Mesoscale Dynamics and Niche Segregation of Two Dinophysis Species in Galician-Portuguese Coastal Waters
Abstract
1. Introduction
2. Results
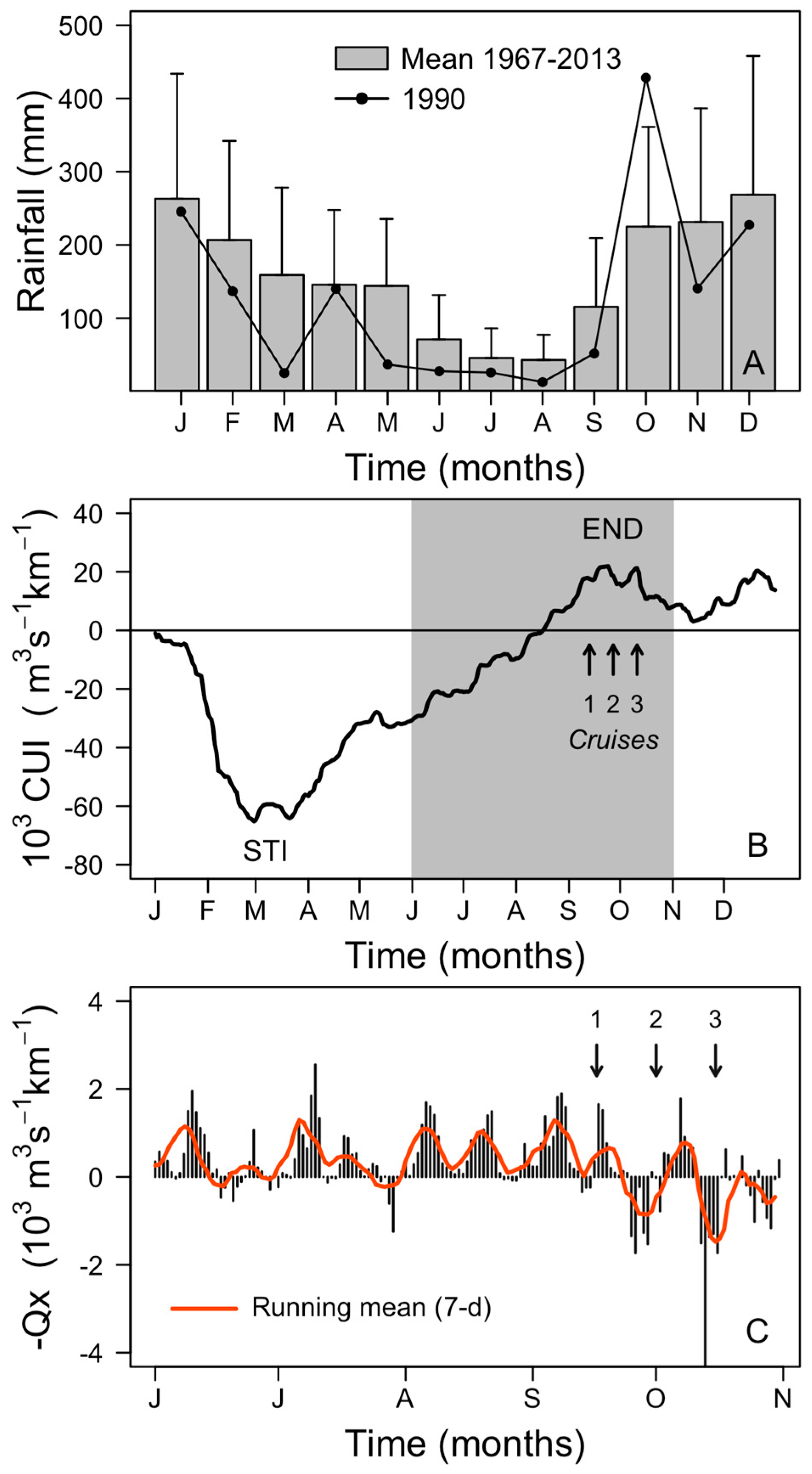
2.1. Meteorological and Hydrographic Conditions
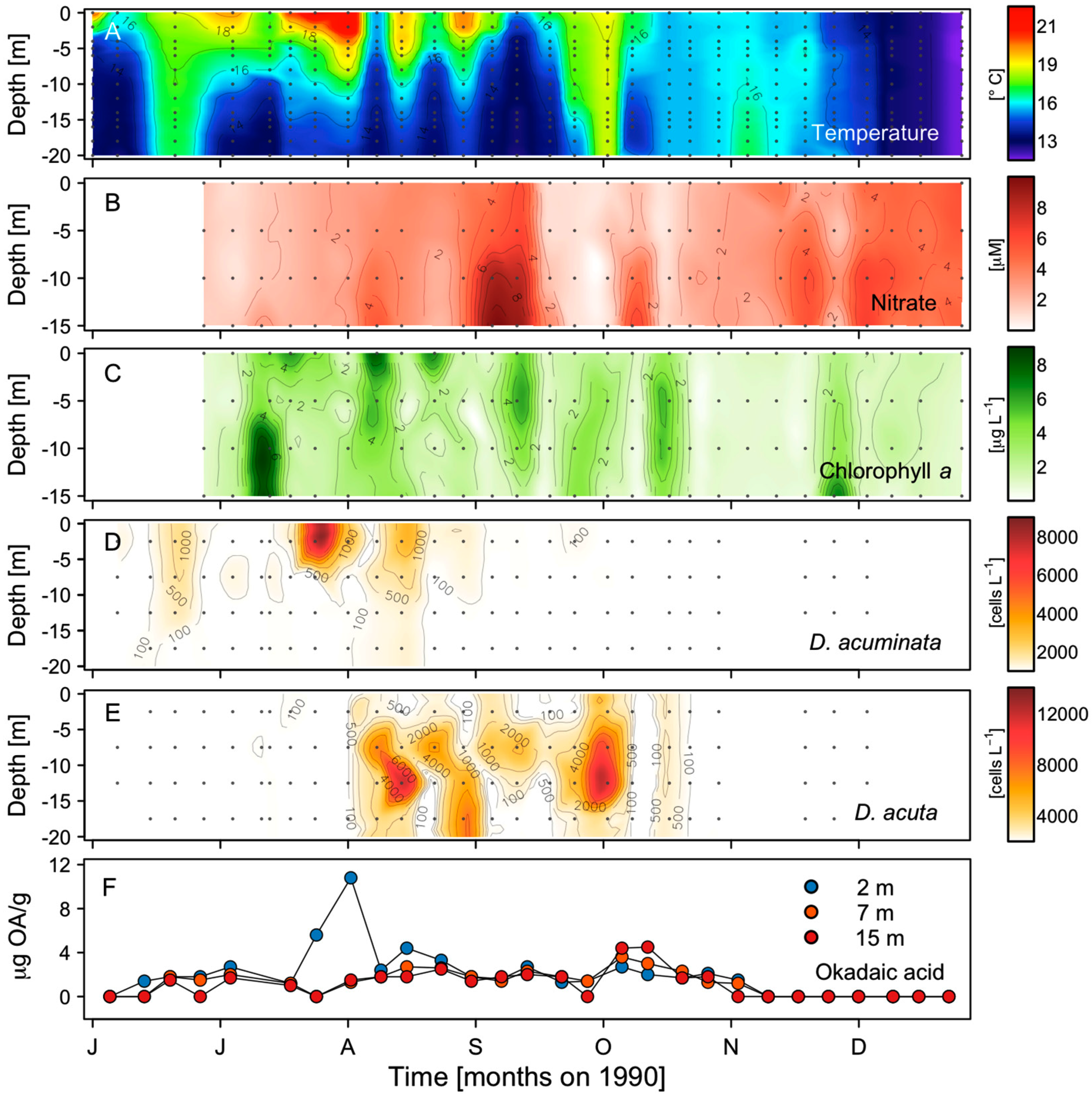
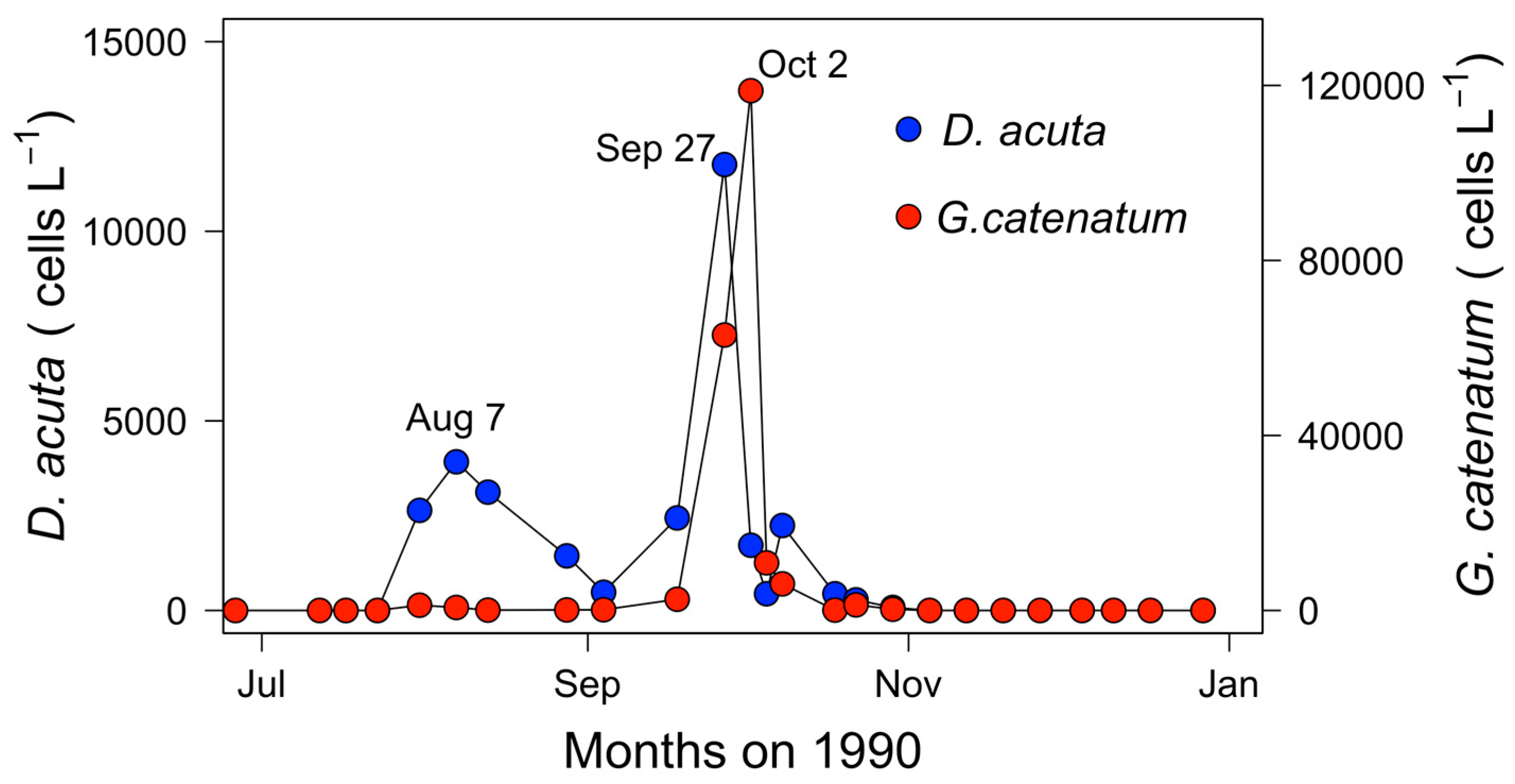
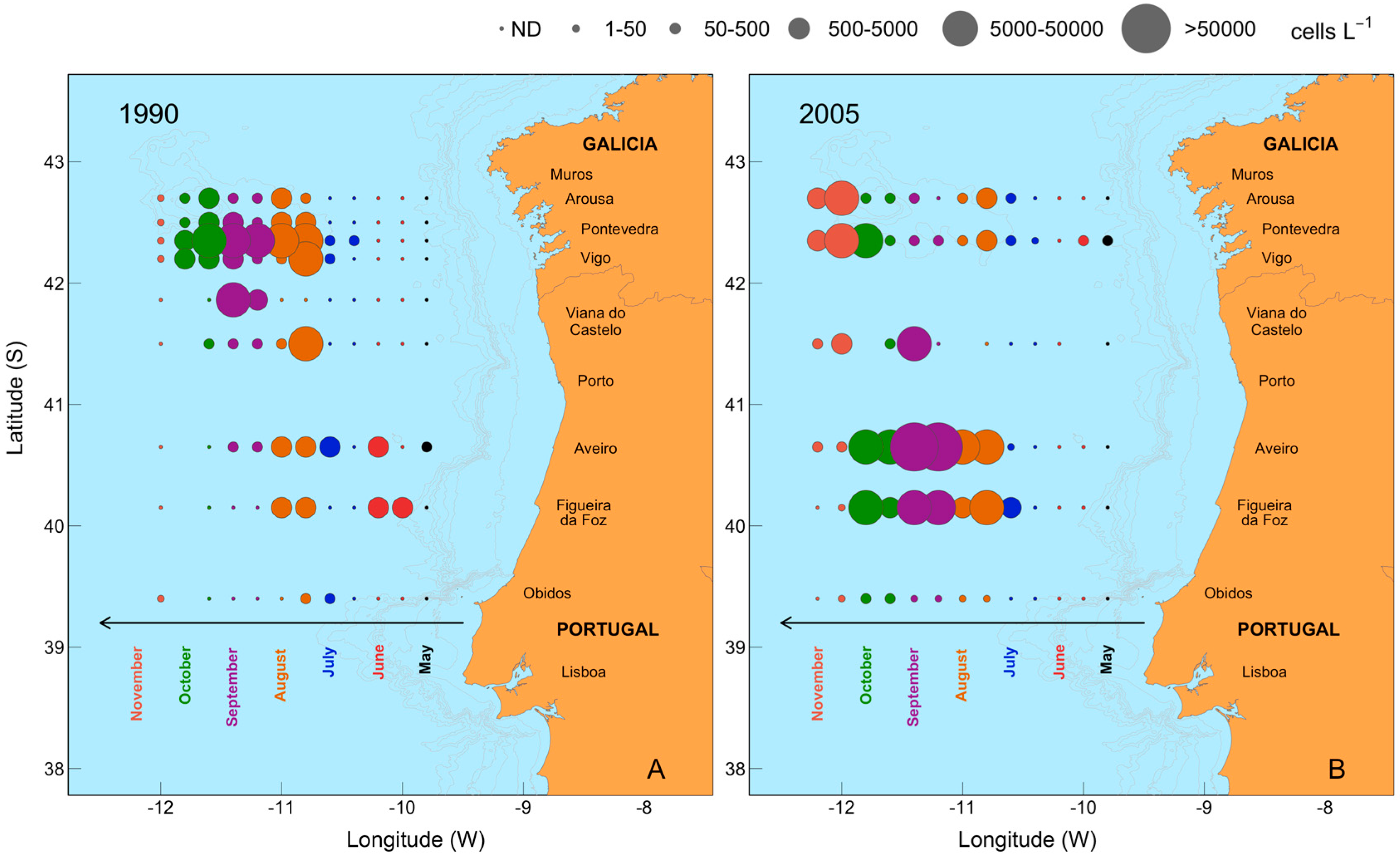
2.2. Seasonal Variability of Dinophysis Species and Microphytoplankton in Ría de Pontevedra
2.3. Distribution of OA with Depth in Raft Mussels
2.4. Hydrodynamic Conditions on Shelf Before, During, and after the Autumn DSP and PSP Events
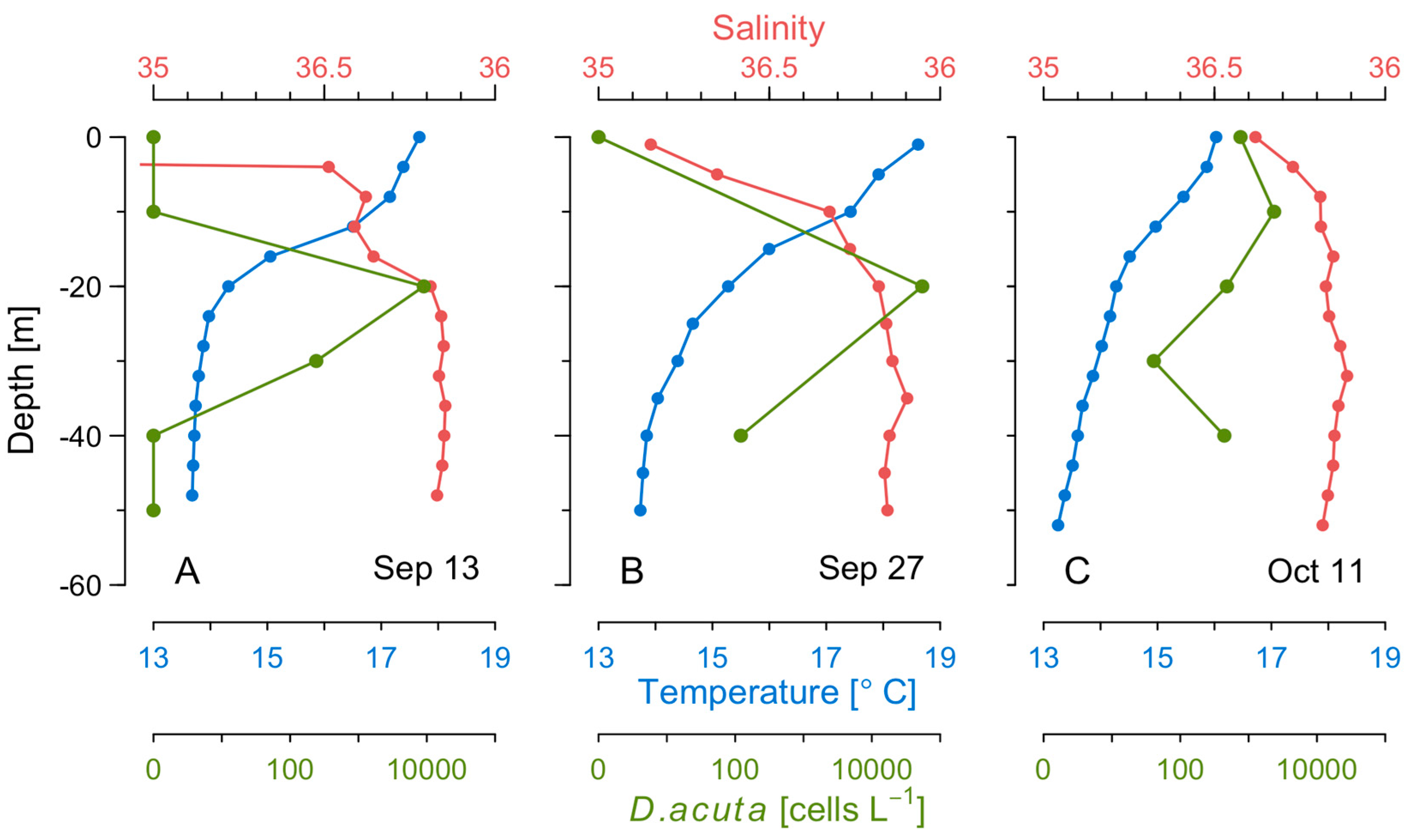
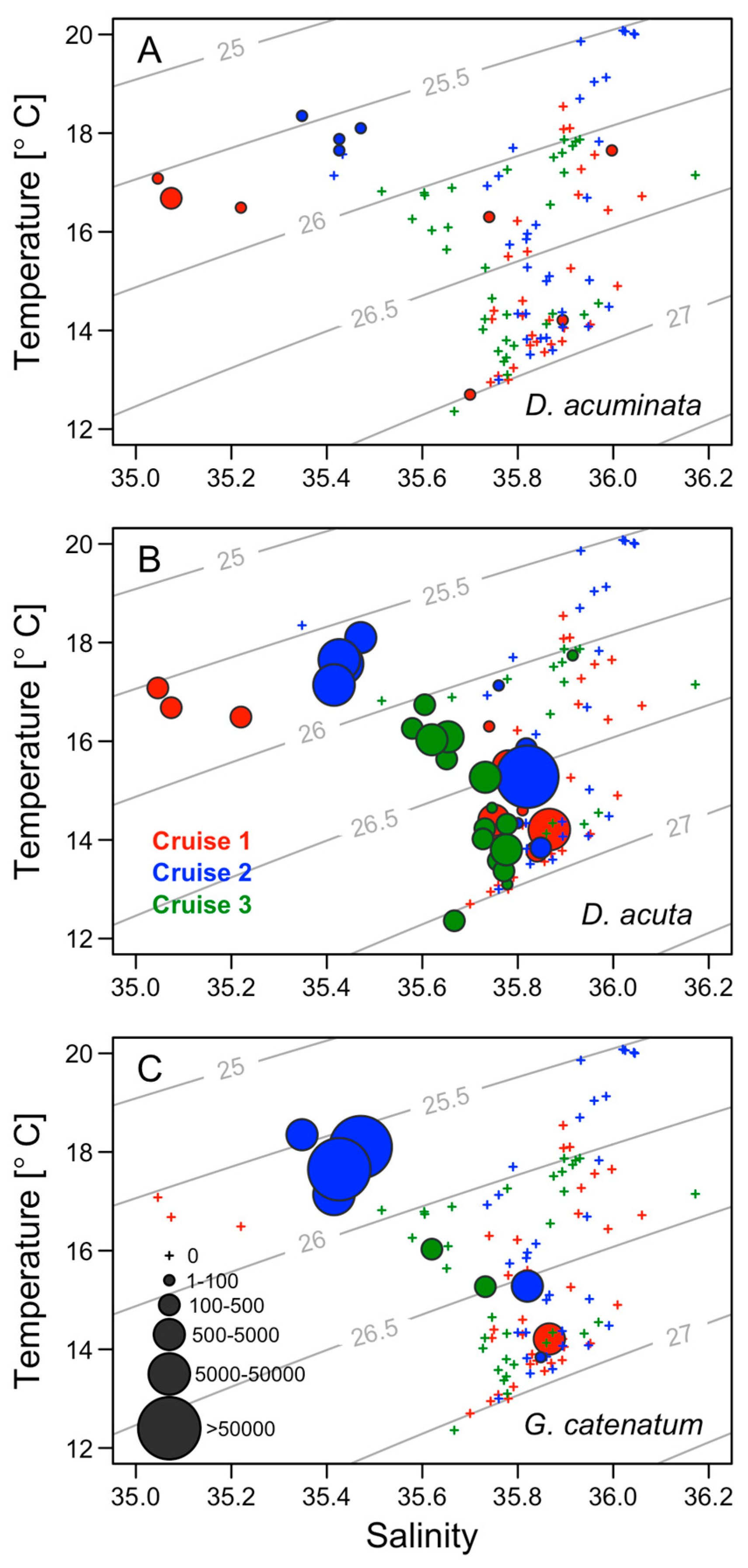
2.5. Thermohaline Conditions Associated with Dinophysis and G. catenatum Shelf Maxima
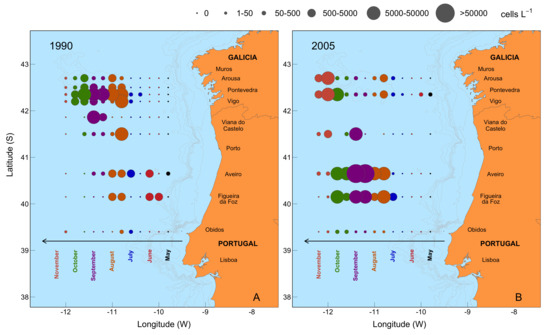
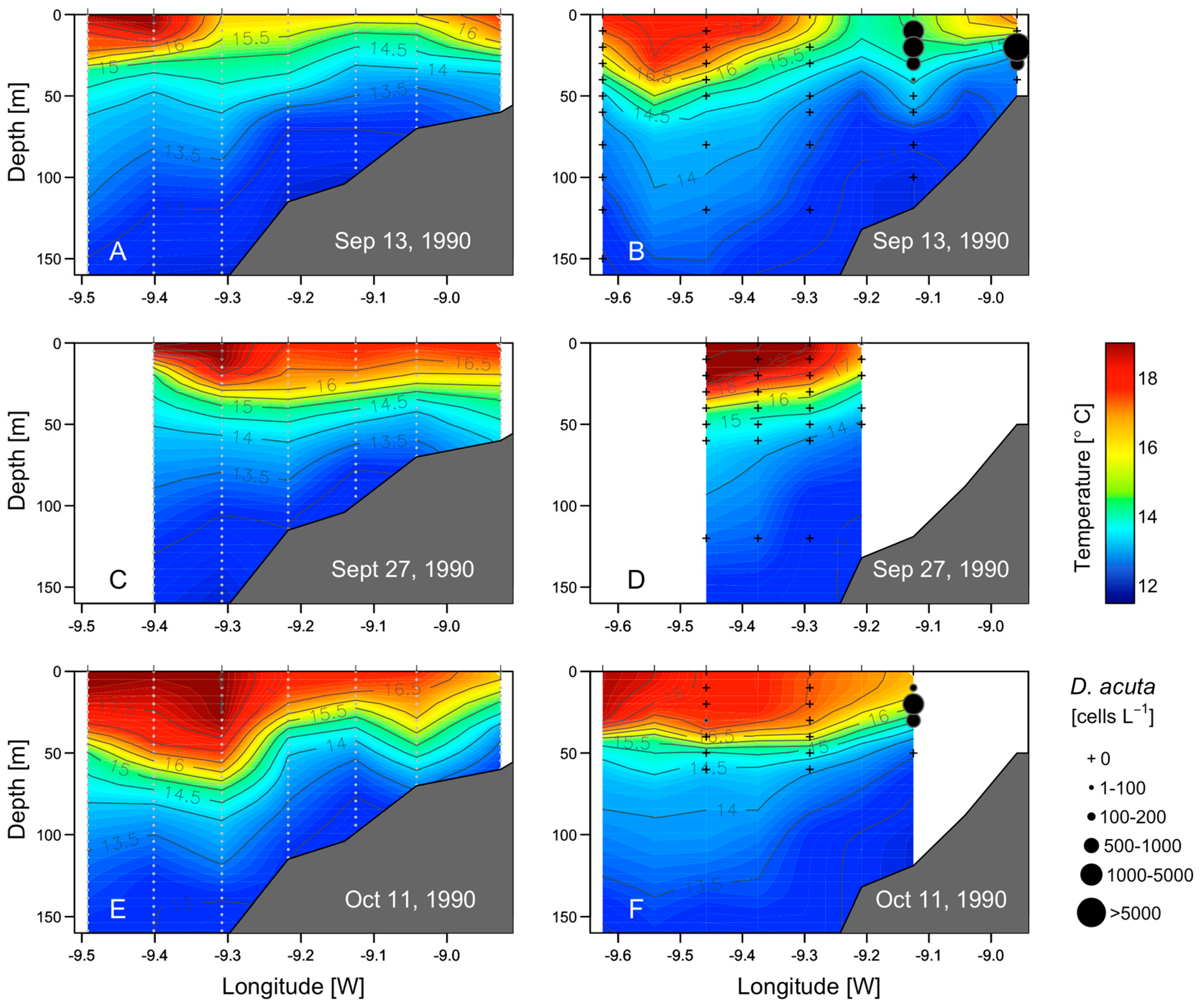
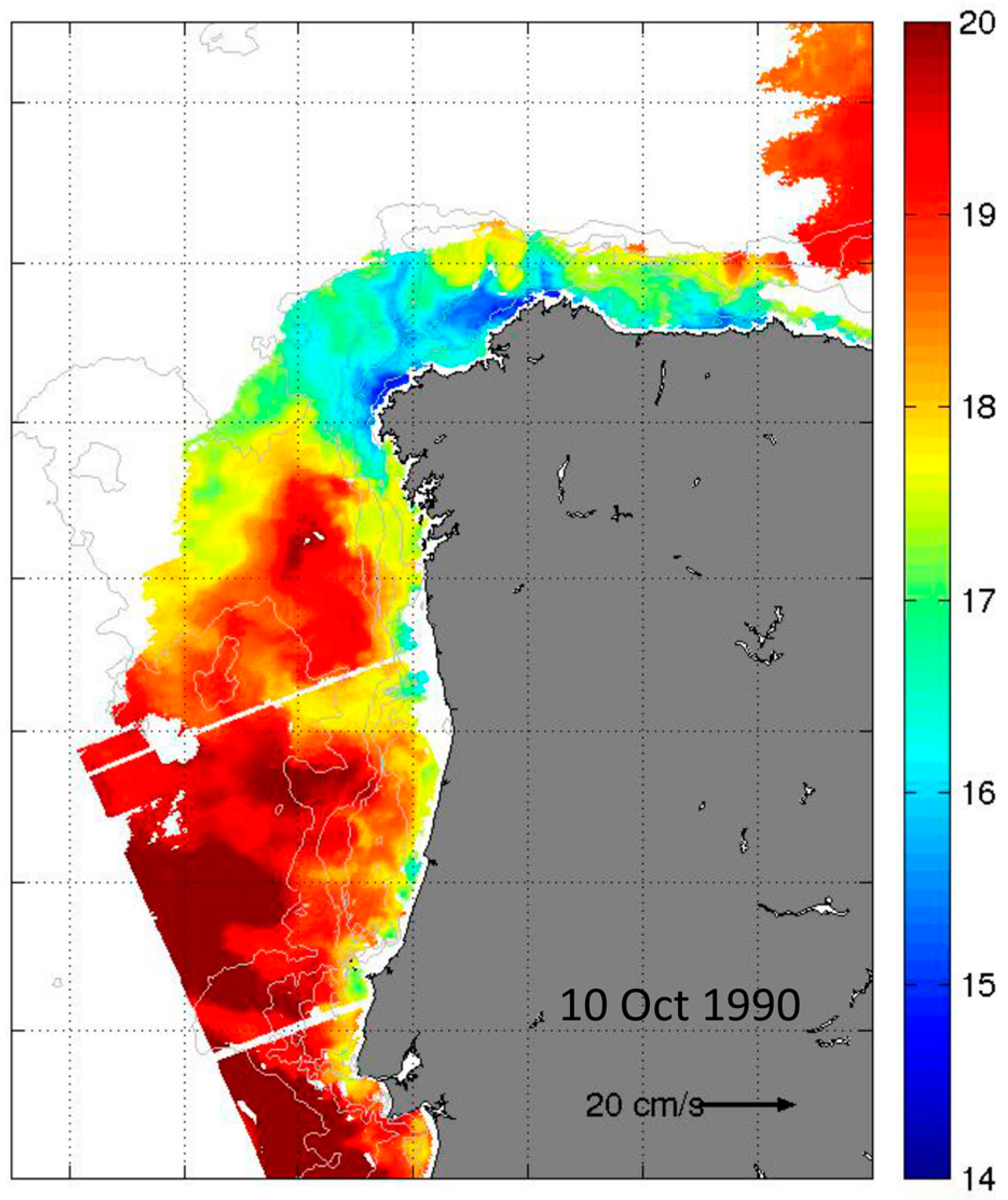
2.6. Mesoscale Dynamics of D. acuta in Galician-Portuguese Coastal Waters
3. Discussion
3.1. Initiation of D. acuta Summer Bloom and the Replacement of D. acuminata
3.2. Niche Partitioning and Specific Requirements of D. acuminata and D. acuta
3.3. Inoculum Source for Bloom Development
3.4. OA Distribution with Depth in Raft Mussels. Implications for Shellfish Exploitation
4. Conclusions
5. Materials and Methods
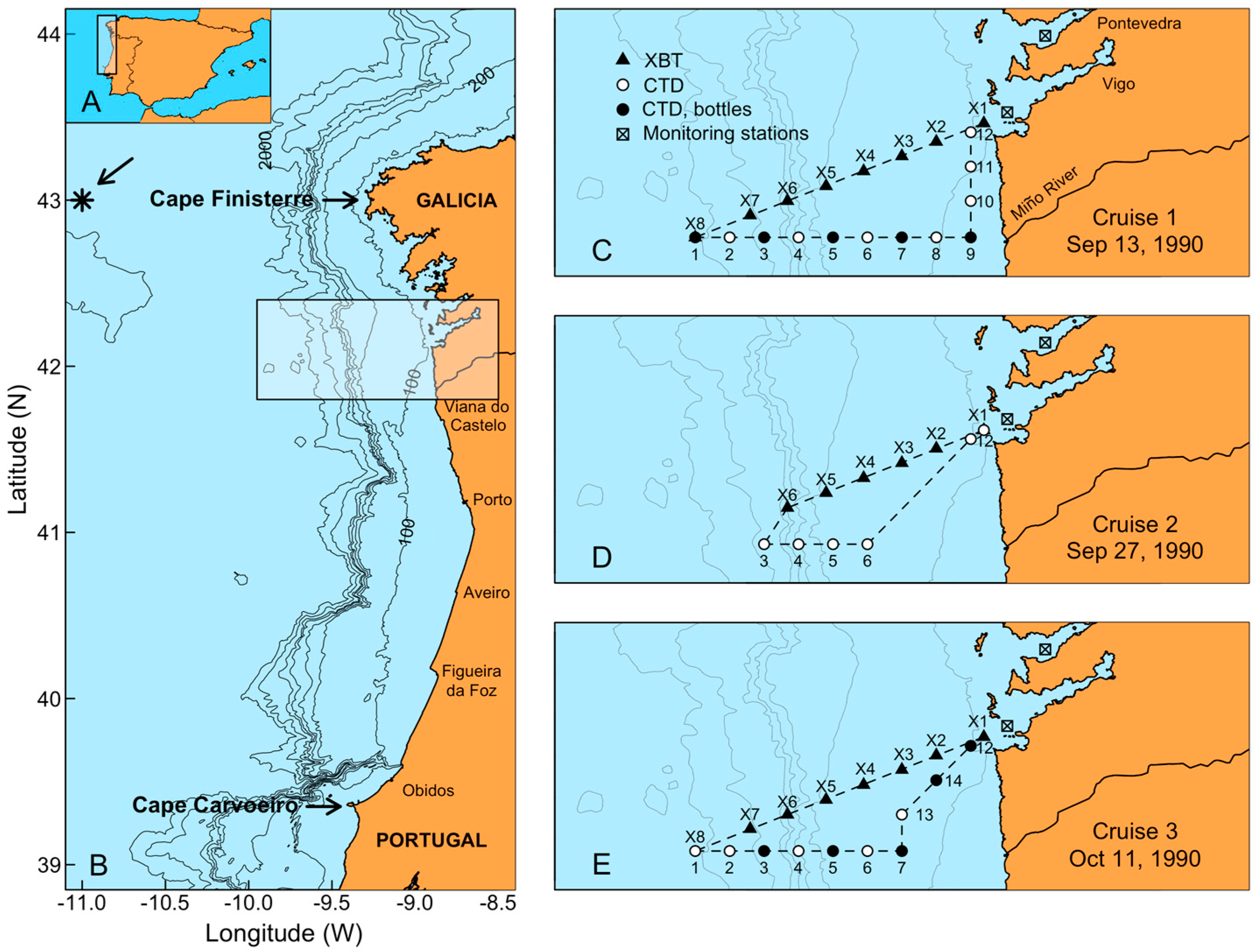
5.1. Study Area
5.2. Meteorological Data
5.3. Satellite Images
5.4. Field Sampling and Phytoplankton Analyses
5.5. Mussel Sampling, Processing, and HPLC Analyses of Okadaic Acid (OA)
5.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- Van Egmond, H.P. Natural toxins: Risks, regulations and the analytical situation in Europe. Anal. Bioanal. Chem. 2004, 378, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Regulation (EC) no. 853/2004 of the European Parliament and of the Council of 29 April 2004. Off. J. Eur. Communities 2004, L139, 55–205. [Google Scholar]
- Blanco, J.; Correa, J.; Muñíz, S.; Mariño, C.; Martín, H.; Arévalo, F. Evaluación del impacto de los métodos y niveles utilizados para el control de toxinas en el mejillón. Revista Galega dos Recursos Mariños 2013, 3, 1–55. [Google Scholar]
- Vale, P.; Botelho, M.J.; Rodrigues, S.M.; Gomes, S.; Sampayo, M. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986–2006): A review of exposure assessment. Harmful Algae 2008, 7, 11–25. [Google Scholar] [CrossRef]
- Wooster, W.S.; Bakun, A.; McLain, D.R. The seasonal upwelling cycle along the eastern boundary of the North Atlantic. J. Mar. Res. 1976, 34, 131–141. [Google Scholar]
