Lipopolysaccharide Structural Differences between Western and Asian Helicobacter pylori Strains
Abstract
:1. Introduction
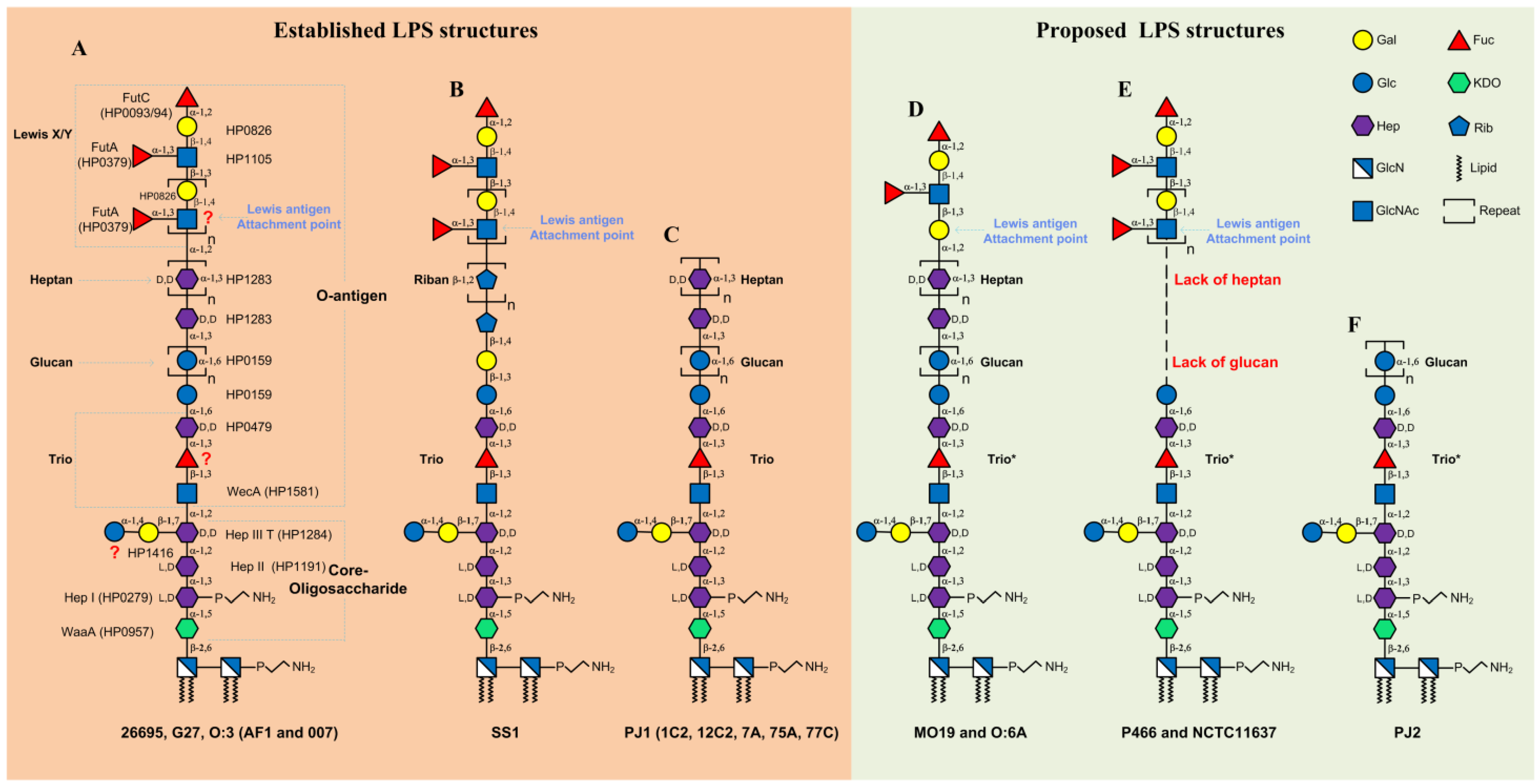
2. LPS Structure in Western H. pylori Strains
2.1. LPS Structure and Biosynthesis in H. pylori Strains 26695 and G27
2.2. LPS Structure in Other Western H. pylori Strains
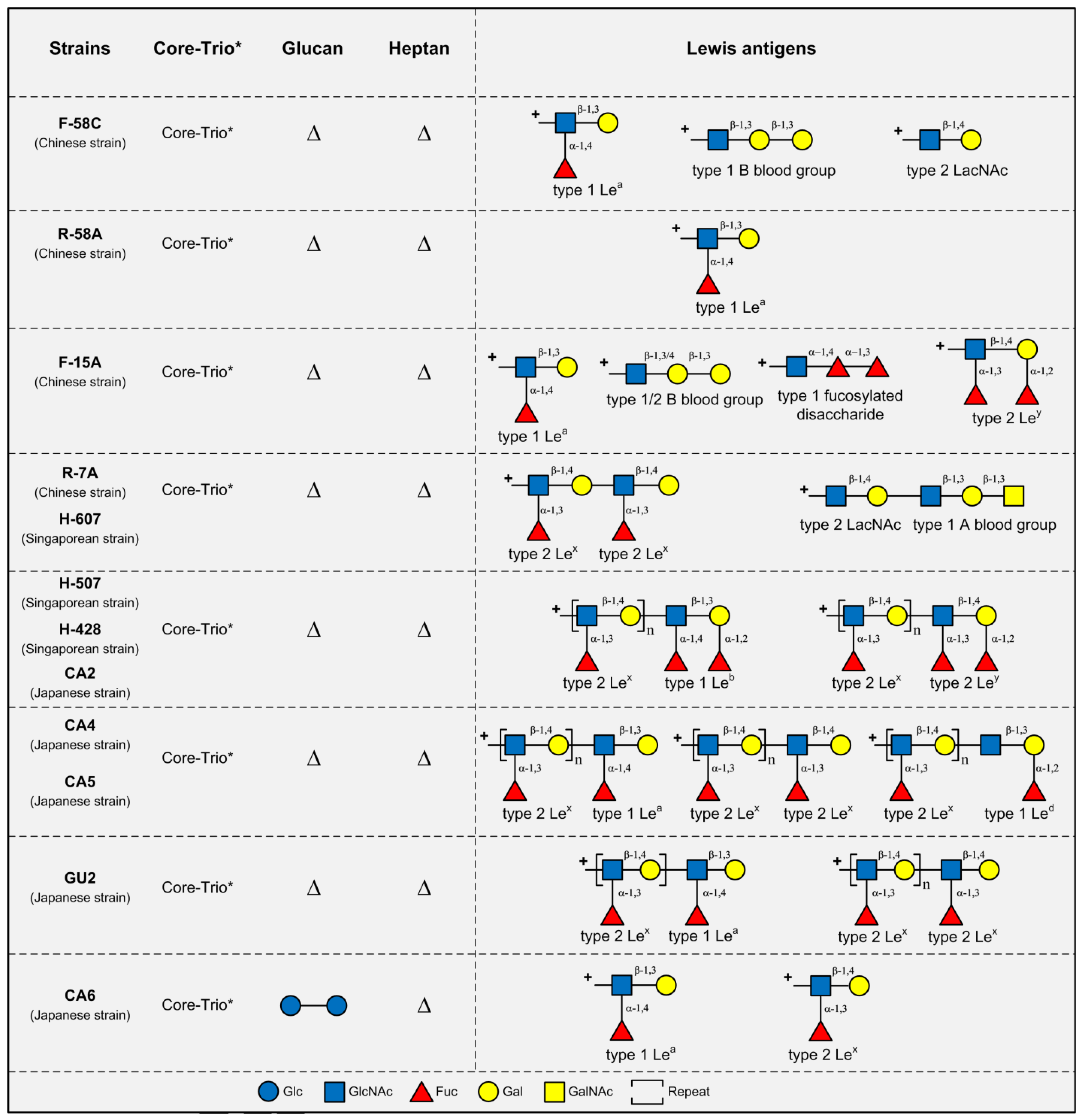
3. LPS Structures in Asian H. pylori Strains
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, T.; Debowski, A.W.; Tang, H.; Nilsson, H.O.; Stubbs, K.A.; Marshall, B.J.; Benghezal, M. Lipopolysaccharide structure and biosynthesis in Helicobacter pylori. Helicobacter 2016, 21, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.W.; Giles, D.K.; Wolf, L.N.; Ecobichon, C.; Boneca, I.G.; Trent, M.S. Helicobacter pylori versus the host: Remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011, 7, e1002454. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, G.O.; Monteiro, M.A.; Shaver, R.T.; Kurjanczyk, L.A.; Penner, J.L. Lipopolysaccharides of Helicobacter pylori serogroups O:3 and O:6--structures of a class of lipopolysaccharides with reference to the location of oligomeric units of D-glycero-alpha-D-manno-heptose residues. Eur. J. Biochem. 1997, 248, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.A.; Appelmelk, B.J.; Rasko, D.A.; Moran, A.P.; Hynes, S.O.; MacLean, L.L.; Chan, K.H.; Michael, F.S.; Logan, S.M.; O’Rourke, J.; et al. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H. pylori P466 carrying sialyl Lewis, X., and H. pylori UA915 expressing Lewis B classification of H. pylori lipopolysaccharides into glycotype families. Eur. J. Biochem. 2000, 267, 305–320. [Google Scholar] [PubMed]
- Aspinall, G.O.; Monteiro, M.A. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: Structures of the O antigen and core oligosaccharide regions. Biochemistry 1996, 35, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.A.; Zheng, P.; Ho, B.; Yokota, S.; Amano, K.; Pan, Z.; Berg, D.E.; Chan, K.H.; MacLean, L.L.; Perry, M.B. Expression of histo-blood group antigens by lipopolysaccharides of Helicobacter pylori strains from Asian hosts: The propensity to express type 1 blood-group antigens. Glycobiology 2000, 10, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, T.; Liao, T.; Debowski, A.W.; Nilsson, H.O.; Fulurija, A.; Haslam, S.M.; Mulloy, B.; Dell, A.; Stubbs, K.A.; et al. The redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. PLoS Pathog. 2017, 13, e1006280. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Chandan, V.; Li, J.; Vinogradov, E. Lipopolysaccharide structure of Helicobacter pylori serogroup O.:3. Carbohydr. Res. 2013, 378, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Chandan, V.; Li, J.; Vinogradov, E. Lipopolysaccharide structures of Helicobacter pylori wild-type strain 26695 and 26695 HP0826: Kan mutant devoid of the O-chain polysaccharide component. Carbohydr. Res. 2011, 346, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Chandan, V.; Li, J.; Vinogradov, E. A reinvestigation of the lipopolysaccharide structure of Helicobacter pylori Strain sydney (SS1). FEBS J. 2011, 278, 3484–3493. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.A.; Chan, K.H.; Rasko, D.A.; Taylor, D.E.; Zheng, P.Y.; Appelmelk, B.J.; Wirth, H.P.; Yang, M.; Blaser, M.J.; Hynes, S.O.; et al. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between H. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J. Biol. Chem. 1998, 273, 11533–11543. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, G.O.; Monteiro, M.A.; Pang, H.; Walsh, E.J.; Moran, A.P. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): Structure of the O antigen chain and core oligosaccharide regions. Biochemistry 1996, 35, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, T.; Liao, T.; Debowski, A.W.; Nilsson, H.O.; Haslam, S.M.; Dell, A.; Stubbs, K.A.; Marshall, B.J.; Benghezal, M. Insights from the redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. Microb. Cell 2017, 4, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, H.; Kiyohara, Y.; Aoyagi, K.; Kato, I.; Iwamoto, H.; Nakayama, K.; Shimizu, H.; Tanizaki, Y.; Arima, H.; Shinohara, N.; et al. Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: The Hisayama study. Arch. Intern. Med. 2000, 160, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Tomb, J.F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Baltrus, D.A.; Amieva, M.R.; Covacci, A.; Lowe, T.M.; Merrell, D.S.; Ottemann, K.M.; Stein, M.; Salama, N.R.; Guillemin, K. The complete genome sequence of Helicobacter pylori strain G27. J. Bacteriol. 2009, 191, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Kocharova, N.A.; Hynes, S.O.; Widmalm, G.; Andersen, L.P.; Jansson, P.E.; Moran, A.P. Structural studies on lipopolysaccharides of serologically non-typable strains of Helicobacter pylori, AF1 and 007, expressing Lewis antigenic determinants. Eur. J. Biochem. 1999, 266, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.A.; St Michael, F.; Rasko, D.A.; Taylor, D.E.; Conlan, J.W.; Chan, K.H.; Logan, S.M.; Appelmelk, B.J.; Perry, M.B. Helicobacter pylori from asymptomatic hosts expressing heptoglycan but lacking Lewis O-chains: Lewis blood-group O-chains may play a role in Helicobacter pylori induced pathology. Biochem. Cell Biol. 2001, 79, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.M.; Conlan, J.W.; Monteiro, M.A.; Wakarchuk, W.W.; Altman, E. Functional genomics of Helicobacter pylori: Identification of a beta-1, 4 galactosyltransferase and generation of mutants with altered lipopolysaccharide. Mol. Microbiol. 