Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study
Abstract
1. Introduction
Objectives
2. Results
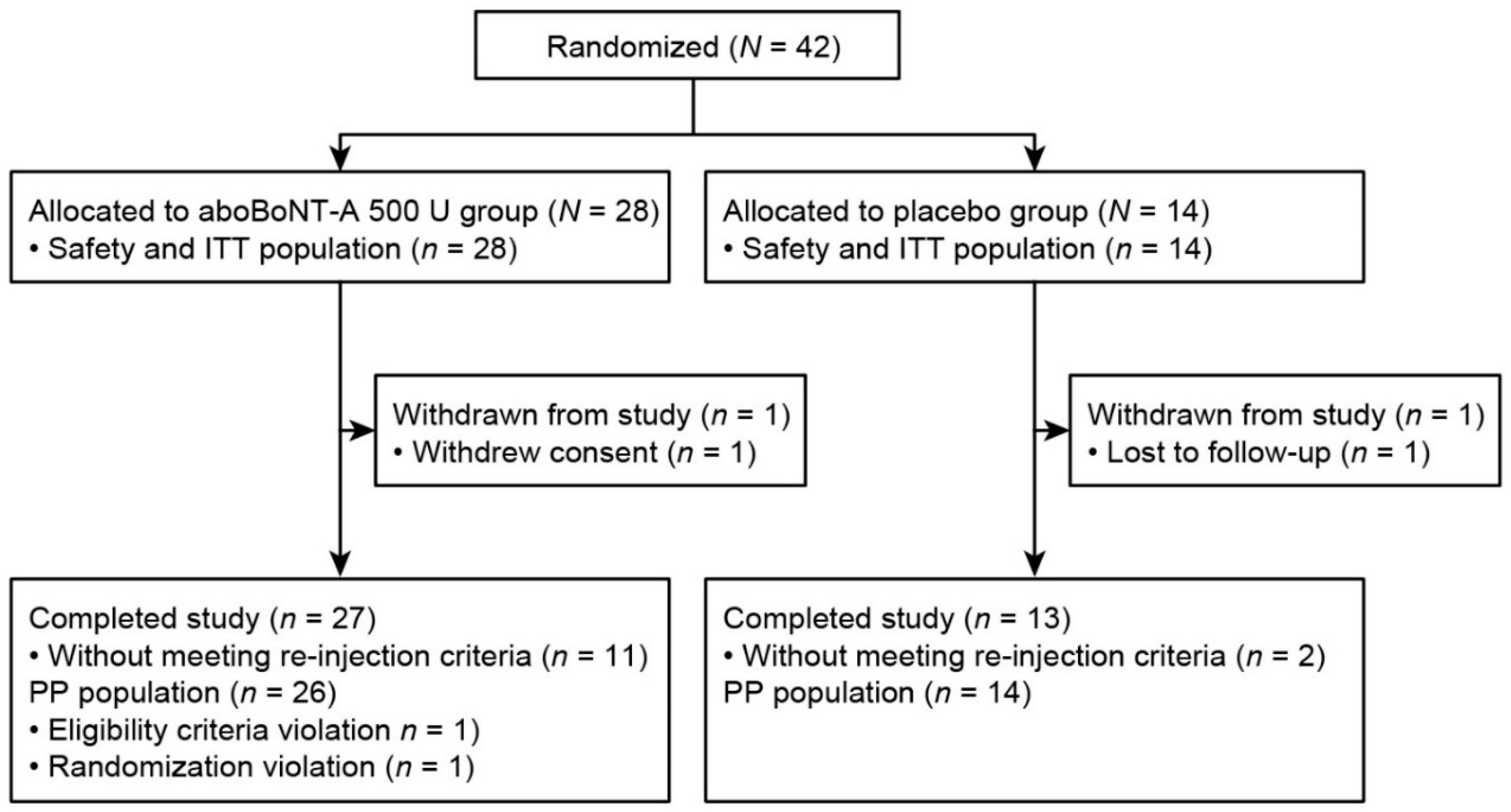
2.1. Baseline Characteristics and Patient Disposition
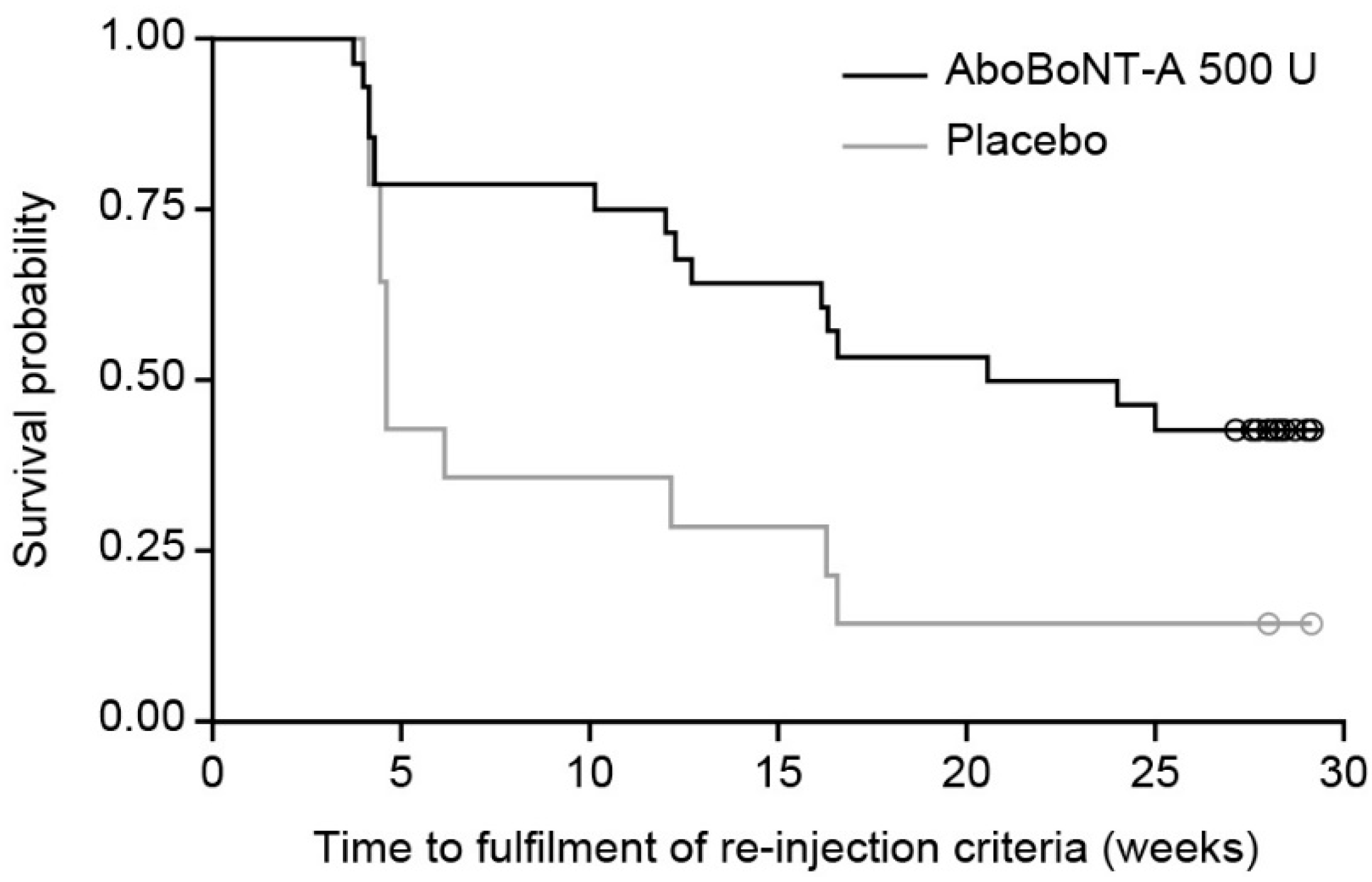
2.2. Time to Re-Injection
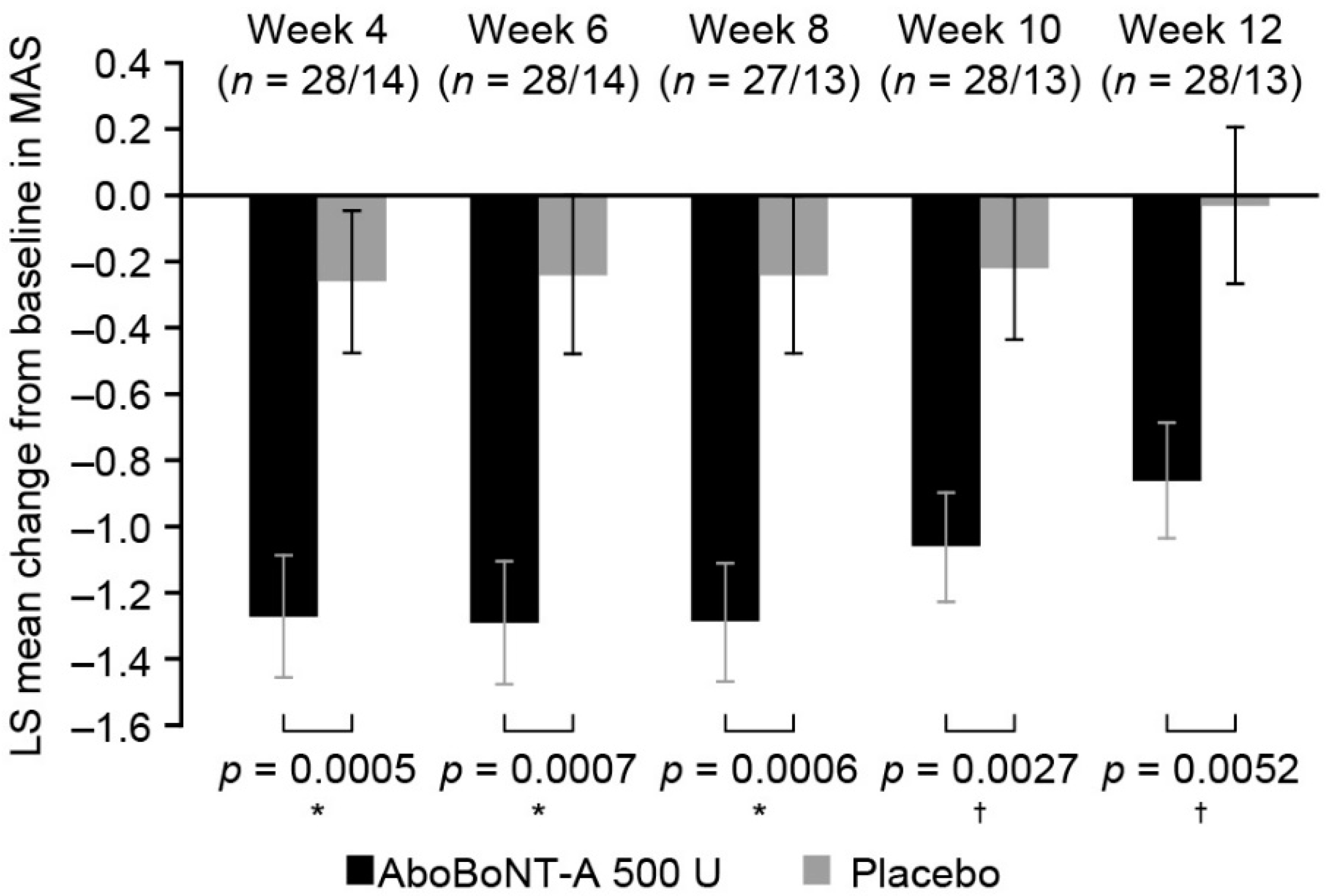
2.3. Assessment of Change in Muscle Tone
2.4. Assessment of Motor Function Recovery
2.5. Changes in Global Assessment
2.6. Concomitant Therapy
2.7. Safety Assessment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Primary Research Question
5.2. Study Design and Participants
- Impaired passive function (score ≥ 1 on a 4-point Likert scale: 0 = no impact, 1 = mild impact, 2 = moderate impact, 3 = severe impact).
- ○
- ‘In general, how much does spasticity impact the following activities of daily living and/or your rehabilitation program: hygiene (i.e., hand, nails, armpit, elbows), dressing the affected limb, positioning the affected limb, splint application or removal?’
- Impaired active function (score ≥ 1 on a 4-point Likert scale, as above).
- ○
- ‘In general, how much does spasticity impact the following activities of daily living and/or your rehabilitation program: reaching, grasping, releasing, gripping, holding, bimanual function, manipulating objects, dexterity, fine motor skills, lifting and carrying?’ [19]
- Presence of involuntary movements, which occur during standing up, walking, and transfers (if unable to stand up/walk) (score ≥ 1 on a 4-point Likert scale: 0 = no involuntary movements, 1 = involuntary movements with mild impact on posture and ambulation, 2 = involuntary movements with moderate impact on posture and ambulation, 3 = involuntary movements with severe impact on posture and ambulation).
- Pain (score ≥ 4 on the NPRS: 0 = no pain to 10 = severe disabling pain, with impacts on movement; score of 4 indicates moderate pain) [37].