- Fiuza, A.F.G.; Hamann, M.; Ambar, I.; del Río, G.D.; González, N.; Cabanas, J.M. Water masses and their circulation off western Iberia during May 1993. Deep Sea Res. I 1998, 45, 1127–1160. [Google Scholar] [CrossRef]
- Ruiz-Villareal, M.; Gonzalez-Pola, C.; Diaz del Rio, G.; Lavin, A.; Otero, P.; Piedracoba, S.; Cabanas, J.M. Oceanographic conditions in North and Northwest Iberia and their influence on the Prestige oil spill. Mar. Pollut. Bull. 2006, 53, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Frouin, R.; Fiuza, A.F.G.; Ambar, I.; Boyd, T.J. Observations of a poleward surface current off the coasts of Portugal and Spain during winter. J. Geophys. Res. 1990, 95, 679–691. [Google Scholar] [CrossRef]
- Haynes, R.; Barton, E.D. A poleward flow along the atlantic coast of the Iberian Penisula. J. Geophys. Res. 1990, 95, 11425–11441. [Google Scholar] [CrossRef]
- Sordo, I.; Barton, E.D.; Cotos, J.M.; Pazos, Y. An inshore poleward current in the NW of the Iberian Peninsula detected form satellite images, and its relation with G. catenatum and D. acuminata blooms in the Galician Rias. Estuar. Coast. Shelf. Sci. 2001, 53, 787–799. [Google Scholar] [CrossRef]
- Peliz, A.; Rosa, T.L.; Santos, A.M.P.; Pissarra, J.L. Fronts, currents and counter-flows in the Western Iberian upwelling system. J. Mar. Syst. 2002, 35, 61–77. [Google Scholar] [CrossRef]
- Figueiras, F.G.; Ríos, A.F. Phytoplankton succession, red tides and the hydrographic regime in the Rias Bajas of Galicia. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 239–244. [Google Scholar]
- Tilstone, G.H.; Míguez, B.M.; Figueiras, F.G.; Fermín, E.G. Diatom dynamics in a coastal ecosystem affected by upwelling: Coupling between species succession, circulation and biogeochemical processes. Mar. Ecol. Prog. Ser. 2000, 205, 23–41. [Google Scholar] [CrossRef]
- Nogueira, E.; Figueiras, F.G. The microplankton succession in the Ría de Vigo revisited: Species assemblages and the role of weather-induced, hydrodynamic variability. J. Mar. Syst. 2005, 54, 139–155. [Google Scholar] [CrossRef]
- Álvarez-Salgado, X.A.; Labarta, U.; Fernández-Reiriz, M.J.; Figueiras, F.G.; Rosón, G.; Piedracoba, S.; Filgueira, R.; Cabanas, J.M. Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (SW Europe). Harmful Algae 2008, 7, 849–855. [Google Scholar] [CrossRef]
- Relvas, P.; Barton, E.D.; Dubert, J.; Oliveira, P.B.; Peliz, A.; da Silva, J.C.B.; Santos, A.M.P. Physical oceanography of the western Iberia ecosystem: Latest views and challenges. Prog. Oceanogr. 2007, 74, 149–173. [Google Scholar] [CrossRef]
