; Ding, Y.; Yu, L. Synergistic interaction between tetrandrine and chemotherapeutic agents and influence of tetrandrine on chemotherapeutic agent-associated genes in human gastric cancer cell lines. Cancer Chemother. Pharmacol. 2007, 60, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Iorns, E.; Lord, C.J.; Ashworth, A. Parallel RNAi and compound screens identify the PDK1 pathway as a target for tamoxifen sensitization. Biochem. J. 2009, 417, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Huan Ren, K.; He, H.W.; Shao, R.G. Involvement of PI3K/AKT/GSK3ß pathway in tetrandrine-induced G1 arrest and apoptosis. Cancer Biol. Ther. 2008, 7, 1073–1078. [Google Scholar]
- Oh, S.H.; Lee, B.H. Induction of apoptosis in human hepatoblastoma cells by tetrandrine via caspase-dependent Bid cleavage and cytochrome c release. Biochem. Pharmacol. 2003, 66, 725–731. [Google Scholar] [CrossRef]
- Cho, H.S.; Chang, S.H.; Chung, Y.S.; Shin, J.Y.; Park, S.J.; Lee, E.S.; Hwang, S.K.; Kwon, J.T.; Tehrani, A.M.; Woo, M. Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells. J. Vet. Sci. 2009, 10, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kittakoop, P.; Mahidol, C.; Ruchirawat, S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr. Top. Med. Chem. 2014, 14, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.M.; Ebrahimi, S.A.; Mahmoudian, M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its beta-carboline alkaloids. J. Pharm. Pharm. Sci. 2002, 5, 19–23. [Google Scholar] [PubMed]
- Heinrich, M.; Barnes, J.; Gibbons, S.; Williamson, E. A Text Book of Fundamentals of Pharmacognosy and Phytotherapy; Elsevier: New York, NY, USA, 2004; Volume 8, pp. 60–105. [Google Scholar]
- Hesse, M. Alkaloids: Nature’s Curse or Blessing; John Wiley & Sons: zürich, Switzerland, 2002. [Google Scholar]
- Evans, W.C. Trease and Evans’ Pharmacognosy E-Book; Elsevier Health Sciences: Edinburgh, UK, 2009. [Google Scholar]
- Robbers, J.E.; Speedie, M.K.; Tyler, V.E. Pharmacognosy and Pharmacobiotechnology; Williams & Wilkins: Baltimore, MD, USA, 1996. [Google Scholar]
- Hraiech, S.; Brégeon, F.; Brunel, J.M.; Rolain, J.-M.; Lepidi, H.; Andrieu, V.; Raoult, D.; Papazian, L.; Roch, A. Antibacterial efficacy of inhaled squalamine in a rat model of chronic Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 2012, 67, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, E.; Hanrahan, C.; Nikonenko, B.; De los Santos, G.; Reddy, V.; Chen, P.; Barbosa, F.; Einck, L.; Nacy, C.; Protopopova, M. Identification of SQ609 as a lead compound from a library of dipiperidines. Bioorg. Med. Chem. Lett. 2011, 21, 5353–5357. