A Systematic Literature Review for Evidence of Aphanizomenon flos-aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017
Abstract
1. Introduction
2. Results
2.1. Toxigenicity
2.1.1. Anatoxin-a
2.1.2. Cylindrospermopsin
2.1.3. Microcystins
2.1.4. Saxitoxin
2.2. Fresh Water
2.3. Dietary Supplements
3. Discussion
4. Conclusions
4.1. Recommendation to Post Educational Signs as a Precautionary Measure at First Sight of Visible Scum
4.2. Recommendation to Limit Harvesting of AFA to Months When Toxicity Is Lowest
4.3. Recommendation to Include AFA in Cell Counts during Visible Blooms
4.4. Recommendation to Reduce Health Advisory Guideline Value for Cyanotoxin Levels
4.5. Recommendations for Proper Species Identification Using 16S rRNA Methods When Toxicity Levels Are Higher than Advisory Levels
4.6. Recommendations for Laboratory-Based Research to Confirm the Ability of AFA to Produce Toxins under Differing Environmental Conditions
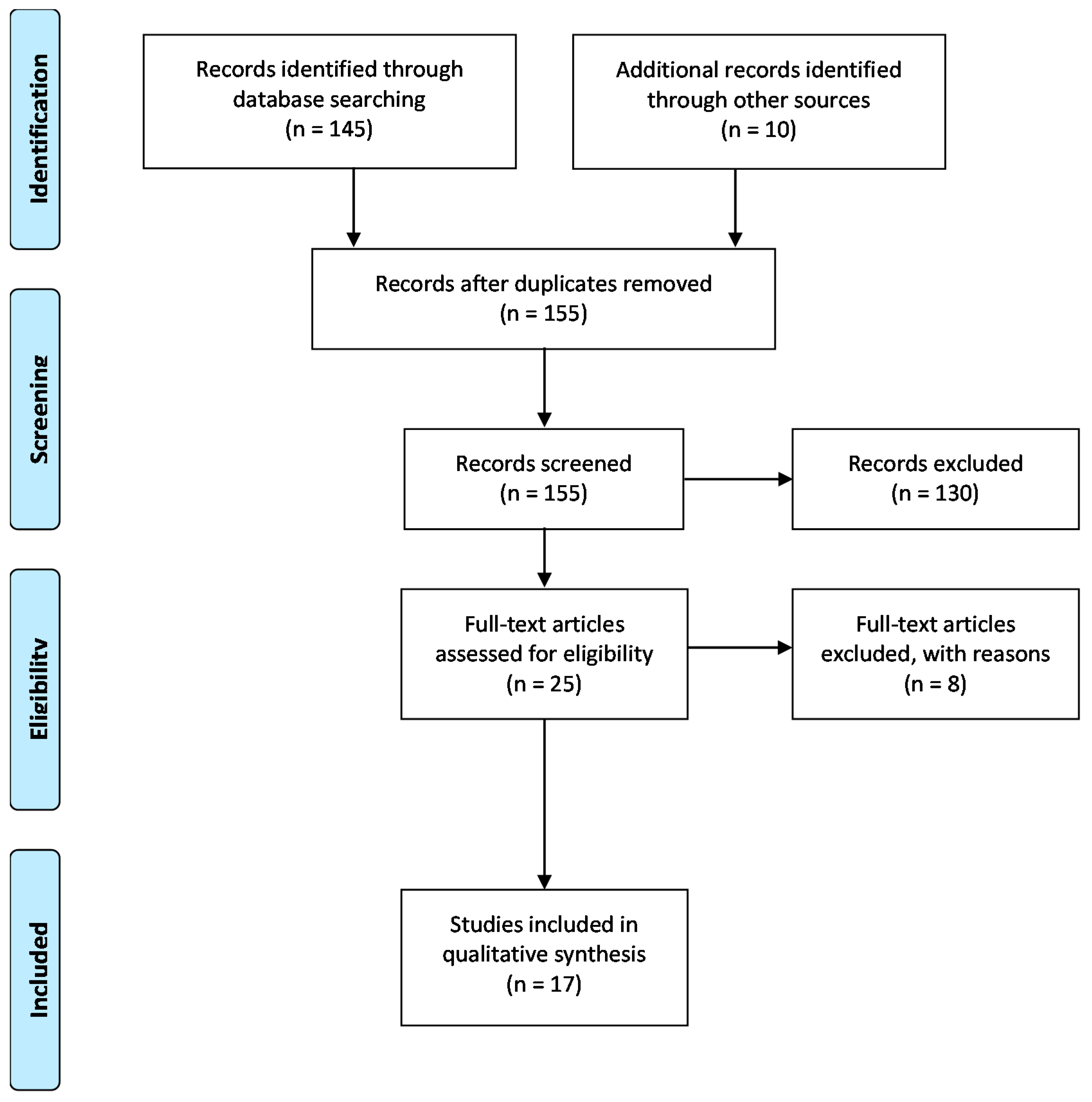
5. Materials and Methods
5.1. Search Strategy
5.2. Inclusion Criteria
5.3. Exclusion Criteria
5.4. Study Quality Assessment
Funding
Acknowledgments
Conflicts of Interest
References
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Health Effects Support Document for the Cyanobacterial Toxin Cylindrospermopsin. 2015. Available online: https://www.epa.gov/sites/production/files/2017-06/documents/cylindrospermopsin-support-report-2015.pdf (accessed on 10 November 2017).
- Mariani, M.A.; Padedda, B.M.; Kaštovský, J.; Buscarinu, P.; Sechi, N.; Virdis, T.; Lugliè, A. Effects of trophic status on microcystin production and the dominance of cyanobacteria in the phytoplankton assemblage of Mediterranean reservoirs. Sci. Rep. 2015, 5, 17964. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Contaminant Candidate List (CCL) and Regulatory Determination. 2016. Available online: https://www.epa.gov/ccl/contaminant-candidate-list-4-ccl-4-0 (accessed on 10 November 2017).
- World Health Organization (WHO). Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management 1999. Available online: http://www.who.int/water_sanitation_health/resourcesquality/toxcyanchap3.pdf (accessed on 1 October 2017).