- Reguera, B.; Bravo, I.; Fraga, S. Autoecology and some life history stages of Dinophysis acuta Ehrenberg. J. Plankton Res. 1995, 17, 999–1015. [Google Scholar] [CrossRef]
- Velo-Suárez, L.; Reguera, B.; Garcés, E.; Wyatt, T. Vertical distribution of division rates in coastal dinoflagellate Dinophysis spp. populations: Implications for modeling. Mar. Ecol. Prog. Ser. 2009, 385, 87–96. [Google Scholar] [CrossRef]
- Díaz, P.A.; Ruiz-Villarreal, M.; Velo-Suárez, L.; Ramilo, I.; Gentien, P.; Lunven, M.; Fernand, L.; Raine, R.; Reguera, B. Tidal and wind-event variability and the distribution of two groups of Pseudo-nitzschia species in an upwelling-influenced Ría. Deep Sea Res. II 2014, 101, 163–179. [Google Scholar] [CrossRef]
- Reguera, B.; Mariño, J.; Campos, J.; Bravo, I.; Fraga, S. Trends in the occurrence of Dinophysis spp. in Galician waters. In Toxic Phytoplankton Blooms in the Sea; Smayda, T., Shimizu, Y., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1993; pp. 559–564. [Google Scholar]
- Palma, A.S.; Vilarinho, M.G.; Moita, M.T. Interannual trends in the longshore distribution of Dinophysis off the Portuguese coast. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and IOC of UNESCO: Vigo, Spain, 1998; pp. 124–127. [Google Scholar]
- Escalera, L.; Reguera, B.; Pazos, Y.; Moroño, A.; Cabanas, J.M. Are different species of Dinophysis selected by climatological conditions? Afr. J. Mar. Sci. 2006, 28, 283–288. [Google Scholar] [CrossRef]
- Moita, M.T.; Silva, A.J. Dynamics of Dinophysis acuta, D. acuminata, D. tripos and Gymnodinium catenatum during an upwelling event off the Northwest Coast of Portugal. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; pp. 169–172. [Google Scholar]
- Pitcher, G.C.; Figueiras, F.G.; Hickey, B.M.; Moita, M.T. The physical oceanography of upwelling systems and the development of harmful algal blooms. Prog. Oceanogr. 2010, 85, 5–32. [Google Scholar] [CrossRef]
- Velo-Suárez, L.; González-Gil, S.; Pazos, Y.; Reguera, B. The growth season of Dinophysis acuminata in an upwelling system embayment: A conceptual model based on in situ measurements. Deep Sea Res. II 2014, 101, 141–151. [Google Scholar] [CrossRef]
- Díaz, P.A.; Reguera, B.; Ruiz-Villarreal, M.; Pazos, Y.; Velo-Suárez, L.; Berger, H.; Sourisseau, M. Climate variability and oceanographic settings associated with interannual variability in the initiation of Dinophysis acuminata blooms. Mar. Drugs 2013, 11, 2964–2981. [Google Scholar] [CrossRef] [PubMed]