2000, 35, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Chandan, V.; Larocque, S.; Aubry, A.; Logan, S.M.; Vinogradov, E.; Li, J. Effect of the HP0159 ORF mutation on the lipopolysaccharide structure and colonizing ability of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2008, 53, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.M.; Altman, E.; Mykytczuk, O.; Brisson, J.R.; Chandan, V.; Schur, M.J.; St Michael, F.; Masson, A.; Leclerc, S.; Hiratsuka, K.; et al. Novel biosynthetic functions of lipopolysaccharide RfaJ homologs from Helicobacter pylori. Glycobiology 2005, 15, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, K.; Logan, S.M.; Conlan, J.W.; Chandan, V.; Aubry, A.; Smirnova, N.; Ulrichsen, H.; Chan, K.H.; Griffith, D.W.; Harrison, B.A.; et al. Identification of a D-glycero-D-manno-heptosyltransferase gene from Helicobacter pylori. J. Bacteriol. 2005, 187, 5156–5165. [Google Scholar] [CrossRef] [PubMed]
- Stead, C.M.; Zhao, J.; Raetz, C.R.; Trent, M.S. Removal of the outer Kdo from Helicobacter pylori lipopolysaccharide and its impact on the bacterial surface. Mol. Microbiol. 2010, 78, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.P.; Shiberu, B.; Ferris, J.A.; Knirel, Y.A.; Senchenkova, S.N.; Perepelov, A.V.; Jansson, P.E.; Goldberg, J.B. Role of Helicobacter pylori RfaJ genes (HP0159 and HP1416) in lipopolysaccharide synthesis. FEMS Microbiol. Lett. 2004, 241, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Langdon, R.; Craig, J.E.; Goldrick, M.; Houldsworth, R.; High, N.J. Analysis of the role of HP0208, a phase-variable open reading frame, and its homologues HP1416 and HP0159 in the biosynthesis of Helicobacter pylori lipopolysaccharide. J. Med. Microbiol. 2005, 54, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Hug, I.; Couturier, M.R.; Rooker, M.M.; Taylor, D.E.; Stein, M.; Feldman, M.F. Helicobacter pylori lipopolysaccharide is synthesized via a novel pathway with an evolutionary connection to protein N-glycosylation. PLoS Pathog. 2010, 6, e1000819. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Chandan, V.; Harrison, B.A.; Vinogradov, E. Structural and immunological characterization of a glycoconjugate based on the delipidated lipopolysaccharide from a nontypeable Helicobacter pylori strain PJ1 containing an extended D-glycero-D-manno-heptan. Carbohydr. Res. 2017, 456, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Appelmelk, B.J.; Martin, S.L.; Monteiro, M.A.; Clayton, C.A.; McColm, A.A.; Zheng, P.Y.; Verboom, T.; Maaskant, J.J.; van den Eijnden, D.H.; Hokke, C.H.; et al. Phase variation in Helicobacter pylori lipopolysaccharide due to changes in the lengths of poly(C) tracts in alpha 3-fucosyltransferase genes. Infect. Immun. 1999, 67, 6715. [Google Scholar]