- Wang, D.Z. Neurotoxins from Marine Dinoflagellates: A Brief Review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Vernocchi, P.; Bonizzi, L.; Carsetti, R.; Castellazzi, A.M.; Dallapiccola, B.; de Vos, W.; Guerzoni, M.E.; Manco, M.; Marseglia, G.L.; et al. Early-life gut microbiota under physiological and pathological conditions: The central role of combined meta-omics-based approaches. J. Proteom. 2012, 75, 4580–4587. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, W.; Li, D.; Shen, Y.; Li, G.; Liu, Y. First report of aphantoxins in China—Waterblooms of toxigenic Aphanizomenon flos-aquae in Lake Dianchi. Ecotoxicol. Environ. Saf. 2006, 65, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Bautista, A.C.; Moore, C.E.; Lin, Y.; Cline, M.G.; Benitah, N.; Puschner, B. Hepatopathy following consumption of a commercially available blue-green algae dietary supplement in a dog. BMC Vet. Res. 2015, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.J.; Kauffman, K.W.; Hall, R.A.; Huang, X.; Chu, F.S. Assessing potential health risks from microcystin toxins in blue-green algae dietary supplements. Environ. Health Perspect. 2000, 108, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Mazija, L.; Fastner, J.; Dietrich, D.R. Toxin content and cytotoxicity of algal dietary supplements. Toxicol. Appl. Pharmacol. 2012, 265, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; Seawright, A.A.; Shaw, G.R. Cyanobacterial poisoning in livestock, wild mammals and birds—An overview. Adv. Exp. Med. Biol. 2008, 619, 613–637. [Google Scholar] [PubMed]
- Watson, S.B.; Miller, C.; Arhonditsis, G.; Boyer, G.L.; Carmichael, W.; Charlton, M.N.; Confesor, R.; Depew, D.C.; Höök, T.O.; Ludsin, S.A.; et al. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae 2016, 56, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Cyanobacteria/Cyanotoxins, Nutrient Policy Data. 2017. Available online: https://www.epa.gov/nutrient-policy-data/cyanobacteriacyanotoxin (accessed on 1 October 2017).