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.; Kelley, C.; Kaul, M.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of substituted 5-methylbenzo [c] phenanthridinium derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7080–7083. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Chowdhury, B.; Bhattacharyya, P. Clausenol and clausenine—Two carbazole alkaloids from Clausena anisata. Phytochemistry 1995, 40, 295–298. [Google Scholar] [CrossRef]
- Nakahara, K.; Trakoontivakorn, G.; Alzoreky, N.S.; Ono, H.; Onishi-Kameyama, M.; Yoshida, M. Antimutagenicity of some edible Thai plants, and a bioactive carbazole alkaloid, mahanine, isolated from Micromelum minutum. J. Agric. Food Chem. 2002, 50, 4796–4802. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lantvit, D.; Hwang, C.H.; Kroll, D.J.; Swanson, S.M.; Franzblau, S.G.; Orjala, J. Indole alkaloids from two cultured cyanobacteria, Westiellopsis sp. and Fischerella muscicola. Bioorgan. Med. Chem. 2012, 20, 5290–5295. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Ashfaq, M.K.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Manly, S.P.; Joshi, V.C.; Walker, L.A.; Muhammad, I. Indolizidine, antiinfective and antiparasitic compounds from Prosopis glandulosa Torr. var. glandulosa. Planta med. 2009, 75, 48. [Google Scholar] [CrossRef]
- Iwasa, K.; Moriyasu, M.; Tachibana, Y.; Kim, H.S.; Wataya, Y.; Wiegrebe, W.; Bastow, K.F.; Cosentino, L.M.; Kozuka, M.; Lee, K.H. Simple isoquinoline and benzylisoquinoline alkaloids as potential antimicrobial, antimalarial, cytotoxic, and anti-HIV agents. Bioorgan. Med. Chem. 2001, 9, 2871–2884. [Google Scholar] [CrossRef]
- Xie, Q.; Johnson, B.R.; Wenckus, C.S.; Fayad, M.I.; Wu, C.D. Efficacy of berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a mixed-culture biofilm in an in vitro tooth model. J. Endod. 2012, 38, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Obiang-Obounou, B.W.; Kang, O.H.; Choi, J.G.; Keum, J.H.; Kim, S.B.; Mun, S.H.; Shin, D.W.; Kim, K.W.; Park, C.B.; Kim, Y.G. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol. Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhang, W.D.; Zhang, C.; Liu, R.H.; Wang, X.W.; Wang, X.L.; Zhu, J.B.; Chen, C.L. Bioavailabilty and pharmacokinetics of four active alkaloids of traditional Chinese medicine Yanhuanglian in rats following intravenous and oral administration. J. Pharma. Biomed. 