Endotoxins from a Pharmacopoeial Point of View
Abstract
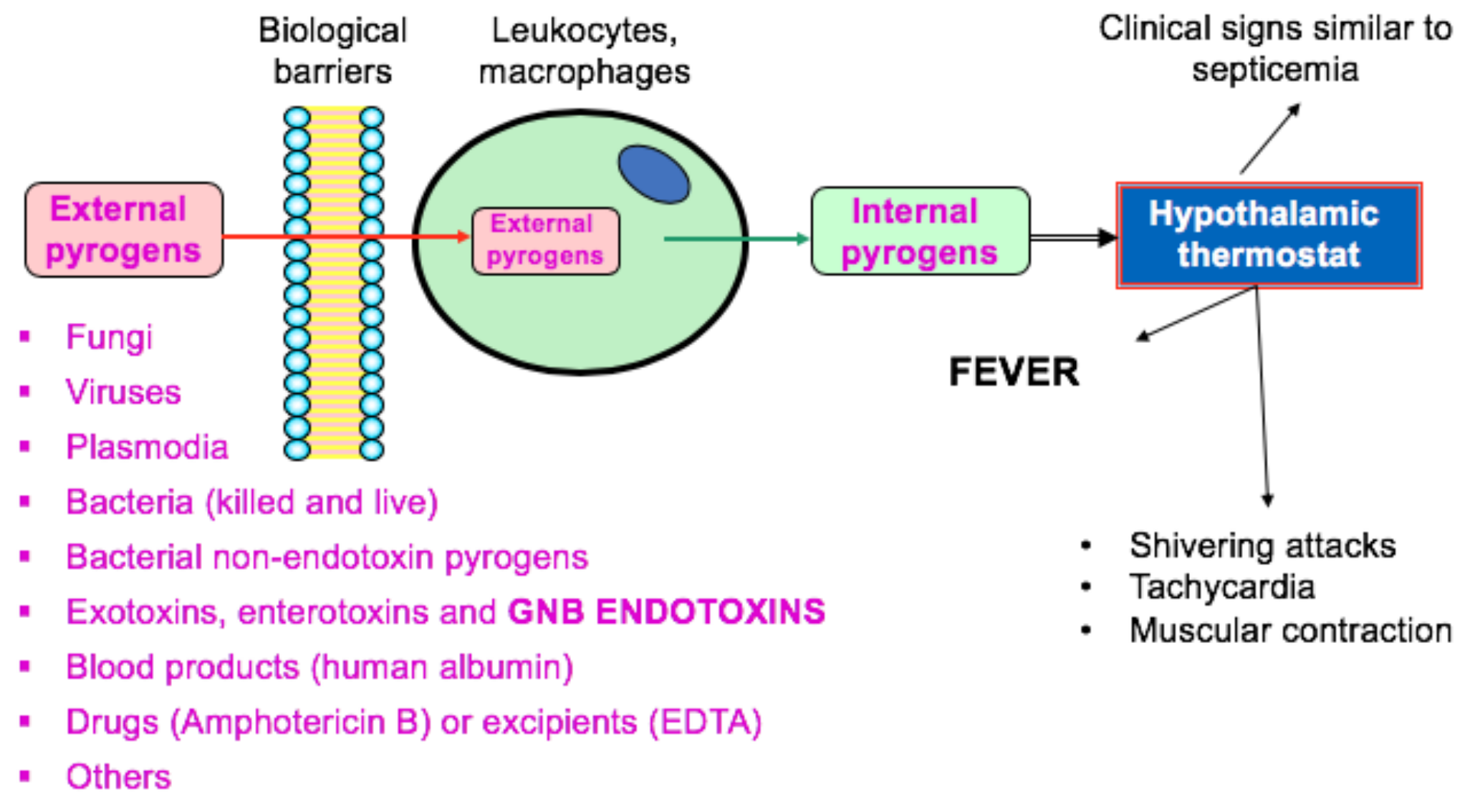
:1. Introduction
2. In Vivo Pyrogen Assay: Rabbit Pyrogen Test (RPT)
3. In Vitro Pyrogen Assay: Bacterial Endotoxins Test (BET)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandel, T. Assesing non-endotoxin microbial pyrogens in relation in pharmaceutical processing. J. GXP Compliance 2015, 19, 1–12. [Google Scholar]
- Tours, N.; Sandle, T. Comparison of dry-heat depyrogenation using three different types of Gram-negative bacterial endotoxin. Eur. J. Parenter. Pharm. Sci. 2008, 13, 17–20. [Google Scholar]
- Ensor, D.S.; Foarde, K.K. The behavior of particles in cleanrooms. In Environmental Monitoring for Cleanrooms and Controlled Environments, 1st ed.; Dixon, A.M., Ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2016; pp. 1–28. ISBN 0-8247-2359-7. [Google Scholar]
- Seibert, F.B. The cause of many febrile reactions following intravenous injections. I. Am. J. Physiol. 1925, 71, 621–651. [Google Scholar] [CrossRef]
- Welch, H.; Calvery, H.D.; McClosky, W.T.; Price, C.W. Method of preparation and test for bacterial pyrogens. J. Am. Pharm. Assoc. 1943, 32, 65–69. [Google Scholar] [CrossRef]
- McClosky, W.T.; Price, C.W.; Van Winkle, W.J.; Welch, H.; Calvery, H.O. Results of the first USP collaborative study of pyrogens. J. Am. Pharm. Assoc. 1943, 32, 69–73. [Google Scholar] [CrossRef]
- Levin, J.; Bang, F.B. A description of cellular coagulation in the limulus. Bull. Johns Hopkins Hosp. 1964, 115, 337–345. [Google Scholar] [PubMed]
- Levin, J.; Bang, F.B. The role of endotoxin in the extracellular coagulation of limulus blood. Bull. Johns Hopkins Hosp. 1964, 115, 265–274. [Google Scholar] [PubMed]
- Altintas, Z.; Abdin, M.J.; Tothill, A.M.; Karim, K.; Tothill, I.E. Ultrasensitive detection of endotoxins using computationally designed nanoMIPs. Anal. Chem. Acta 2016, 935, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Solano, G.; Gómez, A.; León, G. Assessing endotoxins in equine-derived snake antivenoms: Comparison of the USP pyrogen test and the Limulus