- Cires, S.; Ballot, A. A review of the phylogeny, ecology and toxin production of bloom-forming Aphanizomenon spp. and related species within the Nostocales (cyanobacteria). Harmful Algae 2016, 54, 21–43. [Google Scholar] [PubMed]
- WHO. Cyanobacterial Toxins: Microcystin-LR in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2003. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/cyanobactoxins.pdf (accessed on 10 November 2017).
- Preussel, K.; Stüken, A.; Wiedner, C.; Chorus, I.; Fastner, J. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon 2006, 47, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Stuken, A.; Jakobsen, K.S. The cylindrospermopsin gene cluster of Aphanizomenon spp. strain 10E6: Organization and recombination. Microbiology 2010, 156, 2438–2451. [Google Scholar] [PubMed]
- Ballot, A.; Cerasino, L.; Hostyeva, V.; Cirés, S. Variability in the sxt Gene Clusters of PSP Toxin Producing Aphanizomenon gracile Strains from Norway, Spain, Germany and North America. PLoS ONE 2016, 11, e0167552. [Google Scholar] [CrossRef] [PubMed]
- Soto-Liebe, K.; López-Cortés, X.A.; Fuentes-Valdes, J.J.; Stucken, K.; Gonzalez-Nilo, F.; Vásquez, M. In silico analysis of putative paralytic shellfish poisoning toxins export proteins in cyanobacteria. PLoS ONE 2013, 8, e55664. [Google Scholar] [CrossRef]
- Botana, L.M. Marine toxins and the cytoskeleton. FEBS J. 2008, 275, 6059. [Google Scholar] [CrossRef] [PubMed]
- Fawell, J.K.; Mitchell, R.E.; Hill, R.E.; Everett, D.J. The toxicity of cyanobacterial toxins in the mouse: II anatoxin-a. Hum. Exp. Toxicol. 1999, 18, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.O.; Iwamoto, G.A.; Schaeffer, D.J.; Carmichael, W.W.; Beasley, V.R. Pathophysiologic effects of anatoxin-a(s) in anaesthetized rats: The influence of atropine and artificial respiration. Pharmacol. Toxicol. 1990, 67, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Skulberg, O.M.; Namikoshi, M.; Evans, W.R.; Carmichael, W.W.; Rinehart, K.L. Two methyl ester derivatives of microcystins, cyclic heptapeptide hepatotoxins, isolated from Anabaena flos-aquae strain CYA 83/1. Toxicon 1992, 30, 1465–1471. [Google Scholar] [CrossRef]
- Araoz, R.; Molgo, J.; de Marsac, N.T. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Drapeau, C.; Anderson, D.M. Harvesting of Aphanizomenon flos-aquae Ralfs ex Born. & Flah. var. flos-aquae (Cyanobacteria) from Klamath Lake for human dietary use. J. Appl. Phycol. 2000, 12, 585–595. [Google Scholar]
- Fastner, J.; Rücker, J.; Stüken, A.; Preußel, K.; Nixdorf, B.; Chorus, I.; Köhler, A.; Wiedner, C. Occurrence of the cyanobacterial toxin cylindrospermopsin in northeast Germany. Environ. Toxicol. 2007, 22, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.T.; Leao, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.I.; Ohtani, I.; Iwamoto, K.; Suzuki, M.; Watanabe, M.F.; Watanabe, M.; Terao, K. Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method. Toxicon 1994, 32, 73–84. [Google Scholar] [CrossRef]
- Ballot, A.; Ramm, J.; Rundberget, T.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Wiedner, C. Occurrence of non-cylindrospermopsin-producing Aphanizomenon ovalisporum and Anabaena bergii in Lake Kinneret (Israel). J. Plankton Res. 2011, 33, 1736–1746. [Google Scholar] [CrossRef]
- Li, R.; Carmichael, W.W.; Brittain, S.; Eaglesham, G.K.; Shaw, G.R.; Liu, Y.; Watanabe, M.M. First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Raphidiopsis curvata (cyanobacteria). J. Phycol. 2001, 37, 1121–1126. [Google Scholar] [CrossRef]