2006, 41, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zan, K.; Shi, S.P.; Zeng, K.W.; Jiang, Y.; Guan, Y.; Xiao, C.L.; Gao, H.Y.; Wu, L.J.; Tu, P.F. Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia 2013, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Wansi, J.; Mbaveng, A.; Sop, M.K.; Tadjong, A.T.; Beng, V.P.; Etoa, F.X.; Wandji, J.; Meyer, J.M.; Lall, N. Antimicrobial activity of the methanolic extract and compounds from Teclea afzelii (Rutaceae). S. Afr. J. Bot. 2008, 74, 572–576. [Google Scholar] [CrossRef]
- Dekker, K.A.; Inagaki, T.; Gootz, T.D.; Huang, L.H.; Kojima, Y.; Kohlbrenner, W.E.; Matsunaga, Y.; McGuirk, P.R.; Nomura, E.; Sakakibara, T. New Quinolone Compounds from Pseudonocardia sp. with Selective and Potent Anti-Helicobacter pylori Activity. Jpn. J. Antibiot. 1998, 51, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Iwai, T.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J.I. Agelasines O–U, new diterpene alkaloids with a 9-N-methyladenine unit from a marine sponge Agelas sp. Tetrahedron 2012, 68, 9738–9744. [Google Scholar] [CrossRef]
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M.; Forrest, J.N.; McCrimmon, D.; Zasloff, M. Squalamine: An aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Calcul, L.; Longeon, A.; Al Mourabit, A.; Guyot, M.; Bourguet-Kondracki, M.L. Novel alkaloids of the aaptamine class from an Indonesian marine sponge of the genus Xestospongia. Tetrahedron 2003, 59, 6539–6544. [Google Scholar] [CrossRef]
- Alhanout, K.; Malesinki, S.; Vidal, N.; Peyrot, V.; Rolain, J.M.; Brunel, J.M. New insights into the antibacterial mechanism of action of squalamine. J. Antimicrob. Chemother. 2010, 65, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Venkatachalam, S. Inhibition of dihydrofolate reductase and cell growth activity by the phenanthroindolizidine alkaloids pergularinine and tylophorinidine: The in vitro cytotoxicity of these plant alkaloids and their potential as antimicrobial and anticancer agents. Toxicol. Vitr. 2000, 14, 53–59. [Google Scholar] [CrossRef]