- Question to each patient was oriented to obtain a relevant answer. Answers were spontaneous and not condition-dependent (e.g., active/passive movements or during night/day). A support could be used to help patients assess pain.
- ○
- Average pain intensity over 1 week was collected.
5.3. Recruitment and Randomization
5.4. Interventions
5.5. Efficacy Assessments
5.5.1. Primary Efficacy Assessment
5.5.2. Secondary Efficacy Assessments
5.6. Safety Assessments
5.7. Study Schedule
5.8. Statistical Analysis
5.9. Changes in Conduct of the Study and Planned Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosales, R.L. Botulinum Toxin Therapy Manual for Dystonia and Spasticity; Intech Open Access Publishers: Rijeka, Croatia, 2016. [Google Scholar]
- Doan, Q.V.; Brashear, A.; Gillard, P.J.; Varon, S.F.; Vandenburgh, A.M.; Turkel, C.C.; Elovic, E.P. Relationship between disability and health-related quality of life and caregiver burden in patients with upper limb poststroke spasticity. PM R 2012, 4, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zorowitz, R.D.; Gillard, P.J.; Brainin, M. Poststroke spasticity: Sequelae and burden on stroke survivors and caregivers. Neurology 2013, 80, S45–S52. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A. The human and economic burden of poststroke spasticity and muscle overactivity. JCOM 2011, 18, 607–614. [Google Scholar]
- Lundström, E.; Smits, A.; Borg, J.; Terént, A. Four-fold increase in direct costs of stroke survivors with spasticity compared with stroke survivors without spasticity. Stroke 2010, 41, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Chua, K.S.; Lee, J. Symptomatic upper limb spasticity in patients with chronic stroke attending a rehabilitation clinic: Frequency, clinical correlates and predictors. J. Rehabilit. Med. 2010, 42, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Lee, J.; Chua, K.S. Occurrence and temporal evolution of upper limb spasticity in stroke patients admitted to a rehabilitation unit. Arch. Phys. Med. Rehabilit. 2012, 93, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Leathley, M.J.; Gregson, J.M.; Moore, A.P.; Smith, T.L.; Sharma, A.K. Prevalence of spasticity post stroke. Clin. Rehabilit. 2002, 16, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Leathley, M.J.; Gregson, J.M.; Moore, A.P.; Smith, T.L.; Sharma, A.K.; Watkins, C.L. Predicting spasticity after stroke in those surviving to 12 months. Clin. Rehabilit. 2004, 18, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Manack, A.; Brainin, M. Toward an epidemiology of poststroke spasticity. Neurology 2013, 80, S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Welmer, A.K.; Widen Holmqvist, L.; Sommerfeld, D.K. Location and severity of spasticity in the first 1–2 weeks and at 3 and 18 months after stroke. Eur. J. Neurol. 2010, 17, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Kong, K.H.; Goh, K.J.; Kumthornthip, W.; Mok, V.C.; Delgado-De Los Santos, M.M.; Chua, K.S.; Abdullah, S.J.; Zakine, B.; Maisonobe, P.; et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: A randomized controlled trial. Neurorehabilit. Neural Repair 2012, 26, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Sheean, G.; Lannin, N.A.; Turner-Stokes, L.; Rawicki, B.; Snow, B.J.; Cerebral Palsy Institute. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: International consensus statement. Eur. J. Neurol. 2010, 17, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Ward, A.B.; Erztgaard, P.; Bensmail, D.; Hecht, M.J.; Lejeune, T.M.; Schnider, P.; Altavista, M.C.; Cavazza, S.; Deltombe, T.; et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J. Rehabilit. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians; British Society of Rehabilitation Medicine; Chartered Society of Physiotherapy. Association of Chartered Physiotherapists Interested in Neurology. In Spasticity in Adults: Management Using Botulinum Toxin; National guidelines, Royal College of Physicians: London, UK, 2009. [Google Scholar]