- Shaw, G.R.; Sukenik, A.; Livne, A.; Chiswell, R.K.; Smith, M.J.; Seawright, A.A.; Norris, R.L.; Eaglesham, G.K.; Moore, M.R. Blooms of the cylindrospermopsin containing cyanobacterium, Aphanizomenon ovalisporum (Forti), in newly constructed lakes, Queensland, Australia. Environ. Toxicol. 1999, 14, 167–177. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.T.; Xie, C.; Snider, B.B.; Wallace, G.A.; Weinreb, S.M.; Kuhlenkamp, J. In Vitro Hepatotoxicity of the Cyanobacterial Alkaloid Cylindrospermopsin and Related Synthetic Analogues. Toxicol. Sci. 2002, 67, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bazin, E.; Huet, S.; Jarry, G.; Hégarat, L.L.; Munday, J.S.; Humpage, A.R.; Fessard, V. Cytotoxic and genotoxic effects of cylindrospermopsin in mice treated by gavage or intraperitoneal injection. Environ. Toxicol. 2012, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A.R.; Falconer, I.R. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: Determination of no observed adverse effect level for deriving a drinking water guideline value. Environ. Toxicol. 2003, 18, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Meneely, J.P.; Elliott, C.T. Microcystins: Measuring human exposure and the impact on human health. Biomarkers 2013, 18, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Fawell, J.K.; Mitchell, R.E.; Everett, D.J.; Hill, R.E. The toxicity of cyanobacterial toxins in the mouse: I microcystin-LR. Hum. Exp. Toxicol. 1999, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Heinze, R. Toxicity of the cyanobacterial toxin microcystin-LR to rats after 28 days intake with the drinking water. Environ. Toxicol. 1999, 14, 57–60. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Malpas, P.B.; Barton, L.L. Risk assessment of microcystin in dietary Aphanizomenon flos-aquae. Ecotoxicol. Environ. Saf. 1999, 44, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion: Marine Biotoxins in Shellfish-Saxitoxin Group; EFSA: Parma, Italy, 2009; pp. 1–76.
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human exposure to cyanotoxins and their effects on health. Arh Hig Rada Toksikol 2013, 64, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Palus, J.; Dziubałtowska, E.; Stańczyk, M.; Lewińska, D.; Mankiewicz-Boczek, J.; Izydorczyk, K.; Bonisławska, A.; Jurczak, T.; Zalewski, M.; Wąsowicz, W. Biomonitoring of cyanobacterial blooms in Polish water reservoir and the cytotoxicity and genotoxicity of selected cyanobacterial extracts. Int. J. Occup. Med. Environ. Health 2007, 20, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Sulcius, S.; Pilkaitytė, R.; Mazur-Marzec, H.; Kasperovičienė, J.; Ezhova, E.; Błaszczyk, A.; Paškauskas, R. Increased risk of exposure to microcystins in the scum of the filamentous cyanobacterium Aphanizomenon flos-aquae accumulated on the western shoreline of the Curonian Lagoon. Mar. Pollut. Bull. 2015, 99, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Dadheech, P.K.; Selmeczy, G.B.; Vasas, G.; Padisák, J.; Arp, W.; Tapolczai, K.; Casper, P.; Krienitz, L. Presence of potential toxin-producing cyanobacteria in an oligo-mesotrophic lake in Baltic Lake District, Germany: An ecological, genetic and toxicological survey. Toxins 2014, 6, 2912–2931. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.; Carlisle, J.; Linville, R. Toxicological Summary and Suggested Action Levels to Reduce Potential Adverse Health Effects of Six Cyanotoxins; Office of Environmental Health Hazard Assessment, California Environmental Protection Agency: Sacramento, CA, USA, 2012; pp. 1–119.