- Casu, L.; Cottiglia, F.; Leonti, M.; De Logu, A.; Agus, E.; Tse-Dinh, Y.-C.; Lombardo, V.; Sissi, C. Ungeremine effectively targets mammalian as well as bacterial type I and type II topoisomerases. Bioorg. Med. Chem. Lett. 2011, 21, 7041–7044. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Higuchi, K.; Hamasaki, N.; Hamaguchi, M.; Takashima, T.; Tanigawa, T.; Watanabe, T.; Fujiwara, Y.; Tezuka, Y.; Nagaoka, T. In vivo action of novel alkyl methyl quinolone alkaloids against Helicobacter pylori. J. Antimicrob. Chemother. 2002, 50, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Identification of the Target Protein of Agelasine D, a Marine Sponge Diterpene Alkaloid, as an Anti-dormant Mycobacterial Substance. ChemBioChem 2014, 15, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Salmi, C.; Loncle, C.; Vidal, N.; Letourneux, Y.; Fantini, J.; Maresca, M.; Taïeb, N.; Pagès, J.M.; Brunel, J.M. Squalamine: An appropriate strategy against the emergence of multidrug resistant gram-negative bacteria? PLoS ONE 2008, 3, e2765. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.J.; Ghosh, J.S. Antimicrobial activity of Catharanthus roseus—A detailed study. Br. J. Pharmcol. Toxicol. 2010, 1, 40–44. [Google Scholar]
- Hallock, Y.F.; Manfredi, K.P.; Dai, J.R.; Cardellina, J.H.; Gulakowski, R.J.; McMahon, J.B.; Schäffer, M.; Stahl, M.; Gulden, K.P.; Bringmann, G. Michellamines D–F, new HIV-inhibitory dimeric naphthylisoquinoline alkaloids, and korupensamine E, a new antimalarial monomer, from Ancistrocladus korupensis. J. Nat. Prod. 1997, 60, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yang, S.; Jin, L.H.; Bhadury, P.S. Environment-Friendly Antiviral Agents for Plants; Springer Science & Business Media: Guizhou, China, 2011. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.A.; Fleet, G.W.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated alkaloids—Natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265295. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Imakura, Y.; Jia, Y.; Li, D.; Cosentino, L.M.; Lee, K.H. Sesquiterpene Alkaloids from Tripterygium h ypoglaucum and Tripterygium w ilfordii: A New Class of Potent Anti-HIV Agents. J. Nat. Prod. 2000, 63, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Gray, A.I. Antimicrobial constituents from the stem bark of Feronia limonia. Phytochemistry 2002, 59, 73–77. [Google Scholar] [CrossRef]