- Moita, M.T.; Pazos, Y.; Rocha, C.; Nolasco, R.; Oliveira, P.B. Towards predicting Dinophysis blooms off NW Iberia: A decade of events. Harmful Algae 2016, 52, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Moita, M.T.; Sobrinho-Goncalves, L.; Oliveira, P.B.; Palma, S.; Falcao, M. A bloom of Dinophysis acuta in a thin layer off North-West Portugal. Afr. J. Mar. Sci. 2006, 28, 265–269. [Google Scholar] [CrossRef]
- Escalera, L.; Reguera, B.; Moita, T.; Pazos, Y.; Cerejo, M.; Cabanas, J.M.; Ruiz-Villarreal, M. Bloom dynamics of Dinophysis acuta in an upwelling system: In situ growth versus transport. Harmful Algae 2010, 9, 312–322. [Google Scholar] [CrossRef]
- Trainer, V.L.; Pitcher, G.C.; Reguera, B.; Smayda, T.J. The distribution and impacts of harmful algal bloom species in eastern boundary upwelling systems. Prog. Oceanogr. 2010, 85, 33–52. [Google Scholar] [CrossRef]
- Fraga, S.; Anderson, D.; Bravo, I.; Reguera, B.; Steidenger, K.A.; Yentsch, C.M. Influence of upwelling relaxation on dinoglagellates and shellfish toxicity in Ria de Vigo, Spain. Estuar. Coast. Shelf. Sci. 1988, 27, 349–361. [Google Scholar] [CrossRef]
- Díaz, P.A.; Ruiz-Villarreal, M.; Pazos, Y.; Moita, M.T.; Reguera, B. Climate variability and Dinophysis acuta blooms in an upwelling system. Harmful Algae 2016, 53, 145–159. [Google Scholar] [CrossRef]
- Fraga, F.; Bravo, I.; Reguera, B. Poleward surface current at the shelf break and blooms of Gymnodinium catenatum in the Ría de Vigo (NW Spain). In Toxic Blooms in the Sea; Smayda, T., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 245–249. [Google Scholar]
- Pino-Querido, A.; Álvarez-Castro, J.M.; Guerra-Varela, J.; Toro, M.A.; Vera, M.; Pardo, B.G.; Fuentes, J.; Blanco, J.; Martínez, P. Heritability estimation for okadaic acid algal toxin accumulation, mantle color and growth traits in Mediterranean mussel (Mytilus galloprovincialis). Aquaculture 2015, 440, 32–39. [Google Scholar] [CrossRef]
- Cloern, J.E.; Schraga, T.; Lopez, C.; Knowles, N.; Labiosa, R.; Dugdale, R. Climate anomalies generate an exceptional dinoflagellate bloom in San Francisco Bay. Geophys. Res. Lett. 2005, 32, L14608. [Google Scholar] [CrossRef]
- Edwards, M.; Beaugrand, G.; Reid, P.C.; Rowden, A.A.; Jones, M.B. Ocean climate anomalies and the ecology of the North Sea. Mar. Ecol. Prog. Ser. 2002, 239, 1–10. [Google Scholar] [CrossRef]
- Edwards, M.; Johns, D.G.; Leterme, S.C.; Svendsen, E.; Richardson, A.J. Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr. 2006, 51, 820–829. [Google Scholar] [CrossRef]