- Wang, G.; Rasko, D.A.; Sherburne, R.; Taylor, D.E. Molecular genetic basis for the variable expression of Lewis Y antigen in Helicobacter pylori: Analysis of the alpha (1,2) fucosyltransferase gene. Mol. Microbiol. 1999, 31, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.P.; Trent, M.S. Helicobacter pylori lipopolysaccharides and Lewis antigens. In Helicobacter Pylori: Molecular Genetics and Cellular Biology; Yamaoka, Y., Ed.; Caister Academic Press: Poole, UK, 2008; pp. 7–36. ISBN 978-1-904455-31-8. [Google Scholar]
- Chandan, V.; Logan, S.M.; Harrison, B.A.; Vinogradov, E.; Aubry, A.; Stupak, J.; Li, J.; Altman, E. Characterization of a waaF mutant of Helicobacter pylori strain 26695 provides evidence that an extended lipopolysaccharide structure has a limited role in the invasion of gastric cancer cells. Biochem. Cell Biol. 2007, 85, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ge, Z.; Rasko, D.A.; Taylor, D.E. Lewis antigens in Helicobacter pylori: Biosynthesis and phase variation. Mol. Microbiol. 2000, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Skoglund, A.; Moran, A.P.; Annuk, H.; Engstrand, L.; Normark, S. An enzymatic ruler modulates Lewis antigen glycosylation of Helicobacter pylori LPS during persistent infection. Proc. Natl. Acad. Sci. USA 2006, 103, 2863–2868. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Boulton, P.G.; Chan, N.W.; Palcic, M.M.; Taylor, D.E. Novel Helicobacter pylori alpha1,2-fucosyltransferase, a key enzyme in the synthesis of Lewis antigens. Microbiology 1999, 145 Pt 11, 3245–3253. [Google Scholar] [CrossRef]
- Altman, E.; Smirnova, N.; Li, J.J.; Aubry, A.; Logan, S.M. Occurrence of a nontypable Helicobacter pylori strain lacking Lewis blood group O antigens and DD-heptoglycan: Evidence for the role of the core alpha 1,6-glucan chain in colonization. Glycobiology 2003, 13, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Chandan, V.; Harrison, B.A.; Panayotopoulou, E.G.; Roma-Giannikou, E.; Li, J.; Sgouras, D.N. Helicobacter pylori isolates from Greek children express type 2 and type 1 lewis and alpha1,6-glucan antigens in conjunction with a functional type IV secretion system. J. Med. Microbiol. 