- Tabarrini, O.; Manfroni, G.; Fravolini, A.; Cecchetti, V.; Sabatini, S.; De Clercq, E.; Rozenski, J.; Canard, B.; Dutartre, H.; Paeshuyse, J. Synthesis and anti-BVDV activity of acridones as new potential antiviral agents. J. Med. Chem. 2006, 49, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Itoigawa, M.; Sato, A.; Hasan, C.M.; Rashid, M.A.; Tokuda, H.; Mukainaka, T.; Nishino, H.; Furukawa, H. Chemical Constituents of Glycosmis a rborea: Three New Carbazole Alkaloids and Their Biological Activity. J. Nat. Prod. 2004, 67, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Manske, R.H.F.; Holmes, H.L. The Alkaloids: Chemistry and Physiology; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Ududua, U.O.; Monanu, M.O.; Chuku, L.C. Proximate Analysis and Phytochemical Profile of Brachystegia eurycoma Leaves. Asian J. Res. Biochem. 2019, 4, 1–11. [Google Scholar] [CrossRef]
- Petrov, N.M. Antiviral Activity of Plant Extract from Tanacetum Vulgare Against Cucumber Mosaic Virus and Potato Virus Y. J. Biomed Biotechnol. 2016, 5, 189–194. [Google Scholar]
- Rex, J.H.; Rinaldi, M.; Pfaller, M. Resistance of Candida species to fluconazole. Antimicrob. Agents. Chemother. 1995, 39, 1. [Google Scholar] [CrossRef] [PubMed]
- Borris, R.P. Natural products research: Perspectives from a major pharmaceutical company. J. Ethnopharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Lewis, W.H.; Elvin-Lewis, M.P. Medicinal plants as sources of new therapeutics. Ann. Mo. Bot. Gard. 1995, 82, 16–24. [Google Scholar] [CrossRef]
- Anaissie, E.; Bodey, G.; Rinaldi, M. Emerging fungal pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wey, S.B.; Mori, M.; Pfaller, M.A.; Woolson, R.F.; Wenzel, R.P. Hospital-acquired candidemia: The attributable mortality and excess length of stay. Arch. Intern. Med. 1988, 148, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Beck-Sague, C.; Banerjee, S.; Jarvis, W.R. Infectious diseases and mortality among US nursing home residents. Am. J. Public Health 1993, 83, 1739–1742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schultes, R.E. Plants and plant constituents as mind-altering agents throughout history. In Stimulants; Springer: Boston, MA, USA, 1978; pp. 219–241. [Google Scholar]
- Küçükosmanoǧlu Bahçeevli, A.; Kurucu, S.; Kolak, U.; Topçu, G.; Adou, E.; Kingston, D.G. Alkaloids and Aromatics of Cyathobasis f ruticulosa (Bunge) Aellen. J. Nat. Prod. 2005, 68, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Ferheen, S.; Ahmed, E.; Afza, N.; Malik, A.; Shah, M.R.; Nawaz, S.A.; Choudhary, M.I. Haloxylines A and B, antifungal and cholinesterase inhibiting piperidine alkaloids from Haloxylon salicornicum. Chem. Pharm. Bull. 2005, 53, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Azmi, S.; Maurya, S.; Singh, U.; Jha, R.; Pandey, V. Two plant alkaloids isolated fromCorydalis longipes as potential antifungal agents. Folia Microbiol. 