- Roelke, D.; Buyukates, Y. The Diversity of Harmful Algal Bloom-Triggering Mechanisms and the Complexity of Bloom Initiation. Hum. Ecol. Risk Assess. 2001, 7, 1347–1362. [Google Scholar] [CrossRef]
- Park, M.; Kim, S.; Kim, H.; Myung, G.; Kang, Y.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef]
- Hansen, P.J.; Nielsen, L.T.; Johnson, M.; Berge, T.; Flynn, K.R. Acquired phototrophy in Mesodinium and Dinophysis—A review of cellular organization, prey selectivity, nutrient uptake and bioenergetics. Harmful Algae 2013, 28, 126–139. [Google Scholar] [CrossRef]
- González-Gil, S.; Velo-Suárez, L.; Gentien, P.; Ramilo, I.; Reguera, B. Phytoplankton assemblages and characterization of a Dinophysis acuminata population during an upwelling-downwelling cycle. Aquat. Microb. Ecol. 2010, 58, 273–286. [Google Scholar] [CrossRef]
- Harred, L.B.; Campbell, L. Predicting harmful algal blooms: A case study with Dinophysis ovum in the Gulf of Mexico. J. Plankton Res. 2014, 36, 1434–1445. [Google Scholar] [CrossRef]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Margalef, R. Life forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Smayda, T. Adaptations and selection of harmful and other dinoflagellate species in upwelling systems. 2. Motility and migratory behaviour. Prog. Oceanogr. 2010, 85, 71–91. [Google Scholar] [CrossRef]
- Smayda, T. Adaptations and selection of harmful and other dinoflagellate species in upwelling systems 1. Morphology and adaptive polymorphism. Prog. Oceanogr. 2010, 85, 53–70. [Google Scholar] [CrossRef]
- Rial, P.; Laza-Martínez, A.; Reguera, B.; Raho, N.; Rodríguez, F. Origin of cryptophyte plastids in Dinophysis from Galician waters: Results from field and culture experiments. Aquat. Microb. Ecol. 2015, 76, 163–174. [Google Scholar] [CrossRef]
- García-Portela, M.; Riobó, P.; Reguera, B.; Garrido, J.; Blanco, J.; Rodríguez, F. Comparative ecophysiology of Dinophysis acuminata and D. acuta: Effect of light intensity and quality on growth, cellular toxin content and photosynthesis. J. Phycol. 2018. [Google Scholar] [CrossRef]
- García-Portela, M.; Reguera, B.; Ribera d’Alcalà, M.; Rodríguez, F.; Montresor, M. The turbulent life of Dinophysis. In Proceedings of the 18 International Conference on Harmful Algae, Nantes, France, 21–26 October 2018; Abstracts Book. p. 64. [Google Scholar]
- Moita, M.T. Development of toxic dinoflagellates in relation to upwelling patterns off Portugal. In Toxic Phytoplankton Blooms in the Sea; Smayda, T., Shimizu, Y., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1993; pp. 299–304. [Google Scholar]