- Ferreira, F.M.; Soler, J.M.F.; Fidalgo, M.L.; Fernández-Vila, P. PSP toxins from Aphanizomenon flos-aquae (cyanobacteria) collected in the Crestuma-Lever reservoir (Douro river, northern Portugal). Toxicon 2001, 39, 757–761. [Google Scholar] [CrossRef]
- Brient, L.; Lengronne, M.; Bormans, M.; Fastner, J. First occurrence of cylindrospermopsin in freshwater in France. Environ. Toxicol. 2009, 24, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.; Bakker, G.J. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.A.; Carmichael, W.W. Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon 1986, 24, 175–186. [Google Scholar] [CrossRef]
- Pereira, P.; Onodera, H.; Andrinolo, D.; Franca, S.; Araújo, F.; Lagos, N.; Oshima, Y. Paralytic shellfish toxins in the freshwater cyanobacterium Aphanizomenon flos-aquae, isolated from Montargil reservoir, Portugal. Toxicon 2000, 38, 1689–1702. [Google Scholar] [CrossRef]
- Gkelis, S.; Zaoutsos, N. Cyanotoxin occurrence and potentially toxin producing cyanobacteria in freshwaters of Greece: A multi-disciplinary approach. Toxicon 2014, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Farrer, D.; Counter, M.; Hillwig, R.; Cude, C. Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins 2015, 7, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Saker, M.L.; Welker, M.; Vasconcelos, V.M. Multiplex PCR for the detection of toxigenic cyanobacteria in dietary supplements produced for human consumption. Appl. Microbiol. Biotechnol. 2007, 73, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Draisci, R.; Ferretti, E.; Palleschi, L.; Marchiafava, C. Identification of anatoxins in blue-green algae food supplements using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2001, 18, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Rellan, S.; Osswald, J.; Saker, M.; Gago-Martinez, A.; Vasconcelos, V. First detection of anatoxin-a in human and animal dietary supplements containing cyanobacteria. Food Chem. Toxicol. 2009, 47, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Voyakina, E.; Babanazarova, O.; Romanov, R.; Kotovshchikov, A.; Mazur-Marzec, H. Dolichospermum and Aphanizomenon as neurotoxins producers in some Russian freshwaters. Toxicon 2017, 130, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mooney, K.M.; Hamilton, J.T.; Floyd, S.D.; Foy, R.H.; Elliott, C.T. Initial studies on the occurrence of cyanobacteria and microcystins in Irish lakes. Environ. Toxicol. 2011, 26, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Blahova, L.; Oravec, M.; Maršálek, B.; Šejnohova, L.; Šimek, Z.; Bláha, L. The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods. Toxicon 2009, 53, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Carmichael, W.W.; Pereira, P. Morphological and 16S rRNA Gene Evidence for Reclassification of the Paralytic Shellfish Toxin Producing Aphanizomenon Flos-Aquae LMECYA 31 as Aphanizomenon Issatschenkoi (Cyanophyceae). J. Phycol. 2003, 39, 814–818. [Google Scholar] [CrossRef]
- Rucker, J.; Stüken, A.; Nixdorf, B.; Fastner, J.; Chorus, I.; Wiedner, C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 2007, 50, 800–809. [Google Scholar] [CrossRef] [PubMed]
- EPA. Guidelines and Recommendations-Nutrient Policy Data, 2017. 2017. Available online: https://19january2017snapshot.epa.gov/nutrient-policy-data/guidelines-and-recommendations_.html (accessed on 1 October 2017).