2003, 48, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Sung, W.S.; Yeo, S.H.; Kim, H.S.; Lee, I.S.; Woo, E.R.; Lee, D.G. Antifungal effect of amentoflavone derived fromSelaginella tamariscina. Arch. Pharm. Res. 2006, 29, 746. [Google Scholar] [CrossRef] [PubMed]
- Morteza-Semnani, K.; Amin, G.; Shidfar, M.; Hadizadeh, H.; Shafiee, A. Antifungal activity of the methanolic extract and alkaloids of Glaucium oxylobum. Fitoterapia 2003, 74, 493–496. [Google Scholar] [CrossRef]
- Thouvenel, C.; Gantier, J.C.; Duret, P.; Fourneau, C.; Hocquemiller, R.; Ferreira, M.E.; de Arias, A.R.; Fournet, A. Antifungal compounds from Zanthoxylum chiloperone var. angustifolium. Phytother. Res. 2003, 17, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Sarma, B.; Mishra, P.; Ray, A. Antifungal activity of venenatine, an indole alkaloid isolated fromAlstonia venenata. Folia Microbiol. 2000, 45, 173. [Google Scholar] [CrossRef] [PubMed]
- Balls, A.; Hale, W.; Harris, T. A crystalline protein obtained from a lipoprotein of wheat flour. Cereal Chem. 1942, 19, 279–288. [Google Scholar]
- Liu, S.; Oguntimein, B.; Hufford, C.; Clark, A. 3-Methoxysampangine, a novel antifungal copyrine alkaloid from Cleistopholis patens. Antimicrob. Agents Chemother. 1990, 34, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Emile, A.; Waikedre, J.; Herrenknecht, C.; Fourneau, C.; Gantier, J.C.; Hnawia, E.; Cabalion, P.; Hocquemiller, R.; Fournet, A. Bioassay-guided isolation of antifungal alkaloids from Melochia odorata. Phytother. Res. 2007, 21, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.; Schrader, K.; Mamonov, L.; Sitpaeva, G.; Kustova, T.; Dunbar, C.; Wedge, D. Isolation and identification of antifungal and antialgal alkaloids from Haplophyllum sieversii. J. Agric. Food Chem. 2005, 53, 7741–7748. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Boyunegmez Tumer, T.; Ozleyen, A.; Rodríguez-Pérez, C.; M Ezzat, S.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef] [PubMed]
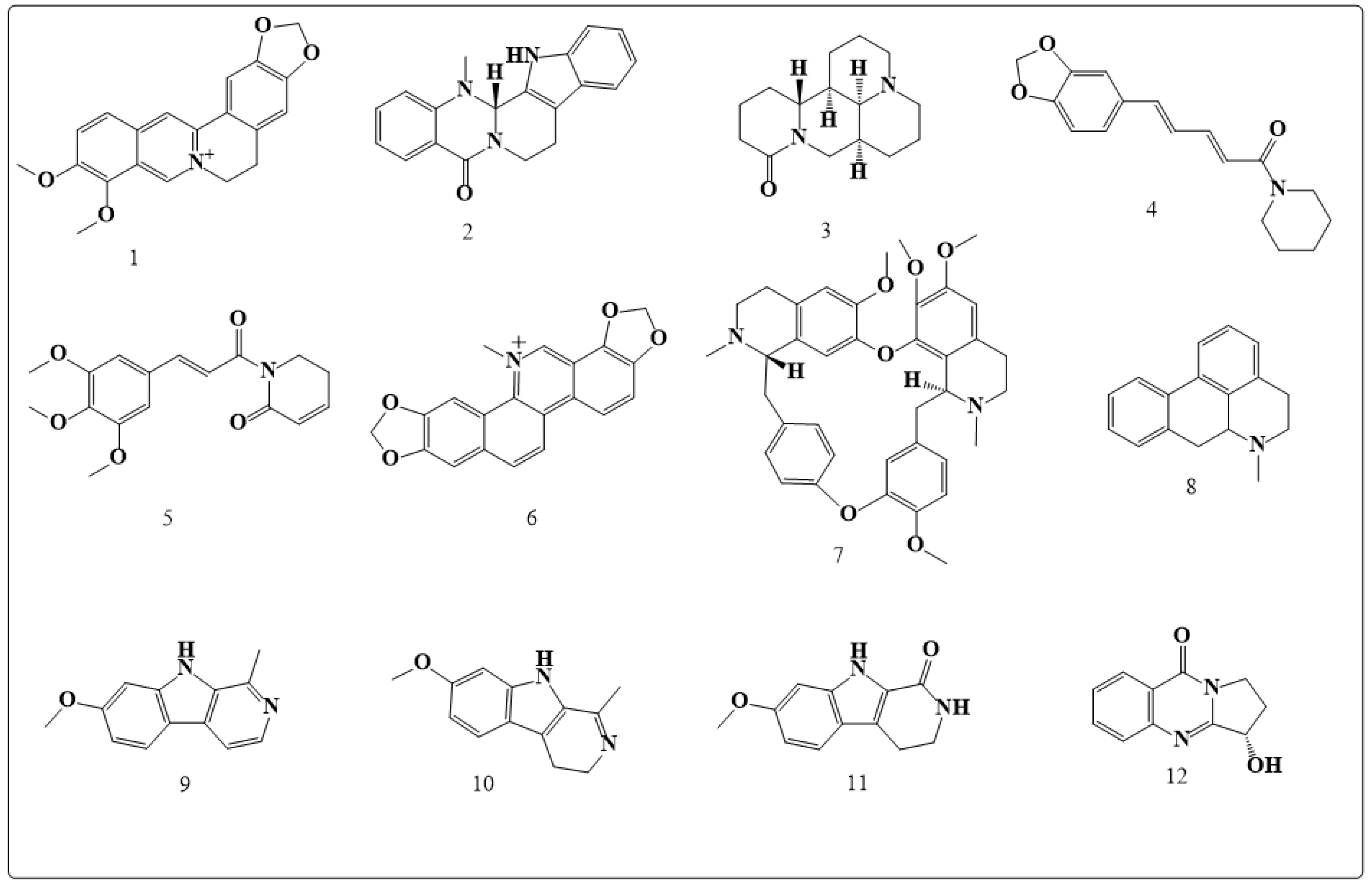
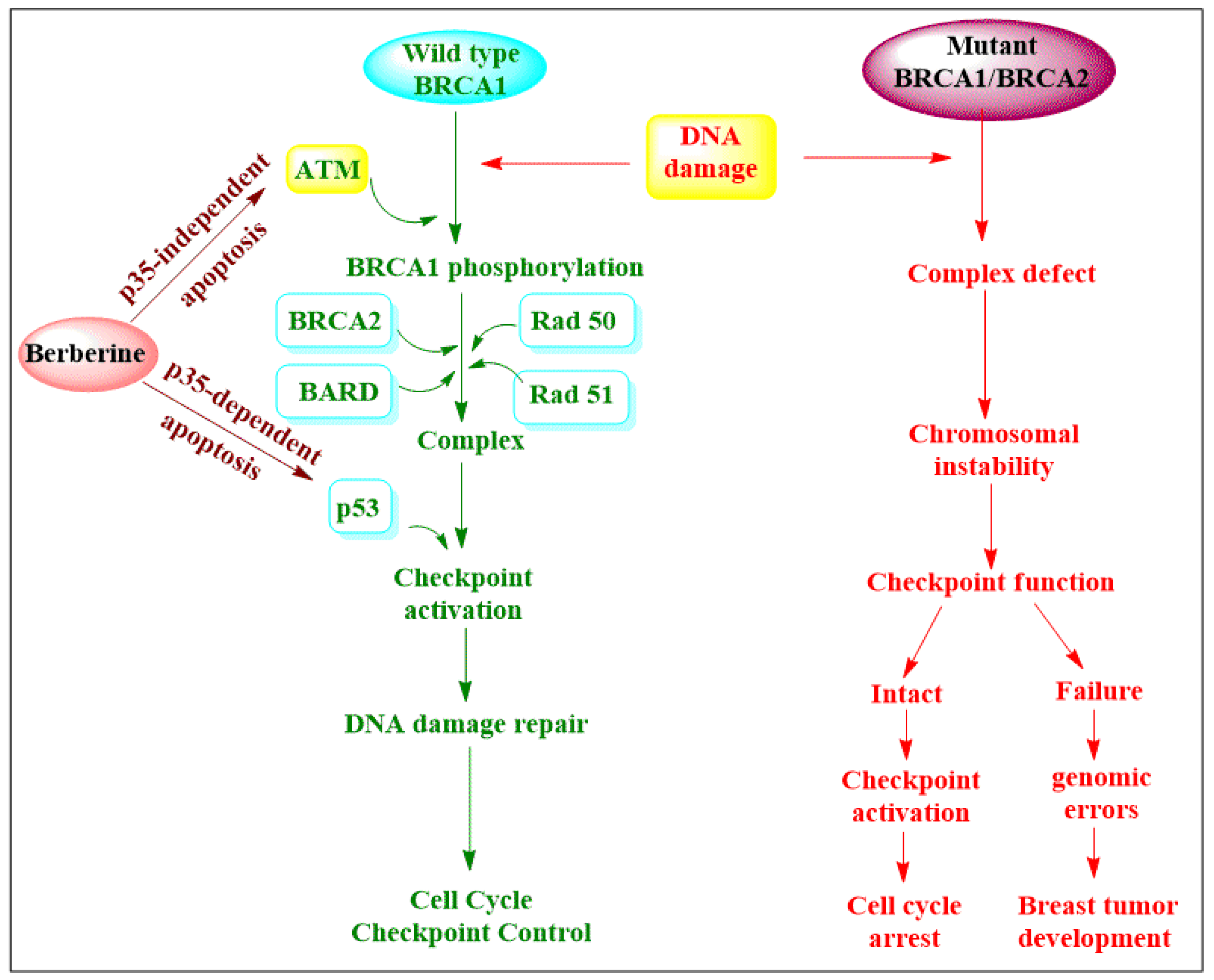
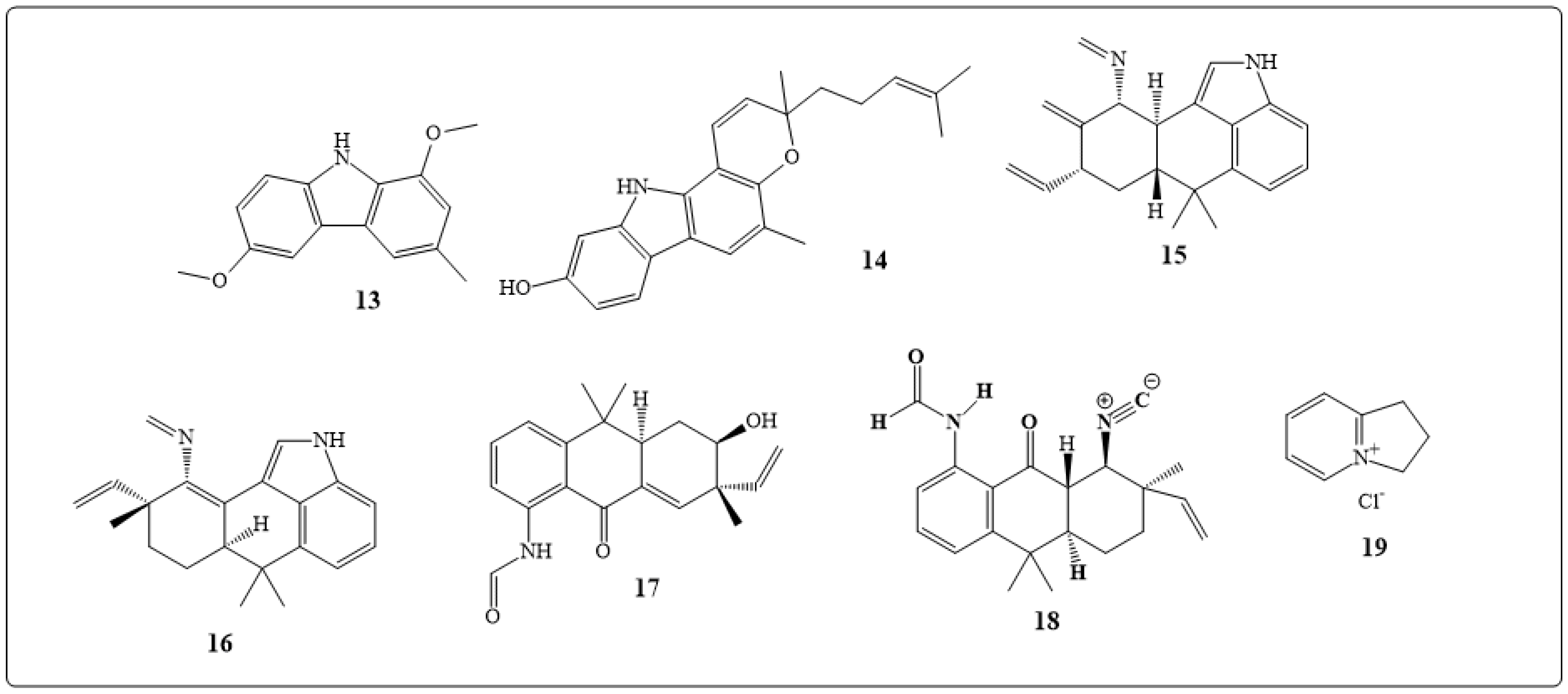
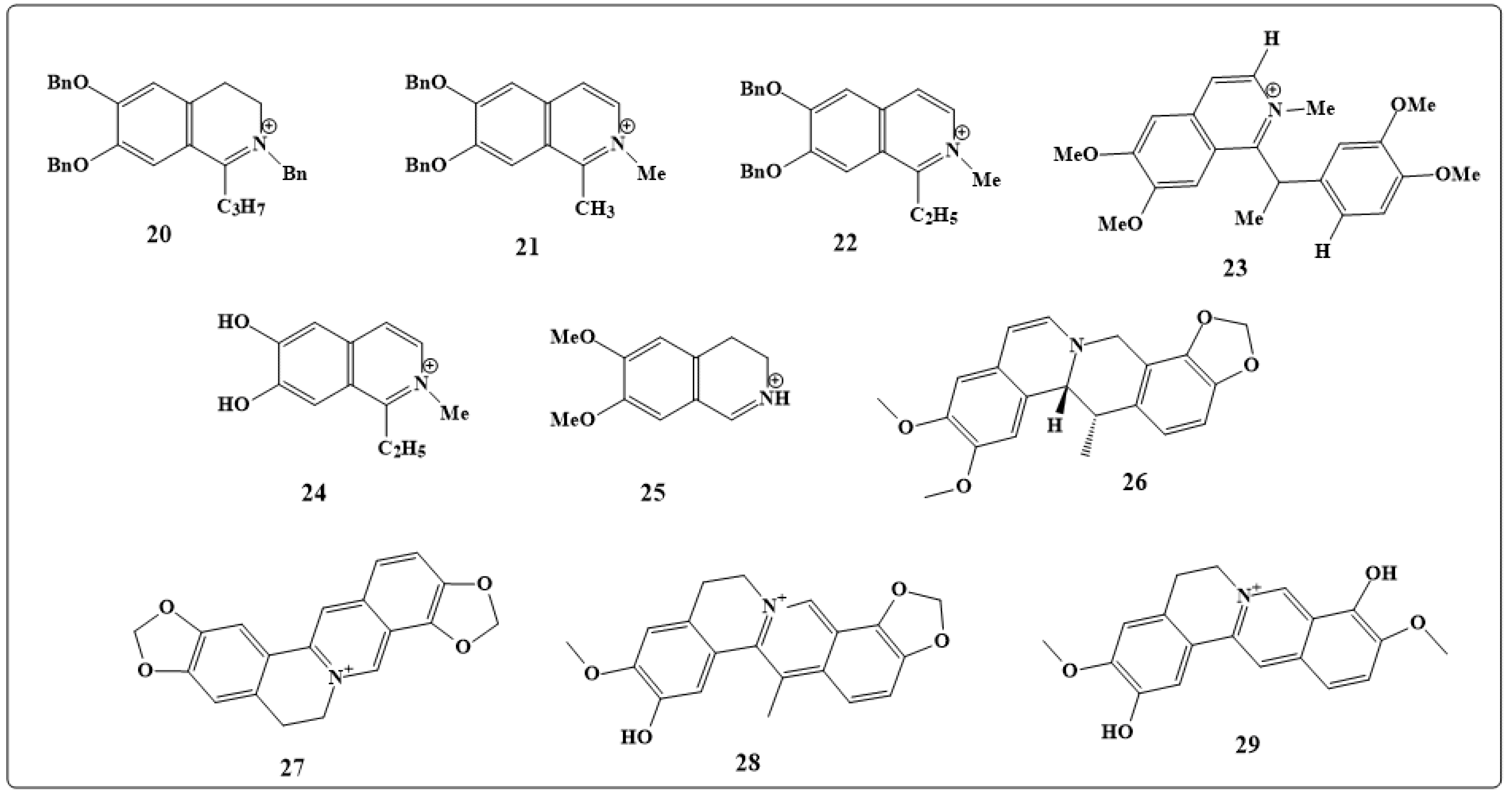
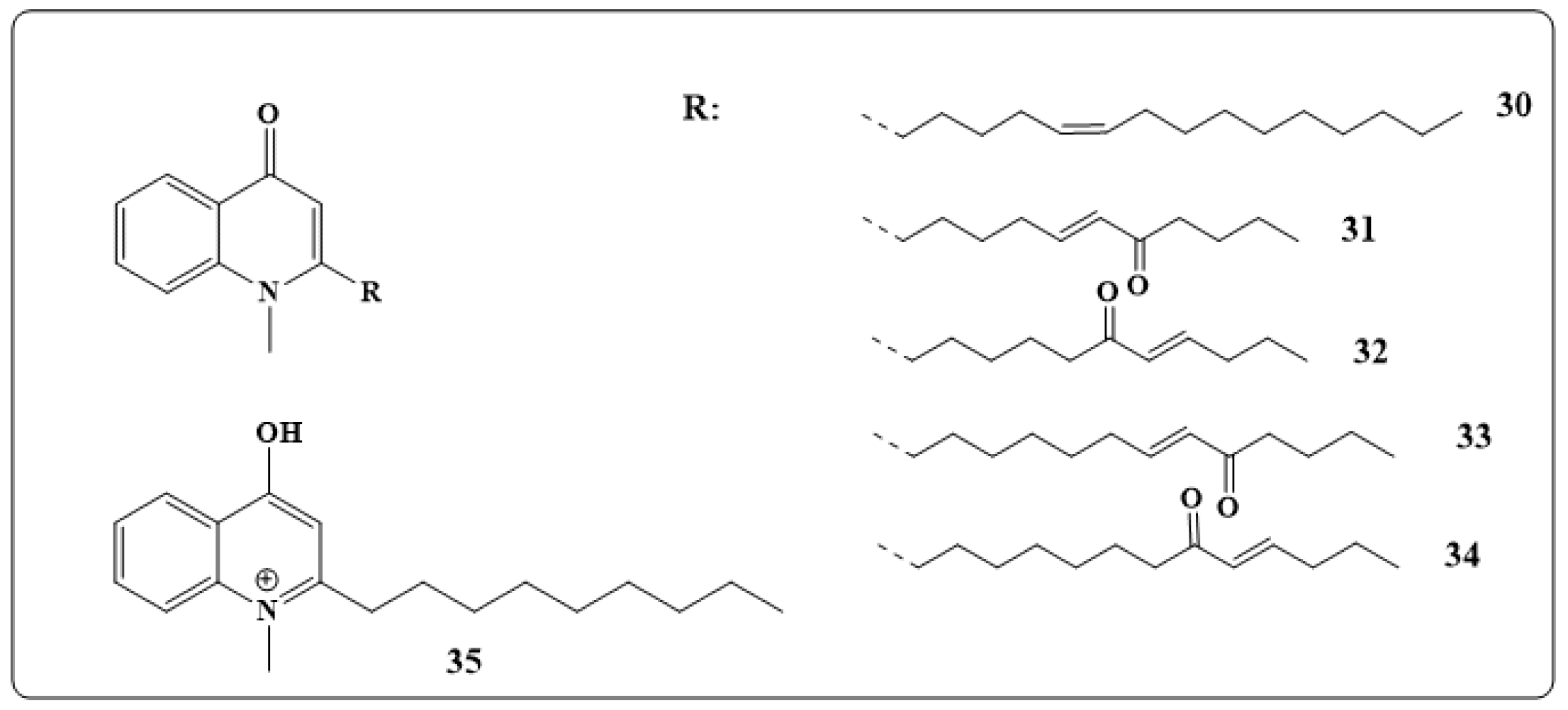
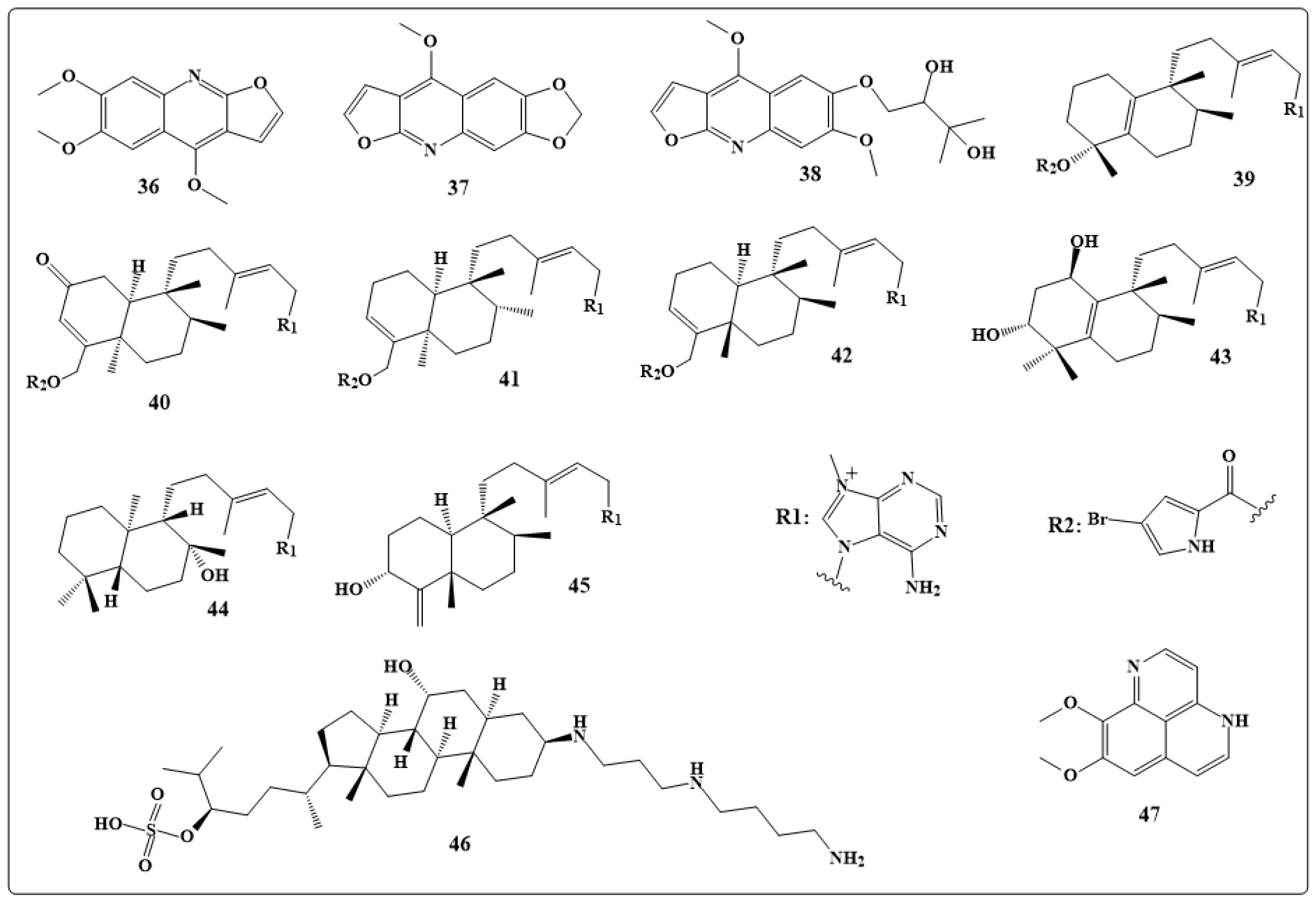
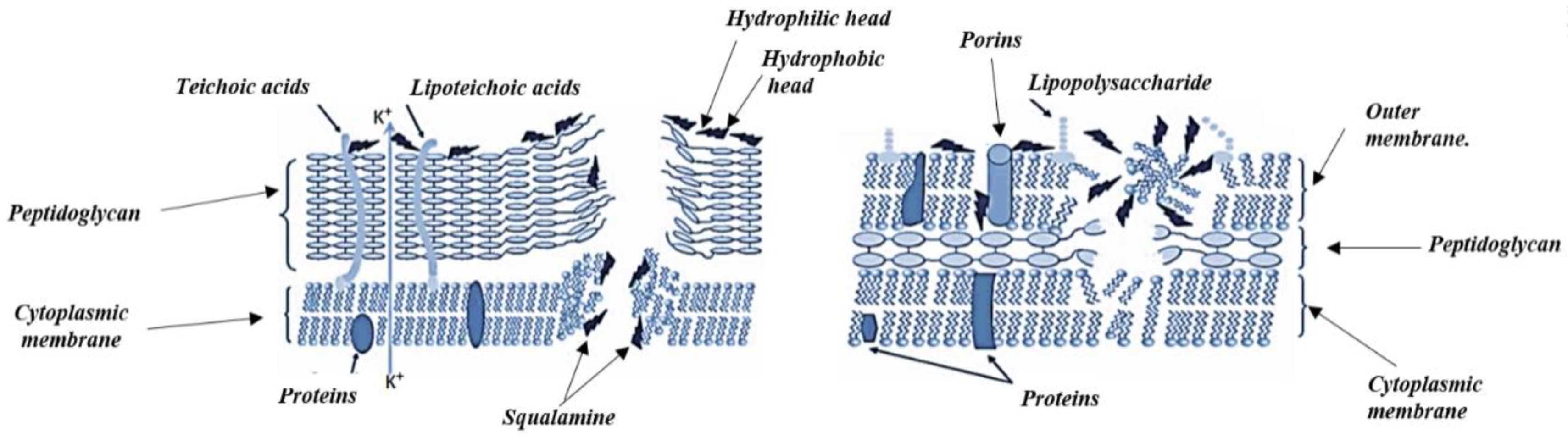