- Moita, M.T.; Oliveira, P.B.; Mendes, J.C.; Palma, A.S. Distribution of chlorophyll a and Gymnodinium catenatum associated with coastal upwelling plumes off central Portugal. Acta Oecol. 2003, 24, S125–S132. [Google Scholar] [CrossRef]
- Ruiz-Villarreal, M.; García-García, L.; Cobas, M.; Díaz, P.A.; Reguera, B. Modeling the hydrodynamic conditions associated with Dinophysis blooms in Galicia (NW Spain). Harmful Algae 2016, 53, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.; Gentien, P.; Fernand, L.; Lunven, M.; Reguera, B.; González-Gil, S.; Raine, R. Scales characterising a high density thin layer of Dinophysis acuta Ehrenberg and its transport within a coastal jet. Hamrful Algae 2012, 15, 36–46. [Google Scholar] [CrossRef]
- Raine, R.; Cosgrove, S.; Fennell, S.; Gregory, C.; Barnett, M.; Purdie, D.; Cave, R. Origins of Dinophysis blooms which impact Irish aquaculture. In Marine and Fresh-Water Harmful Algae, Proceedings of the 17th International Conference on Harmful Algae, Santa Catarina, Brazil, 9–14 October 2016; Proença, L.A.O., Hallegraeff, G.M., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2017; pp. 46–49. [Google Scholar]
- Swan, S.C.; Turner, A.D.; Bresnan, E.; Whyte, C.; Paterson, R.F.; McNeill, S.; Mitchell, E.; Davidson, K. Dinophysis acuta in Scottish coastal waters and its influence on Diarrhetic Shellfish Toxin profiles. Toxins 2018, 10, 399. [Google Scholar] [CrossRef]
- Smayda, T. Turbulence, watermass stratification and harmful algal blooms: An alternative view and frontal zones as “pelagic seed banks”. Harmful Algae 2002, 1, 95–112. [Google Scholar] [CrossRef]
- Reguera, B.; Díaz, P.A.; Escalera, L.; Ramilo, I.; Cabanas, J.M.; Ruiz-Villarreal, M. Winter distributions of Dinophysis populations: Do they help predict the onset of the bloom? In Marine and Freshwater Harmful Algae 2014, Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; MacKenzie, L.A., Ed.; Cawthron Institute: Nelson, New Zealand, the International Society for the Study of Harmful Algae (ISSHA); 2014; pp. 128–131. [Google Scholar]
- Reguera, B.; Bravo, I.; Marcaillou-le, C.; Masselin, P. Monitoring of Dinophysis spp. and vertical distribution of okadaic acid on mussel rafts in Ría de Pontevedra (NW Spain). In Toxic phytoplankton Blooms in the Sea; Smayda, T., Shimizu, Y., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1993; pp. 553–558. [Google Scholar]