Amoebocyte Lysate assay (LAL). Toxicon 2015, 105, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Fingola, F.F.; Albertino, S.R.G.; Abrantes, S.M.P.; Zamith, H.P.S. Intralaboratory validation of kinetic chromogenic Limulus amebocyte lysate assay for bacterial endotoxin determination in anti-bothropic serum. J. Pharm. Biomed. Anal. 2013, 85, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P.; Chaturvedula, L.M.; Sritharan, V.; Gupta, S. In vitro flow-through assay for rapid detection of endotoxin in human sera: A proof-of-concept. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Mujika, M.; Zuzuarregui, A.; Sánchez-Gómez, S.; Martínez de Tejada, G.; Arana, S.; Pérez-Lorenzo, E. Screening and selection of synthetic peptides for a novel and optimized endotoxin detection method. J. Biotechnol. 2014, 186, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Caldeira da Siva, C.; Franca Presgrave, O.A.; Hartung, T.; Lage de Moraes, A.M.; Fernandes Delgado, I. Applicability of the Monocyte Activation Test (MAT) for hyperimmune sera in the routine of the quality control laboratory: Comparison with the Rabbit Pyrogen Test (RPT). Toxicol. Vitr. 2016, 32, 70–75. [Google Scholar]
- Reich, J.; Lang, P.; Grallert, H.; Motschmann, H. Masking of endotoxin in surfactant samples: Effects on Limulus-based detection systems. Biologicals 2016, 44, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Bolden, J.; Knight, M.; Stockman, S.; Omokoko, B. Results of a harmonized endotoxin recovery study protocol evaluation by 14 BioPhorum Operations Group (BPOG) member companies. Biologicals 2017, 48, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jia, J.; Li, C.; Xue, J.; Sun, J.; Wang, K.; Gan, Y.; Xu, J.; Shi, Y.; Liang, X. LAL test and RPT for endotoxin detection of CPT-11/DSPE-mPEG2000 nanoformulation: What if traditional methods are not applicable? Asian J. Pharm. Sci. 2018, in press. [Google Scholar] [CrossRef]
- 2.6.30. Monocyte Activation Test. In European Pharmacopeia 9.0., 9th ed.; Council of Europe: Strasbourg, France, 2017; pp. 193–194. ISBN 978-9-2871-8133-6.
- Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers. U.S. Food and Drug Administration, 2012. Available online: www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 3 August 2018).
- Hartung, T.; Wendel, A. Detection of Pyrogens using human whole blood. Altex 1995, 12, 70–75. [Google Scholar] [PubMed]
- Schindler, S.; von Aulock, S.; Daneshian, M.; Hartung, T. Development, validation and applications of the monocyte activation test for pyrogens based on human whole blood. Altex 2009, 26, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Peterbauer, A.; Schindler, S.; Fennrich, S.; Poole, S.; Mistry, Y.; Montag-Lessing, T.; Spreitzer, I.; Löschner, B.; Van Aalderen, M.; et al. International validation of novel pyrogen tests based on human monocytoid cells. J. Immunol. Methods 2005, 298, 161–173. [Google Scholar] [CrossRef] [PubMed]
- De Mattos, K.A.; Navega, E.C.A.; Silva, V.F.; Almeida, A.S.; Da Silva, C.C.; Presgrave, O.A.F.; Junior, D.D.S.G.; Delgado, I.F. Applicability of the monocyte activation test (MAT) in the quality control of the 17DD yellow fever vaccine. Altern. Lab. Anim. 2018, 46, 23–37. [Google Scholar] [PubMed]
- Nordgren, I.K. Leukoreduction system chambers provide a valuable source of functional monocytes for the monocyte activation test by comparison with internationally validated methods. J. Immunol. Methods 2016, 428, 42–49. [Google Scholar] [CrossRef] [PubMed]
- <151> Pyrogen Test. In U.S. Pharmacopeia 41; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2018; pp. 6083–6085. ISBN 978-3-7692-7022-8.
- 2.6.8. Pyrogens. In European Pharmacopeia 9.0., 9th ed.; Council of Europe: Strasbourg, France, 2017; pp. 193–194. ISBN 978-9-2871-8133-6.
- 4.04 Pyrogen Test. In The Japanese Pharmacopoeia, 17th ed; The Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016; pp. 120–194. Available online: www.pmda.go.jp/english (accessed on 23 May 2018).
- 3.4 Test for bacterial endotoxins. In The International Pharmacopoeia, 7th ed.; WHO Department of Essential Medicines and Health Products: Geneva, Switzerland, 2017; Available online: www.who.int/phint/ (accessed on 21 May 2018).
- Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions on Bacterial Endotoxins Test. General Chapter. Q4b Annex 14. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2012. Available online: www.ich.org (accessed on 30 May 2018).
- 3.5 Test for pyrogens. In The International Pharmacopoeia, 7th ed.; WHO Department of Essential Medicines and Health Products: Geneva, Switzerland, 2017; Available online: www.who.int/phint/ (accessed on 21 May 2018).
- <85> Bacterial Endotoxins Test. In U.S. Pharmacopeia 41; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2018; pp. 6011–6017. ISBN 978-3-7692-7022-8.
