Further Understanding of Degradation Pathways of Microcystin-LR by an Indigenous Sphingopyxis sp. in Environmentally Relevant Pollution Concentrations
Abstract
1. Introduction
2. Results
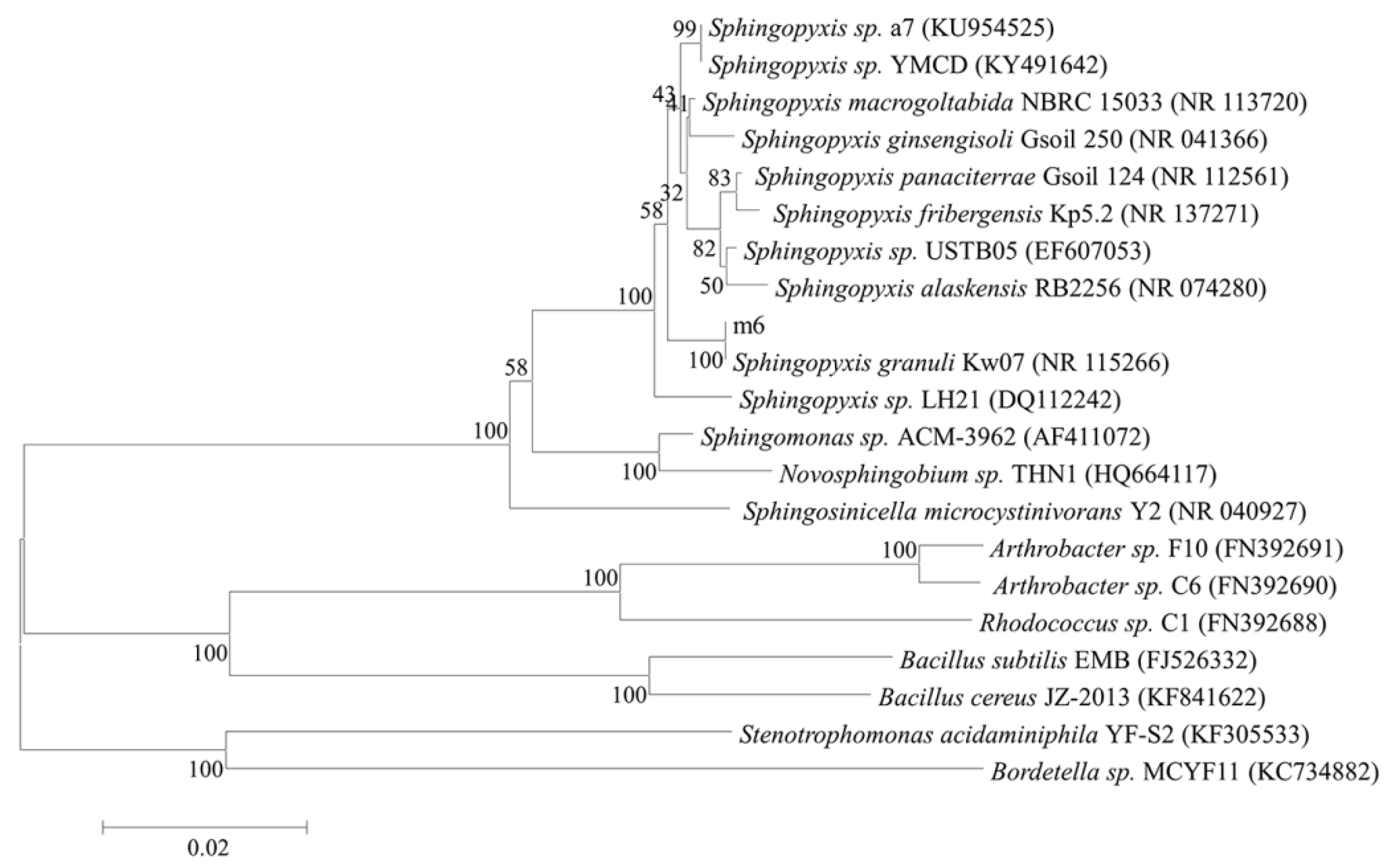
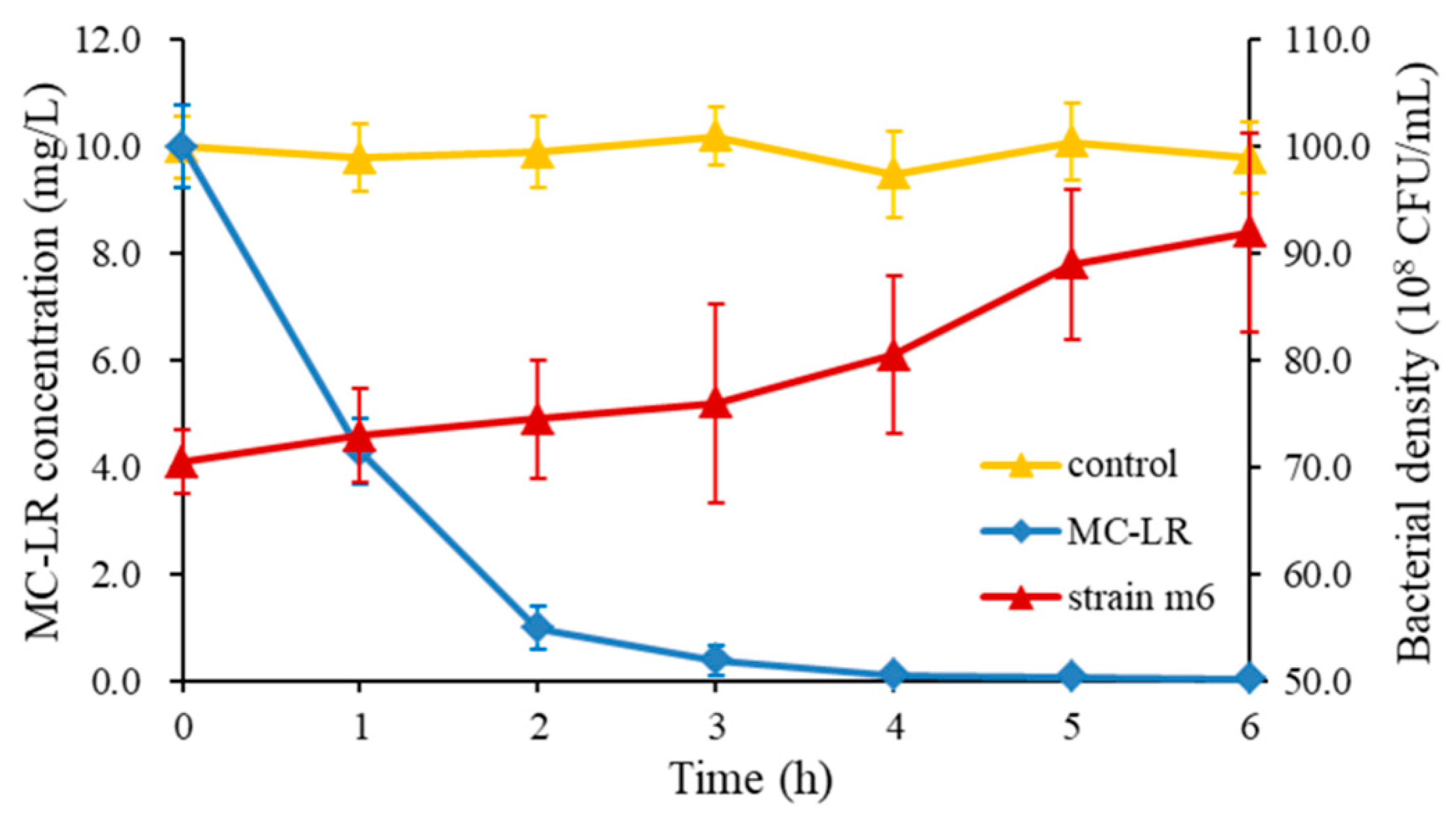
2.1. Bacterial Identification and the Maximum MC- LR Degrading Capability
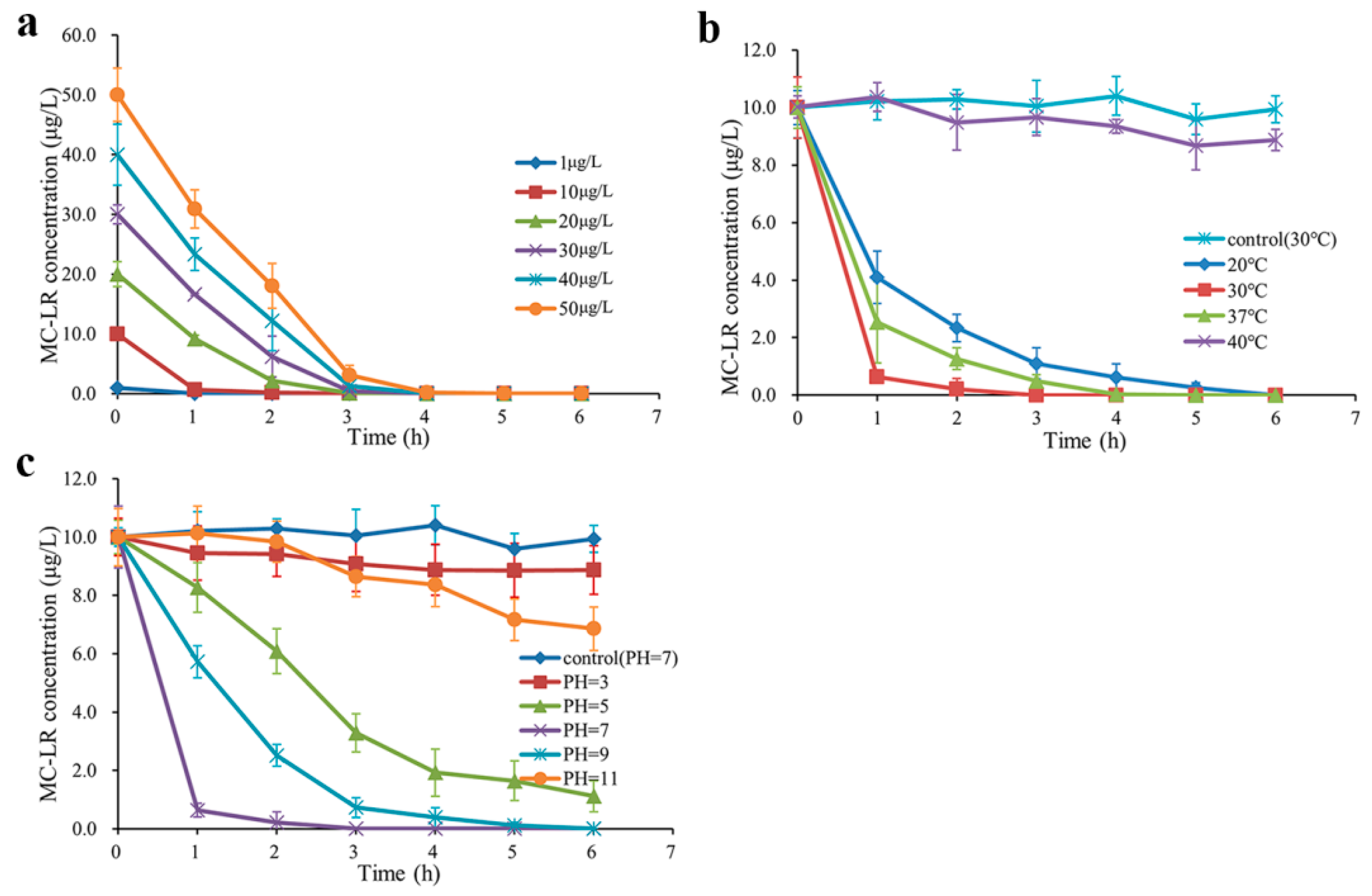
2.2. MC-LR Degrading Activities in Environmentally Relevant Pollution Concentrations under Various Conditions