- Lee, J.S.; Igarashi, T.; Fraga, S.; Dahl, E.; Hovgaard, P.; Yasumoto, T. Determination of diarrhetic shellfish toxins in various dinoflagellate species. J. Appl. Physiol. 1989, 1, 147–152. [Google Scholar] [CrossRef]
- Hu, T.; Doyle, J.; Jackson, D.; Marr, J.; Nixon, E.; Pleasance, S.; Quilliam, M.; Walter, J.; Wright, J. Isolation of a new diarrhetic shellfish poison from Irish mussels. J. Chem. Soc. Chem. Commun. 1992, 54, 39–41. [Google Scholar] [CrossRef]
- Fernández, M.L.; Míguez, A.; Martínez, A.; Moroño, A.; Arévalo, F.; Pazos, Y.; Salgado, C.; Correa, J.; Blanco, J.; González-Gil, S.; et al. First report of pectenotoxin-2 in phytoplankton net-hauls and mussels from the Galician Rías Baixas during proliferations of Dinophysis acuta and D. caudata. In Molluscan Shellfish Safety; Villalba, A., Reguera, B., Romalde, J., Beiras, R., Eds.; Consellería de Pesca e Asuntos Marítimos da Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 2003; pp. 75–83. ISBN 84-453-3638-X. [Google Scholar]
- Fernández, M.; Reguera, B.; González-Gil, S.; Míguez, A. Pectenotoxin-2 in single-cell isolates of Dinophysis caudata and Dinophysis acuta from the Galician Rías (NW Spain). Toxicon 2006, 48, 477–490. [Google Scholar] [CrossRef]
- Díaz, P.A.; Ruiz-Villarreal, M.; Rodriguez, F.; Garrido, J.L.; Mouriño-Carballido, B.; Riobó, P.; Reguera, B. Fine scale physical-biological interactions in a Dinophysis acuminata population during an upwelling-relaxation transition. In Marine and Fresh-Water Harmful Algae, Proceedings of the 17th International Conference on Harmful Algae, Santa Catarina, Brazil, 9–14 October 2016; Proença, L.A.O., Hallegraeff, G.M., Eds.; ISSHA and IOC of UNESCO: Paris, France, 2017; pp. 50–53. ISBN 978-87-990827-6-6. [Google Scholar]
- Natural banks. Available online: www.fao.org (accessed on 30 October 2018).
- Spanish Meteorological Agency. Available online: www.aemet.es (accessed on 30 July 2018).
- Spanish Institute of Oceanography (IEO). Available online: www.indicedeafloramiento.ieo.es (accessed on 30 July 2018).
- Bograd, S.T.; Schroeder, I.; Sarkar, N.; Qiu, X.; Sydeman, W.J.; Schwing, F.B. Phenology of coastal upwelling in the California Current. Geophys. Res. Lett. 2009, 36, L01602. [Google Scholar] [CrossRef]
- Rayner, N.A.; Parker, D.E.; Horton, E.B.; Folland, C.K.; Alexander, L.V.; Rowell, D.P.; Kent, E.C.; Kaplan, A. Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. J. Geophys. Res. 2003, 108, 4407. [Google Scholar] [CrossRef]