- 4.01 Bacterial Endotoxins Test. In the Japanese Pharmacopoeia, 17th ed.; The Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016; pp. 110–114. Available online: www.pmda.go.jp/english (accessed on 23 May 2018).
- 2.6.14. Bacterial Endotoxins. In European Pharmacopeia 9.0., 9th ed.; Council of Europe: Strasbourg, France, 2017; pp. 204–207. ISBN 978-9-2871-8133-6.
| Experimental | IP | USP | JP | EP |
|---|---|---|---|---|
| Number | 3 | 3 | 3 | 3 |
| Condition | Healthy | Healthy | Healthy | Healthy |
| Age | Adult | Mature | Mature | Adult |
| Weight | - | - | ≥1.5 kg | ≥ 1.5kg |
| Sex | - | - | - | either |
| Variety | The same, ideally | - | - | - |
| Housing: | ||||
| temperature | uniform (±2 °C) | uniform (20–23 °C) (±3 °C) | constant = 20 °C–27 °C | uniform, appropriate |
| humidity | uniform | - | - | - |
| watering | ad libitum | - | - | - |
| feeding | usual food ad libitum | - | constant diet | complete, balanced, antibiotics free diet |
| Environs | Not exciting | Not exciting | Not exciting | Quiet |
| Individually or in group | Individually | Individually | Individually | Individually |
| Rejection reasons | ▪ Last use, in a pyrogen test, in the last 48 h ▪ T rise in the last test ≥ 0.5 °C ▪ Last use in a pyrogen-positive test in the previous 2 weeks | ▪ Last use, in a pyrogen test, in the last 48 h ▪ T rise ≥ 0.6 °C in a pyrogen test in the previous 2 weeks ▪ Last use in a pyrogen-positive test in the previous 2 weeks | ▪ Last use, in a pyrogen test, in the last 48 h ▪ Last use in a pyrogen-positive test ▪ Loss of body mass in the previous week | ▪ Loss of body mass in the previous week ▪ Last use, in a pyrogen test, in the last 3 days ▪ Last use, in a pyrogen-positive test, in the last 3 weeks ▪ Use, in a pyrogen test, where the rabbits’ temperature mean rise > 1.2 °C |
| Experimental | IP | USP | JP | EP |
|---|---|---|---|---|
| Test room | Housing area or similar separate room | Separate area designated solely for pyrogen testing | Separate room | Housing area or separate room (at least 18h of previous staying) |
| Room T | Similar to the housing T | Similar to the housing T | Similar to the housing T | Within 3 °C of the housing T |
| Instrument | Accurate thermometer or T-recording device | Accurate T-sensing device (clinical thermometer or thermistor probe) | Rectal thermometer or T-measuring apparatus | Thermometer or electrical device |
| Precision | 0.1 °C | ± 0.1 °C | ≤ ± 0.1 °C | 0.1 °C |
| Time | Sufficient to reach a maximum T | Sufficient to reach a maximum T and < 5 min | - | An electrical device may be left throughout the test. |
| Site and depth | Rectum, ≈ 6 cm | Rectum, ≥ 7.5 cm | Rectum, 6–9 cm constant | Rectum, ≈ 5 cm constant |
| Restraint | By a loosely fitting neck stock | With lightly fitting neck stock | By a loosely fitting neck stock | By a loosely fitting neck stock (at least 1h before and throughout the test) |
| Posture | Natural resting | Natural resting | Natural resting | Normal |