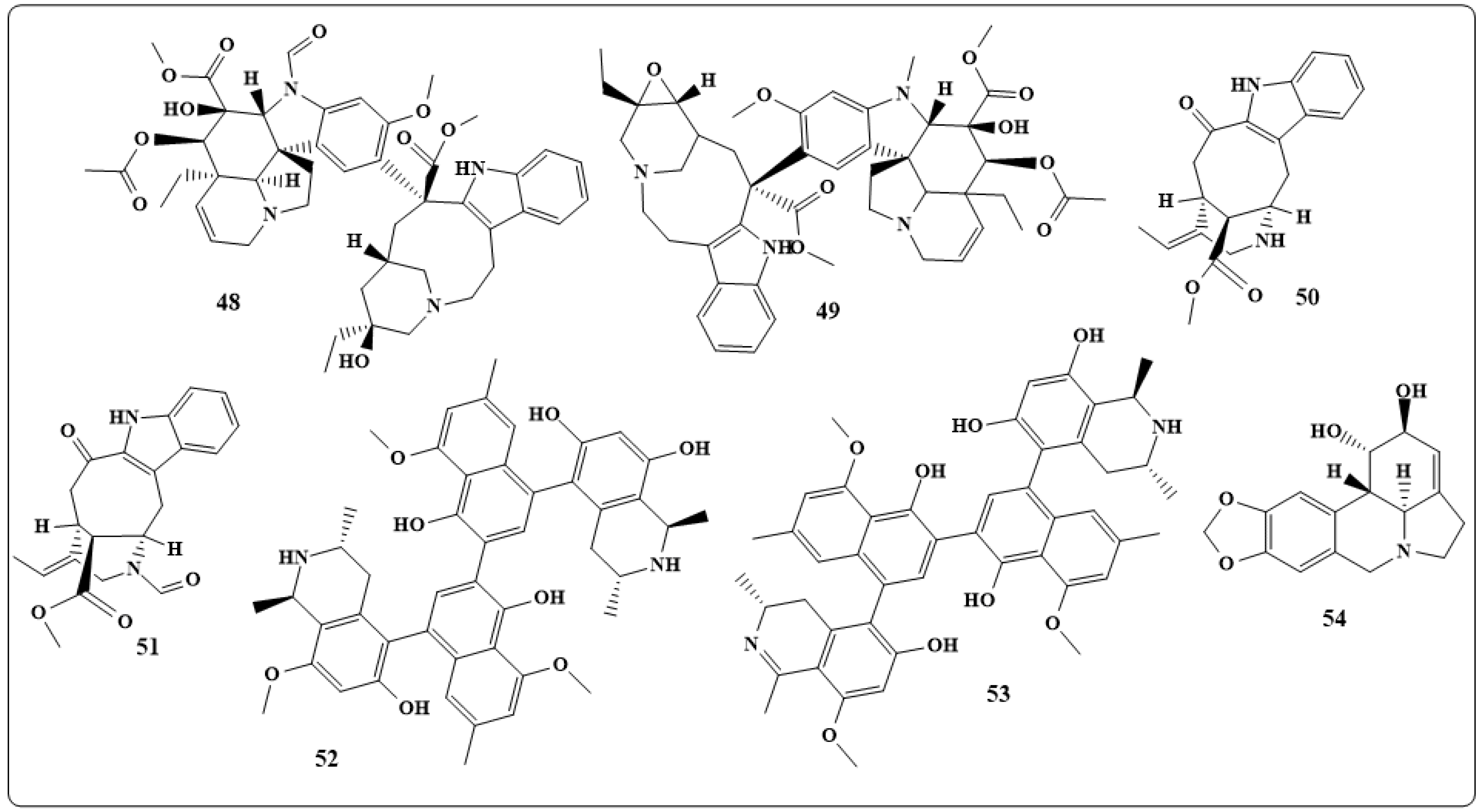
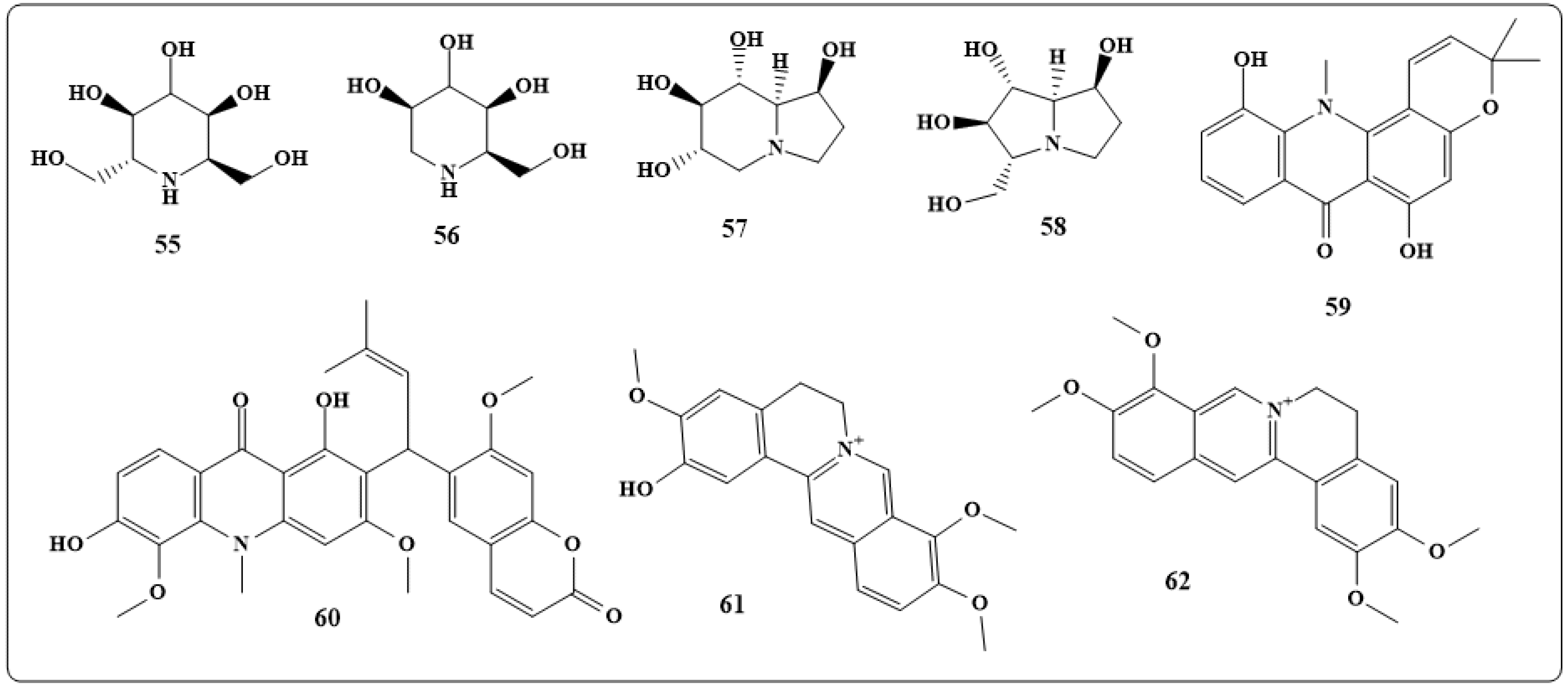
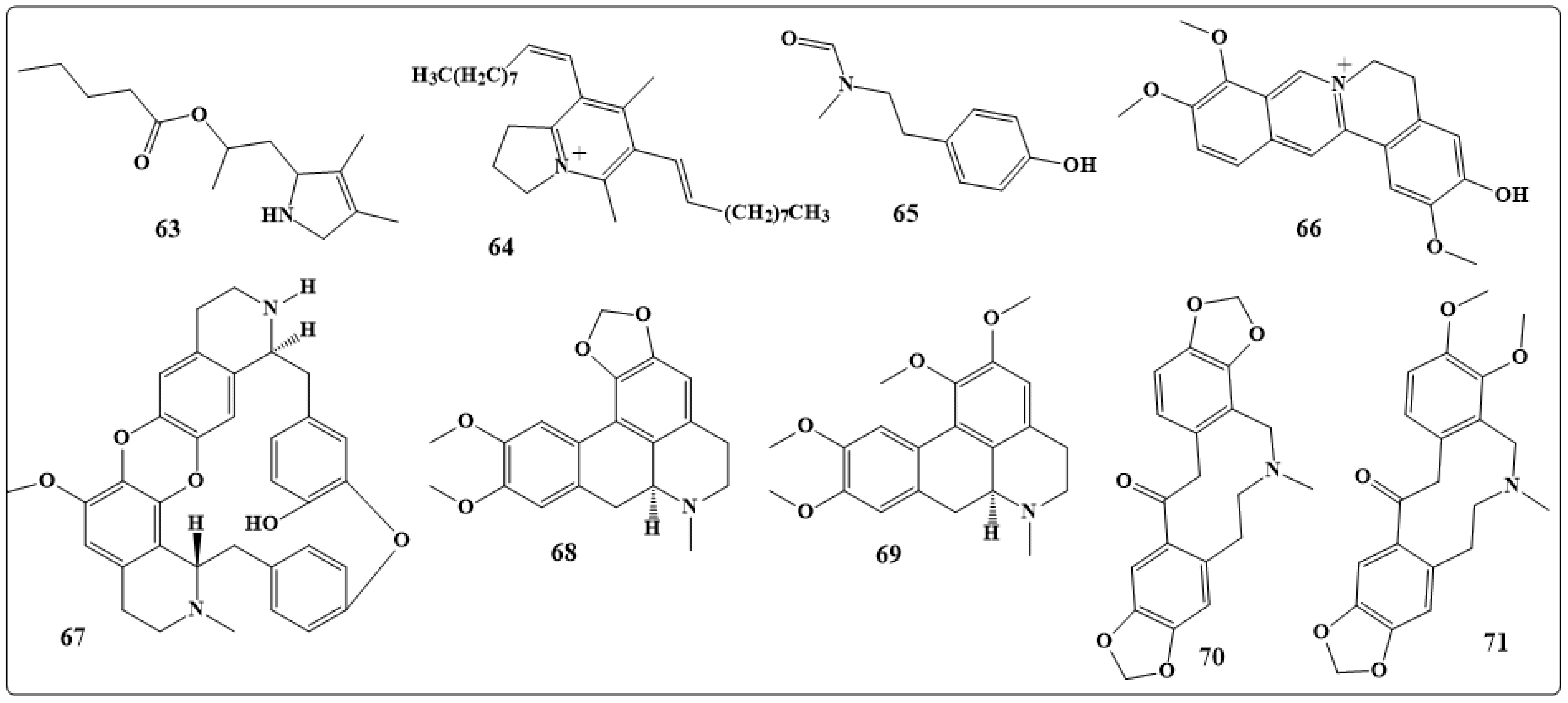
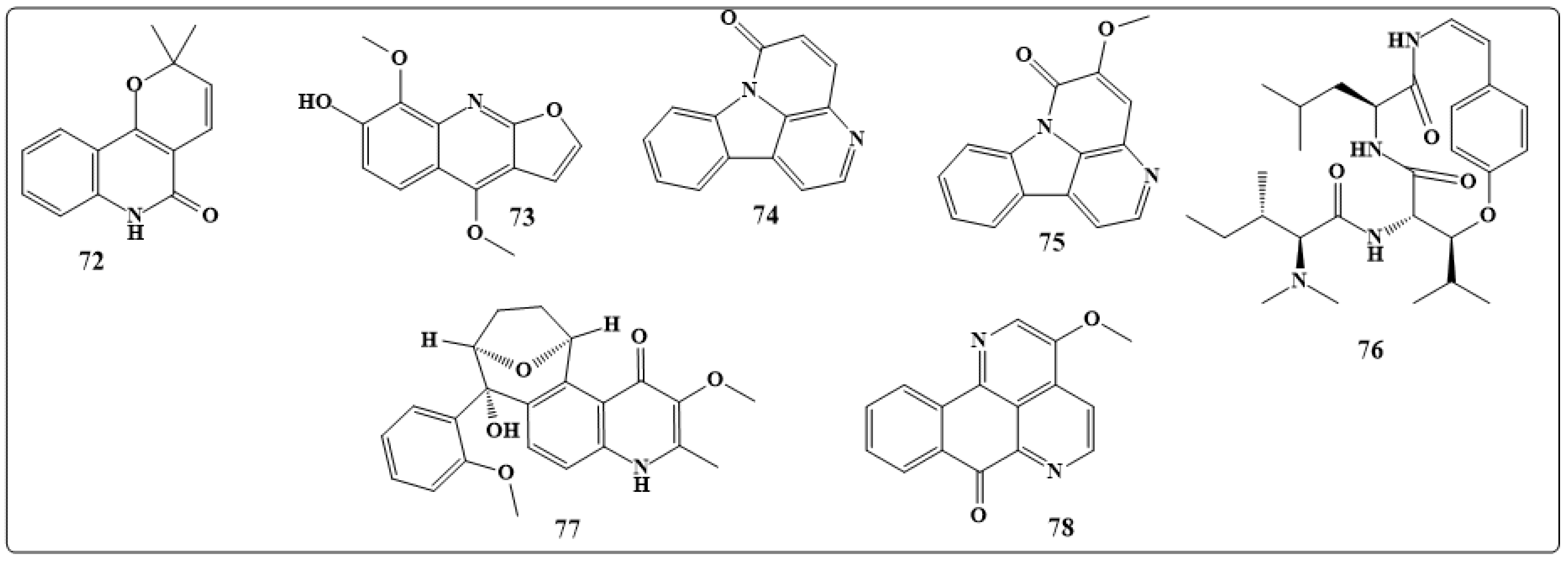
| Alkaloids | Anticancer Activity |
|---|---|
| Berberine Matrine | Inhibits the proliferation of breast, lung, colon and liver cancer cell lines Inducing the cell cycle arrest or apoptosis in cancer cell Inhibits the proliferation of cancer cell by G1 cell cycle arrest or apoptosis |
| Piplartine | Antitumor related to its antiproliferative effect |
| Piperine Sanguinarine Tetrandrine | Antitumor and immunomodulatory Induces cell cycle arrest at different phases or apoptosis in a variety of cancer cell Induces different phases of cell cycle arrest depends on cancer cell types |
| Aporphine | Antitumor activity against small cell, lung cancer and breast cancer cells, in addition to a high antitumor activity against epidermoid carcinoma |
| Apomorphine | Antioxidant and antipoliferative activities |
| Harmine, harmaline harmalacidine, vasicinone | Inhibit topoisomerase 1 resulting in antiproliferation of cancer cells |
| Evodiamine Sanguinarine | Induce cell cycle arrest or apoptosis, inhibiting the angiogenesis, invasion, and metastasis in a variety of cancer cell lines |
| Matrine | Inhibits the proliferation of various types of cancer cells mainly through the mediation of G1 cell cycle arrest or apoptosis |
| Tetrandrine | Induces apoptosis in many human cancer cells, including leukemia, bladder, colon, hepatoma, and lung |
| Alkaloids | Antibacterial Activity |
|---|---|
| Clausenol, Kokusaginine, Maculine, Kolbisine, squalamine, Aaptamine | Active against Gram-positive and negative bacteria and fungi |
| R- Mahanine | Antimicrobial activity against Bacillus cereus and Staphylococcus aureus |
| hapalindole X, deschlorohapalin-dole I, 13-hydroxy dechlorofonto-namide, hapalonamide H | Potent activity against both Mycobacterium tuberculosis and Candida albicans |
| Indolizdine | Antibacterial activities against Cryptococcus neoformans, Aspergillus fumigatus, methicillin-resistant Staphylococcus aureus and Mycobacterium intracellular |
| Isoquinolines | Antimalarial, cytotoxic, and anti-HIV effects. |
| Sanguinarine, | Antibacterial activity against methicillin-resistant Staphylococcus aureus |
| Alkaloids | Antiviral Activity |
|---|---|
| Leurocristine | Active against mengovirus extracellular virucidal, poliovirus, vaccinia, and influenza viruses |