- Catherine, Q.; Susanna, W.; Isidora, E.S.; Mark, H.; Aurelie, V.; Jean-François, H. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, C.; Wang, G.; Li, D.; Li, G.; Liu, Y. Zebrafish neurotoxicity from aphantoxins—Cyanobacterial paralytic shellfish poisons (PSPs) from Aphanizomenon flos-aquae DC-1. Environ. Toxicol. 2013, 28, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [PubMed]
| Lead Author (Year) | Location | Findings | Method of Detection | Conclusions |
|---|---|---|---|---|
| Roy-Lachapelle (2017) | Canada | Out of 18 products tested, 8 contained cyanotoxins at levels exceeding WHO’s TDI. Supplements containing AFA had MC concentrations between 0.8 and 8.2 μg/g. Low amounts of BMAA (neurotoxin) were also found. | Lemieux and Adda oxidation. Chemical derivatization, laser diode thermal desorption, and liquid chromatography. | Some dietary products could be harmful upon long-term consumption due to the presence of cyanotoxins. A critical need exists for better monitoring for all BGAS, and guidelines for maximum intake. |
| Chernova (2017) | Russia | Aphanizomenon flos-aquae found to produce ANTX in Sestroretskij Razliv Lake | PCR and LC-MS/MS and Restriction Fragment Length Polymorphism (RFLP) analysis | Both dominant species Aphanizomenon flos-aquae and Dolichospermum planctonicum are ANTX producers. |
| Cires (2016) | Global | Aphanizomenon spp. known toxin producer specifically: CYN (11–41% of total toxins, up to 58–63% under certain conditions), ANTX (7–47% of total toxins), and SXTs (7–35% of total toxins) | Literature Review. | Although Aphanizomenon spp. are known toxin producers, the toxigenicity of AFA is still uncertain. |
| Mariani (2015) | Sardinia | AFA and Aphanocapsa spp. dominated total cyanobacteria. | ELISA, Mass spectrometer. | Species composition during periods of maximum MC concentration differed from typical in other Mediterranean sites. |
| Sulcius (2015) | Lithuania/Russia | Concentrations of cyanotoxins in scum materials increased from ~30~300 fold compared to bloom samples. AFA comprised ~19% of total cyanobacteria biomass. The most common toxin-producing cyanobacteria from Curonian Lagoon belong to the genera of Aphanizomenon spp., Microcystis, and Planktothrix. | Microscopic, and chemical. Extraction of saxitoxins with 4 mM ammonium formate buffer and acetonitrile 2:3 ratio, Mass spectrometer, information dependent acquisition mode, and multiple reaction monitoring. | Larger concentrations of cyanotoxins were found in scum compared to blooms. |
| Dadheech (2014) | Germany | Although AFA dominated total phytoplankton at >80% contribution to total biomass, AFA did not show amplification for the mcyE gene, or STX and ANTX production. | Molecular analysis:16S rRNA sequencing, BLAST identification. | Differences seen in dominant taxon of field sample from Dolichospermum circinale in 2011 to AFA in 2012, with reduction in total MC content seen from 27.32 μg/L to 4.25 μg/L. |
| Gkelis (2014) | Greece | C. raciborskii and AFA are potential STX producers. A. gracile confirmed STX producer. MC: 3.9–108 μg/L, CYNs: 0.3–2.8 μg/L, and STXs: 0.4–1.2 μg/L Aphanizomenon spp. STX gene cluster may be biogeographically differentiated by county. | Microscopy, molecular, and immunologic methods: ELISA. | AFA was not found to be the dominant species in blooms, or a producer of toxins. Co-occurrence of more than one cyanotoxins in sites used for drinking water, agriculture, or recreation represent potential health risks. |
| Heussner (2012) | Germany | All AFA products tested positive for MCs and the mcyE gene. The contamination levels of the MC-positive samples were ≤1 μg MC-LR equivalents per g dry weight. | Colorimetric protein phosphatase inhibition assay (cPPIA), Adda-ELISA, Cell Culture, Liquid chromatography tandem mass spectrometry (LC-MS/MS), DNA extraction and PCR. | Recommendation for prohibition of marketing and sale of AFA-based dietary supplements in order to prevent acute and chronic exposure to MCs. |