| Feeding | Not allowed (2h before and during test) | Not allowed | Not allowed (several hours before and during test) | Not allowed (previous overnight and during test) |
| Watering | Allowed | Allowed, may be restricted | - | Not allowed |
| Experimental | IP | USP | JP | EP |
|---|---|---|---|---|
| Pretraining for rabbits not previously used | Same test omitting the injection, 1–3 days before | Same test omitting the injection, not more than 7 days before; for rabbits never used before | Same test omitting the injection, 1–3 days before | Same test injecting pyrogen-free 9 g/L solution of sodium chloride R, 1–3 days before. Non-use period = 2 weeks |
| Pre-injection conditioning time | ≥ 1 h | - | ≥ 48 h | 18 h |
| Control T (CT) | Mean of two T readings (T1, T2) at an interval of 30 min in the 40 min preceding the injection | Taken no more than 30 min prior to the injection | Mean of two T readings (T1, T2) at an interval of 30 min in the 40 min preceding the injection | Mean of two T readings (T1, T2) at an interval of 30 min in the 40 min preceding the injection |
| Rabbit selection criteria | ▪ ΔCT among rabbits ≤ 1.0 °C ▪ T1-T2 ≤ mean ± 0.2 °C ▪ 38 °C ≤ CT ≤ 39.8 °C | ▪ ΔCT among rabbits ≤ 1.0 °C ▪ CT < 39.8 °C | ▪ T1-T2 ≤ ± 0.2 °C ▪ CT ≤ 39.8 °C | ▪ ΔT ≤ 0.6 °C in the pretraining ▪ T1-T2 ≤ ± 0.2 °C ▪ 38 °C ≤ CT ≤ 39.8 °C ▪ ΔCT among rabbits ≤ 1.0 °C |
| Syringe, needle and glassware | Free of pyrogens by any suitable method (250 °C, 30 min) | Free of pyrogens by any suitable method (250 °C, 30 min) | Free of pyrogens | Thorough wash and heating in a hot-air oven (250 °C, 30 min or 200 °C, 1h) |
| Test material | Solution of the substance being examined | Either the product or the product treated as directed in the monograph | Solution of the substance being examined. When hypotonic, may be made isotonic. | Sterile solution of the substance being examined |
| Tested product amount | As specified in the monograph | As prescribed in the monograph | - | As prescribed in the monograph |
| Volume injected | 10 mL/kg (or as specified in the monograph) | 10 mL/kg (or as specified in the monograph) | 10 mL/kg (or as specified in the monograph) | 0.5 mL/kg–10 mL/kg |
| Test solution T | ≈ 38 °C | 37 °C ± 2 °C | 37 °C ± 2 °C | ≈ 38 °C |
| Injection site | Marginal vein of the ear | Ear vein | Marginal vein of the ear | Marginal vein of the ear |
| Injecting time | ≤ 4 min (or as specified in the monograph) | ≤ 10 min | ≤ 10 min | ≤ 4 min (or as specified in the monograph) |
| Measurement period, after injection | 3 h | 3 h | 3 h | 3 h |
| Measurement frequency | Continuously or every 30 min | Every 30 min between 1 and 3 h subsequent to the injection | ≤ 30 min | ≤ 30 min (starting at least 90 min before the injection) |
| Rabbit T rise (TR) = response | ▪ TR = Tmax − CT ▪ TR = 0 when Tmax < CT (Tmax = maximum T recorded after injection/rabbit) | ▪ TR = T − CT ▪ TR = 0 when T < CT (T = any T recorded after injection/rabbit) | ▪ TR = Tmax − CT ▪ TR = 0 when Tmax < CT (Tmax = maximum T recorded after injection/rabbit) | ▪ TR = Tmax − CT ▪ TR = 0 when Tmax < CT (Tmax = maximum T recorded after injection/rabbit) |