- Rosales, R.L.; Efendy, F.; Teleg, E.S.; Delos Santos, M.M.; Rosales, M.C.; Ostrea, M.; Tanglao, M.J.; Ng, A.R. Botulinum toxin as early intervention for spasticity after stroke or non-progressive brain lesion: A meta-analysis. J. Neurol. Sci. 2016, 371, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ashford, S.; Fheodoroff, K.; Jacinto, J.; Turner-Stokes, L. Common goal areas in the treatment of upper limb spasticity: A multicentre analysis. Clin. Rehabilit. 2016, 30, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Brashear, A.; Marciniak, C.; Jech, R.; Banach, M.; Marque, P.; Grandoulier, A.S.; Vilain, C.; Picaut, P. Duration of effect of abobotulinumtoxinA (Dysport) in adult patients with upper limb spasticity (ULS) post stroke or traumatic brain injury. Toxicon 2016, 123, S35. [Google Scholar] [CrossRef]
- Gracies, J.M.; O’Dell, M.; Vecchio, M.; Hedera, P.; Kocer, S.; Rudzinska-Bar, M.; Rubin, B.; Timerbaeva, S.L.; Lusakowska, A.; Boyer, F.C.; et al. Effects of repeated abobotulinumtoxinA injections in upper limb spasticity. Muscle Nerve 2018, 57, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Fheodoroff, K.; Jacinto, J.; Geurts, A.; Molteni, F.; Franco, J.H.; Santiago, T.; Rosales, R.; Gracies, J.M. How can we improve current practice in spastic paresis? Eur. Neurol. Rev. 2016, 11, 79–86. [Google Scholar] [CrossRef]
- Rosales, R.L.; Kanovsky, P.; Fernandez, H.H. What’s the “catch” in upper-limb post-stroke spasticity: Expanding the role of botulinum toxin applications. Parkinsonism Relat. Disord. 2011, 17, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L. Dystonia, spasticity and botulinum toxin therapy: Rationale, evidences and clinical context. In Dystonia—The Many Facets; Rosales, R.L., Ed.; InTech: Chicago, IL, USA, 2012. [Google Scholar] [CrossRef]
- Veverka, T.; Hlustik, P.; Hok, P.; Otruba, P.; Tudos, Z.; Zapletalova, J.; Krobot, A.; Kanovsky, P. Cortical activity modulation by botulinum toxin type A in patients with post-stroke arm spasticity: Real and imagined hand movement. J. Neurol. Sci. 2014, 346, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Mach, H.; Frohlich, S.; Behrend, S.; Werner, C.; Melzer, I. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling finger flexor stiffness six months later: A randomized controlled trial. Clin. Rehabilit. 2012, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; Harvey, L.A.; Rawicki, B. Rehabilitation therapies after botulinum toxin-A injection to manage limb spasticity: A systematic review. Phys. Ther. 2014, 94, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.B.; Finlayson, H.; Sudol, M.; O’Connor, R. Systematic review of adjunct therapies to improve outcomes following botulinum toxin injection for treatment of limb spasticity. Clin. Rehabilit. 2016, 30, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R. Botulinum toxin therapy as an early intervention for post-stroke spasticity: Beyond a functional viewpoint. J. Neurol. Sci. 2017, 382, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Noyes, K.; Weinstock-Guttman, B. Impact of diagnosis and early treatment on the course of multiple sclerosis. Am. J. Manag. Care 2013, 19, s321–s331. [Google Scholar] [PubMed]
- Dressler, D.; Bhidayasiri, R.; Bohlega, S.; Chahidi, A.; Chung, T.M.; Ebke, M.; Jacinto, L.J.; Kaji, R.; Kocer, S.; Kanovsky, P.; et al. Botulinum toxin therapy for treatment of spasticity in multiple sclerosis: Review and recommendations of the IAB-Interdisciplinary Working Group for Movement Disorders task force. J. Neurol. 2017, 264, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, B.B.; Cozens, J.A.; Chamberlain, M.A.; Bamford, J.M. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: A randomised double blind placebo controlled trial. J. Neurol. Neurosurg. Psychiatr. 2000, 69, 217–221. [Google Scholar] [CrossRef]