| Mooney (2011) | Ireland | AFA, Gomaphosphaeria spp. and Microcystis aeruginosa were the most dominant cyanobacterial species associated with high MC concentration. AFA was dominant in 1/14 sites with lake area of 382 km2, and MC concentration of 1652 ng/μg Chla | Synoptic survey of 14 sites, used high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). | Further studies are recommended to use molecular detection methods to determine whether AFA is a MC producer. It is unknown which species produced the toxins, only recorded dominant cyanobacteria and total toxin per area. |
| Blahova (2009) | France | CYN was found at 3 localities with Aphanizomenon spp. sub-dominated water blooms. Concentrations determined by ELISA (0.4–4 μg/L) were systematically higher than concentrations determined by LC/MS (0.01–0.3 μg/L). | ELISA and LC/MS. | AFA is a potential producer of CYN. |
| Brient (2009) | Czech Republic | AFA var klebahnii found to be a potential producer of CYN. Intracellular concentrations of CYN ranged between 1.55 and 1.95 μg/L. | LC-MS/MS. | AFA is a potential producer of CYN. |
| Palus (2007) | Poland | AFA dominated blooms August–October. The concentration of MC in water did not exceed 1 μg/L, Cyanobacteria co-occurrence found with E. coli. | Protein phosphatase inhibition assay (PPIA), ELISA and HPLC. | Phytoplankton biomass and genotoxicity of CyanoHABs should be assessed to avoid public health issues. |
| Fastner (2007) | Germany | Concentrations reached up to 73.2 μg CYN/g dry weight. Study confirmed AFA is a CYN-producing species frequently inhabiting water bodies in temperate climatic regions. | Microscopy, Mass-spectrometer. | Aphanizomenon spp. may be an important CYN toxin producer in Germany waters. A world hazard analysis and risk assessment is recommended to confirm by geographic regions. |
| Saker (2007) | Australia and Canada | mcyA gene was detected in all 12 AFA dietary supplements, suggesting contamination by Microcystis spp. | Multiplex PCR. | Dietary supplements containing AFA are more at risk for contamination by Microcystis spp., and should be monitored. Laboratory and toxicological analysis of Upper Klamath Lake cyanobacteria would provide useful information to inform and protect human health. |
| Preussel (2006) | Germany | Toxin CYN detected in the range of 2.3–6.6 mg/g of cellular dry weight. | LC-MS/MS analysis and detection of PCR products of gene fragments. | First report of CYN in AFA strains. |
| Ferreira (2001) | Portugal | Presence of PSP toxins: GTX4, GTX1, GTX3, and Cs toxin present either in cells of AFA or in other toxic isolates. | High performance liquid chromatography (HPLC) using 2 isocratic elution systems. | AFA known STX producer, but A. circinalis is also found to be potential STX producer. More work is needed to understand the toxicological profiles of cyanobacteria. |
| Liu (2006) | China | STXs produced by AFA bloom. Significant glutathione-S-transferase (GST) and lactate dehydrogenase (LDH) increases, together with decrease of the glutathione (GSH) level, were measured. | High performance liquid chromatography with post-column fluorescence derivatization (HPLC-FLD) and liquid chromatographic mass spectrometry technique (LC-MS). | The results indicate a potential role of STXs intoxicating and metabolizing in test animals. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyon-Colbert, A.; Su, S.; Cude, C. A Systematic Literature Review for Evidence of Aphanizomenon flos-aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017. Toxins 2018, 10, 254. https://doi.org/10.3390/toxins10070254
Lyon-Colbert A, Su S, Cude C. A Systematic Literature Review for Evidence of Aphanizomenon flos-aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017. Toxins. 2018; 10(7):254. https://doi.org/10.3390/toxins10070254
Chicago/Turabian StyleLyon-Colbert, Amber, Shelley Su, and Curtis Cude. 2018. "A Systematic Literature Review for Evidence of Aphanizomenon flos-aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017" Toxins 10, no. 7: 254. https://doi.org/10.3390/toxins10070254
APA StyleLyon-Colbert, A., Su, S., & Cude, C. (2018). A Systematic Literature Review for Evidence of Aphanizomenon flos-aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017. Toxins, 10(7), 254. https://doi.org/10.3390/toxins10070254