| Experimental | IP | USP | JP | EP |
|---|---|---|---|---|
| Number of rabbits extra-groups | 1 | 1 | Up to 2 | Up to 3 |
| Number of rabbits per extra-group | 5 | 5 | 3 | 3 |
| Case 1 and judgment | ▪ No individual TR ≥ 0.6 °C and Σ TR ≤ 1.4 °C ▪ Absence of pyrogens | ▪ No individual TR ≥ 0.5 °C ▪ Absence of pyrogens | ▪ Σ TR ≤ 1.3 °C ▪ Pyrogen-negative | ▪ Σ TR (n = 3, 6, 9 or 12) ≤ 1.15, 2.80, 4.45 or 6.60 (°C) respectively ▪ Product passes |
| Case 2 and judgment | ▪ 1 or 2 individual TR ≥ 0.6 °C or Σ TR (n = 3) > 1.4 °C ▪ Test 5 other rabbits | ▪ Any individual TR ≥ 0.5 °C ▪ Test 5 other rabbits | ▪ Σ TR > 2.5 °C ▪ Pyrogen-positive | ▪ Σ TR (n = 3, 6, 9 or 12) > 2.65, 4.30, 5.95 or 6.60 (°C) respectively ▪ Product fails |
| Case 3 and judgment | ▪ Not more than 3 of the TR (n = 8) ≥ 0.6 °C and Σ TR (n = 8) ≤ 3.7 °C ▪ Absence of pyrogens | ▪ Not more than 3 of the TR (n = 8) ≥ 0.5 °C and Σ TR (n = 8) ≤ 3.3 °C ▪ Absence of pyrogens | ▪ 1.3 °C < Σ TR < 2.5 °C ▪ Test 3 other rabbits | ▪ Σ TR does not meet neither case 1 nor case 2 ▪ Test 3 other rabbits up to 12 |
| Case 4 and judgment | - | - | ▪ Σ TR (n = 6) ≤ 3.0 °C ▪ Pyrogen-negative | - |
| Case 5 and judgment | - | - | ▪ Σ TR (n = 6) > 4.2 °C ▪ Pyrogen-positive | - |
| Case 6 and judgment | - | - | ▪ 3.0 °C < Σ TR (n = 6) < 4.2 °C ▪ Test 3 other rabbits | - |
| Case 7 and judgment | - | - | ▪ Σ TR (n = 9) ≤ 5.0 °C ▪ Pyrogen-negative | - |
| Case 8 and judgment | - | - | ▪ Σ TR (n = 9) > 5.0 °C ▪ Pyrogen-positive | - |
| Experimental | IP-USP-EP | JP |
|---|---|---|
| Gel-clot techniques: valid test conditions | The lowest concentration of the standard solutions shows a (-) result | When 0.25λ of the standard solution shows a (-) result |
| Photometric quantitative techniques: requirements | ▪ Sol. C comply assurance of criteria ▪ endotoxin recovery: 50–200% ▪ Sol. D: ≤ blank value of the lysate employed or < endotoxin detection limit | ▪ |r| of sol. C: ≥ 0.980 ▪ endotoxin recovery: 50–200% ▪ Sol. D: ≤ blank value of the lysate employed or < endotoxin detection limit |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, E.; Garcia-Recio, V.; Jiménez, P.; Garrosa, M.; Girbés, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Endotoxins from a Pharmacopoeial Point of View. Toxins 2018, 10, 331. https://doi.org/10.3390/toxins10080331
Franco E, Garcia-Recio V, Jiménez P, Garrosa M, Girbés T, Cordoba-Diaz M, Cordoba-Diaz D. Endotoxins from a Pharmacopoeial Point of View. Toxins. 2018; 10(8):331. https://doi.org/10.3390/toxins10080331
Chicago/Turabian StyleFranco, Elvira, Verónica Garcia-Recio, Pilar Jiménez, Manuel Garrosa, Tomás Girbés, Manuel Cordoba-Diaz, and Damián Cordoba-Diaz. 2018. "Endotoxins from a Pharmacopoeial Point of View" Toxins 10, no. 8: 331. https://doi.org/10.3390/toxins10080331
APA StyleFranco, E., Garcia-Recio, V., Jiménez, P., Garrosa, M., Girbés, T., Cordoba-Diaz, M., & Cordoba-Diaz, D. (2018). Endotoxins from a Pharmacopoeial Point of View. Toxins, 10(8), 331. https://doi.org/10.3390/toxins10080331