- Simpson, D.M.; Gracies, J.M.; Graham, H.K.; Miyasaki, J.M.; Naumann, M.; Russman, B.; Simpson, L.L.; So, Y. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.; Simpson, J.; Ispoglou, S.; Sturman, S.G.; Pandyan, A.D. The early use of botulinum toxin in post-stroke spasticity: Study protocol for a randomised controlled trial. Trials 2014, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Balcaitiene, J.; Berard, H.; Maisonobe, P.; Goh, K.J.; Kumthornthip, W.; Rosales, R.L. Effect of early use of AbobotulinumtoxinA after stroke on spasticity progression: Protocol for a randomised controlled pilot study in adult subjects with moderate to severe upper limb spasticity (ONTIME pilot). Contempor. Clin. Trials Commun. 2017, 6, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, M.; Beebe, A. Pain: Clinical Manual for Nursing Practice; Mosby: St. Louis, MO, USA, 1989; p. 353. [Google Scholar]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabilit. Med. 1975, 7, 13–31. [Google Scholar]
| Results, n (%) | AbobotulinumtoxinA 500 U (N = 28) | Placebo (N = 14) | ||||
|---|---|---|---|---|---|---|
| Symptomatic Spasticity (n = 16) | Asymptomatic Spasticity (n = 6) | All (n = 22) | Symptomatic Spasticity (n = 4) | Asymptomatic Spasticity (n = 2) | All (n = 6) | |
| Much better | 1 (6.3) | 1 (16.7) | 2 (9.1) | 0 | 0 | 0 |
| Better | 14 (87.5) | 4 (66.7) | 18 (81.8) | 3 (75.0) | 2 (100.0) | 5 (83.3) |
| No change | 0 | 1 (16.7) | 1 (4.5) | 1 (25.0) | 0 | 1 (16.7) |
| Worse | 1 (6.3) | 0 | 1 (4.5) | 0 | 0 | 0 |
| Much worse | 0 | 0 | 0 | 0 | 0 | 0 |
| Cochran-Mantel-Haenszel p-value = 0.6128 | ||||||
| AbobotulinumtoxinA 500 U (N = 28) | Placebo (N = 14) | All patients (N = 42) | |
| Any adverse events | 8 (28.6), 17 | 4 (28.6), 6 | 12 (28.6), 23 |
| Any serious adverse events | 3 (10.7), 4 | 0 | 3 (7.1), 4 |
| Any TEAEs | 7 (25.0), 16 | 4 (28.6), 6 | 11 (26.2), 22 |
| Intensity of TEAEs | |||
| Severe | 1 (3.6), 2 | 0 | 1 (2.4), 2 |
| Moderate | 6 (21.4), 11 | 1 (7.1), 1 | 7 (16.7), 12 |
| Mild | 3 (10.7), 3 | 3 (21.4), 5 | 6 (14.3), 8 |
| Related TEAEs | 0 | 0 | 0 |
| Reported TEAEs: | |||
| Head injury | 2 (7.1), 2 | 0 | 2 (4.8), 2 |
| Insomnia | 2 (7.1), 2 | 0 | 2 (4.8), 2 |
| Fall | 1 (3.6), 1 | 1 (7.1), 1 | 2 (4.8), 2 |
| Urinary tract infection | 1 (3.6), 1 | 1 (7.1), 2 | 2 (4.8), 3 |
| Asthma | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Constipation | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Cough | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Hypertensive crisis | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Hypokalemia | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Pain | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Pneumonia | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Pyrexia | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Tachycardia | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Vomiting | 1 (3.6), 1 | 0 | 1 (2.4), 1 |
| Dizziness | 0 | 1 (7.1), 1 | 1 (2.4), 1 |
| Epistaxis | 0 | 1 (7.1), 1 | 1 (2.4), 1 |
| Neuralgia | 0 | 1 (7.1), 1 | 1 (2.4), 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales, R.L.; Balcaitiene, J.; Berard, H.; Maisonobe, P.; Goh, K.J.; Kumthornthip, W.; Mazlan, M.; Latif, L.A.; Delos Santos, M.M.D.; Chotiyarnwong, C.; et al. Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study. Toxins 2018, 10, 253. https://doi.org/10.3390/toxins10070253
Rosales RL, Balcaitiene J, Berard H, Maisonobe P, Goh KJ, Kumthornthip W, Mazlan M, Latif LA, Delos Santos MMD, Chotiyarnwong C, et al. Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study. Toxins. 2018; 10(7):253. https://doi.org/10.3390/toxins10070253
Chicago/Turabian StyleRosales, Raymond L, Jovita Balcaitiene, Hugues Berard, Pascal Maisonobe, Khean Jin Goh, Witsanu Kumthornthip, Mazlina Mazlan, Lydia Abdul Latif, Mary Mildred D. Delos Santos, Chayaporn Chotiyarnwong, and et al. 2018. "Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study" Toxins 10, no. 7: 253. https://doi.org/10.3390/toxins10070253
APA StyleRosales, R. L., Balcaitiene, J., Berard, H., Maisonobe, P., Goh, K. J., Kumthornthip, W., Mazlan, M., Latif, L. A., Delos Santos, M. M. D., Chotiyarnwong, C., Tanvijit, P., Nuez, O., & Kong, K. H. (2018). Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study. Toxins, 10(7), 253. https://doi.org/10.3390/toxins10070253