2012, 61, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Harrison, B.A.; Chandan, V.; Slinger, R. Lipopolysaccharide glycotyping of clarithromycin-resistant and clarithromycin-susceptible Canadian isolates of Helicobacter pylori. Can. J. Microbiol. 2014, 60, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Fernandez, H.; Chandan, V.; Harrison, B.A.; Schuster, M.W.; Rademacher, L.O.; Toledo, C. Analysis of Helicobacter pylori isolates from Chile: Occurrence of selective type 1 Lewis b antigen expression in lipopolysaccharide. J. Med. Microbiol. 2008, 57, 585–591. [Google Scholar] [CrossRef] [PubMed]
| GT Genes | Proposed/Demonstrated Functions | References |
|---|---|---|
| HP0279 | Hep I transferase, assembling the core-oligosaccharide | [24,32] |
| HP1191 | Hep II transferase, assembling the core-oligosaccharide | [25,33] |
| HP1284 | Hep III transferase, assembling the core-oligosaccharide | [15] |
| HP1416 | Glc transferase, assembling the core-oligosaccharide | [26,27] |
| HP1581 | GlcNAc transferase (WecA), initiating the O-antigen assembly | [15,28] |
| HP0479 | Hep transferase, assembling the Trio motif | [24] |
| HP0159 | Glc transferase, assembling the glucan structure | [22,26,27] |
| HP1283 | Hep transferase, assembling the heptan structure | [29] |
| HP0826 | β(1,4)Gal transferase, assembling the Lewis chain | [11,21] |
| HP1105 | β(1,3)GlcNAc transferase, assembling the Lewis chain | [23] |
| HP0379 | FutA, α(1,3/4)Fuc transferase, assembling the Lewis chain | [30,34,35] |
| HP0651 | FutB, α(1,3/4)Fuc transferase, assembling the Lewis chain | [30,34,35] |
| HP0093/94 | FutC, α(1,2)Fuc transferase, assembling the Lewis chain | [31,34,35,36] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Tang, H.; Debowski, A.W.; Stubbs, K.A.; Marshall, B.J.; Benghezal, M. Lipopolysaccharide Structural Differences between Western and Asian Helicobacter pylori Strains. Toxins 2018, 10, 364. https://doi.org/10.3390/toxins10090364
Li H, Tang H, Debowski AW, Stubbs KA, Marshall BJ, Benghezal M. Lipopolysaccharide Structural Differences between Western and Asian Helicobacter pylori Strains. Toxins. 2018; 10(9):364. https://doi.org/10.3390/toxins10090364
Chicago/Turabian StyleLi, Hong, Hong Tang, Aleksandra W. Debowski, Keith A. Stubbs, Barry J. Marshall, and Mohammed Benghezal. 2018. "Lipopolysaccharide Structural Differences between Western and Asian Helicobacter pylori Strains" Toxins 10, no. 9: 364. https://doi.org/10.3390/toxins10090364
APA StyleLi, H., Tang, H., Debowski, A. W., Stubbs, K. A., Marshall, B. J., & Benghezal, M. (2018). Lipopolysaccharide Structural Differences between Western and Asian Helicobacter pylori Strains. Toxins, 10(9), 364. https://doi.org/10.3390/toxins10090364