| Periformyline | Inhibits poliovirus type viruses |
| Perivine | Exhibits polio extracellular virucidal activity against vaccinia |
| Vincaleucoblastine | Possesses extracellular virucidal activity against poliovirus vaccinia and influenza virus. |
| Michellamines D and F | HIV-inhibitory |
| Homonojirimycin | Inhibitor of several a-glucosidases |
| Deoxymanojirimycin | Inhibitor of glycoprocessing mannosidase |
| Castanospermine, australine | Reduce the ability of the human immunodeficiency virus (HIV) to infect cultured cells, and have potential for treating AIDS |
| Sesquiterpene | Anti-HIV activity |
| 5-hydroxynoracronycine, Acrimarine F | Remarkable inhibitory effects on Epstein-Barr virus activation |
| Columbamine, Berberine, Palmitine | Inhibitors alkaloids against HIV-1 |
| Alkaloids | Antifungal Activity |
|---|---|
| 2-(3,4-dimethyl-2,5-dihydro-1H-pyrrol-2-yl)-1-methylethyl pentanoate | Activity against Aspergillus and Candida species |
| 6,8-didec-(1Z)-enyl-5,7-dimethyl-2,3-dihydro-1H-indolizinium | Good activity against a drug-resistant strain of C. albicans |
| β-carboline, Cocsoline | Effective against all fungal species and marginal activity |
| Dicentrine, glaucine, Protopine, alpha-allocryptopin | Good activity against Microsporumgypseum, Microsporumcanis, T. mentagrophytes and Epidermophytonfloccosum |
| Canthin-6-one, 5-methoxy-canthin-6-one | Antifungal activity against C. albicans, A. fumigatus and T. mentagrophytes |
| Frangulanine, Waltherione, quinolinone alkaloids | Exhibit antifungal activities against a broad spectrum of pathogenic fungi |
| 3-methoxisampangin | Significant antifungal activity against C. albicans, A. fumigatus, and C. neoformans |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. https://doi.org/10.3390/toxins11110656
Thawabteh A, Juma S, Bader M, Karaman D, Scrano L, Bufo SA, Karaman R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins. 2019; 11(11):656. https://doi.org/10.3390/toxins11110656
Chicago/Turabian StyleThawabteh, Amin, Salma Juma, Mariam Bader, Donia Karaman, Laura Scrano, Sabino A. Bufo, and Rafik Karaman. 2019. "The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens" Toxins 11, no. 11: 656. https://doi.org/10.3390/toxins11110656
APA StyleThawabteh, A., Juma, S., Bader, M., Karaman, D., Scrano, L., Bufo, S. A., & Karaman, R. (2019). The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins, 11(11), 656. https://doi.org/10.3390/toxins11110656