- Gordon, H.R.; McCluney, W.R. Estimation of the depth of sunlight penetration in the sea for remote sensing. Appl. Opt. 1975, 14, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, O. A Dividable Hose for Phytoplankton Sampling; Report of the Working Group on Phytoplankton and Management of Their Effects; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1986; C.M.1986/L: 26, Annex III. [Google Scholar]
- Lovegrove, T. An improved form of sedimentation apparatus for use with an inverted microscope. ICES J. Mar. Sci. 1960, 25, 279–284. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkomnung der quantitativen phytoplankton-Methodik. Mitt. Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
- Lee, J.S.; Yanagi, T.; Kenma, R.; Yasumoto, T. Fluorometric determination of diarrhetic shellfish toxins by High-Performance Liquid Chomatography. Agric. Biol. Chem. 1987, 51, 877–881. [Google Scholar]
- Kelley, D. Oce: Analysis of Oceanographic Data, R package Version 0.9-14; 2014. Available online: http://CRAN.R-project.org/package=oce (accessed on 5 May 2018).
- Bivand, R. Maptools: Tools for Reading and Handling Spatial Objects, R package version 0.9-2; 2017. Available online: https://CRAN.R-project.org/package=maptools/ (accessed on 15 September 2018).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. Available online: http://www.r-project.org/ (accessed on 15 September 2018).
- CRAN repository. Available online: www.r-project.org (accessed on 15 September 2018).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, P.A.; Reguera, B.; Moita, T.; Bravo, I.; Ruiz-Villarreal, M.; Fraga, S. Mesoscale Dynamics and Niche Segregation of Two Dinophysis Species in Galician-Portuguese Coastal Waters. Toxins 2019, 11, 37. https://doi.org/10.3390/toxins11010037
Díaz PA, Reguera B, Moita T, Bravo I, Ruiz-Villarreal M, Fraga S. Mesoscale Dynamics and Niche Segregation of Two Dinophysis Species in Galician-Portuguese Coastal Waters. Toxins. 2019; 11(1):37. https://doi.org/10.3390/toxins11010037
Chicago/Turabian StyleDíaz, Patricio A., Beatriz Reguera, Teresa Moita, Isabel Bravo, Manuel Ruiz-Villarreal, and Santiago Fraga. 2019. "Mesoscale Dynamics and Niche Segregation of Two Dinophysis Species in Galician-Portuguese Coastal Waters" Toxins 11, no. 1: 37. https://doi.org/10.3390/toxins11010037
APA StyleDíaz, P. A., Reguera, B., Moita, T., Bravo, I., Ruiz-Villarreal, M., & Fraga, S. (2019). Mesoscale Dynamics and Niche Segregation of Two Dinophysis Species in Galician-Portuguese Coastal Waters. Toxins, 11(1), 37. https://doi.org/10.3390/toxins11010037