2.3. Detection of Degradation Products of MC-LR
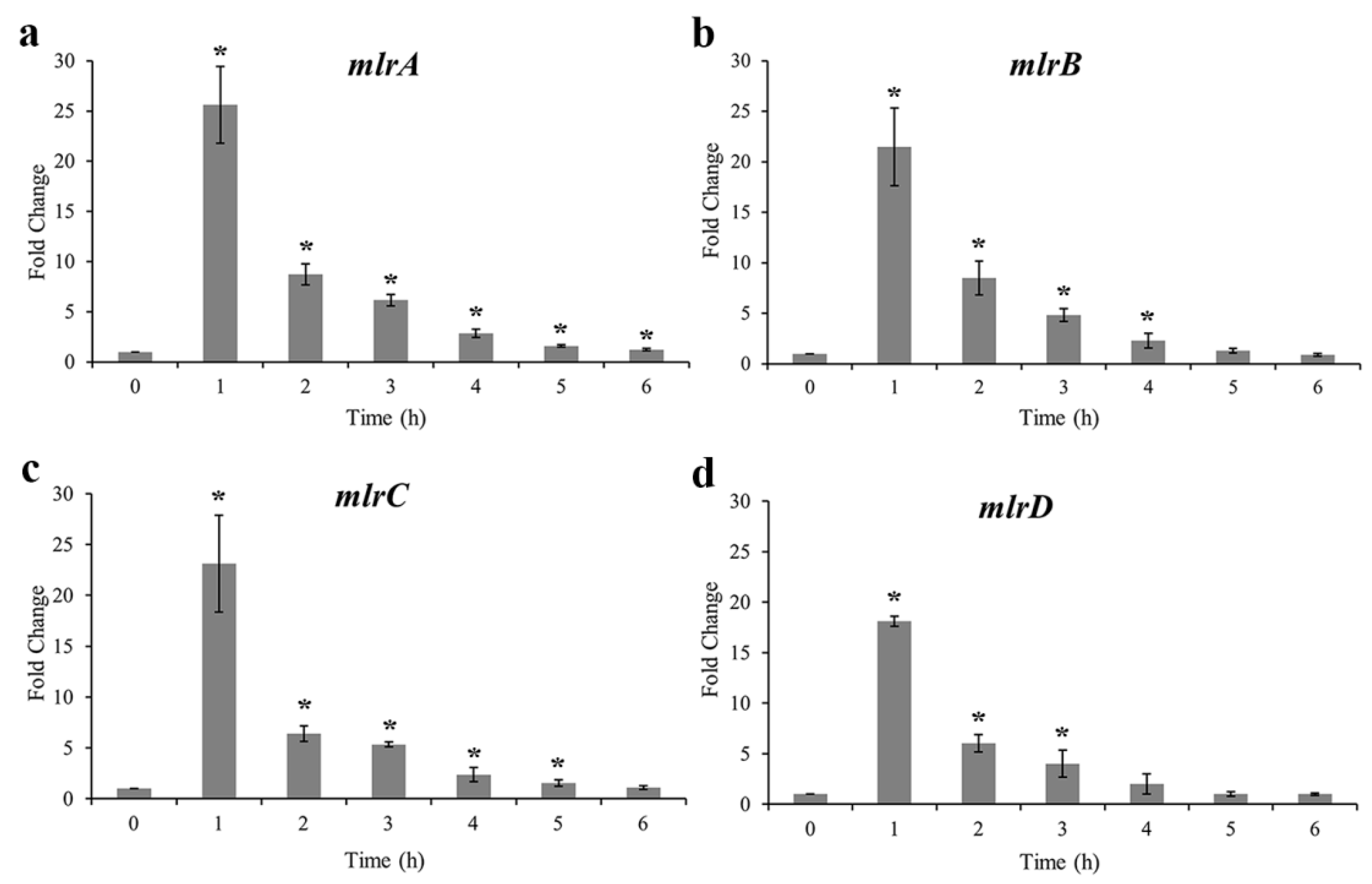
2.4. MC-Degrading Genes and Their Expression Profiles
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Standard Toxin and Reagents
5.2. Acquisition of a Functional Bacterium and Evaluation of MC-LR Degrading Capability
5.3. MC-LR Degradation Experiments under Environmentally Relevant Pollution Concentrations
5.4. Determination of MC-LR and Degradation Products
5.5. Detection of MC-Degrading Genes and Analysis of Their Expression Profiles
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Paerl, H.W.; Otten, T.G. Environmental science. Blooms bite the hand that feeds them. Science 2013, 342, 433–434. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.D.; Tyler, A.N.; Gilvear, D.J.; Willby, N.J. Using remote sensing to aid the assessment of human health risks from blooms of potentially toxic cyanobacteria. Environ. Sci. Technol. 2009, 43, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Sandvik, M.; Haande, S.; Nonga, H.; Ballot, A. LC-MS analysis with thiol derivatization to differentiate [dhb(7)]- from [mdha(7)]-microcystins: Analysis of cyanobacterial blooms, planktothrix cultures and european crayfish from lake steinsfjorden, norway. Environ. Sci. Technol. 2013, 47, 4080–4087. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-lr is a potent and specific inhibitor of protein phosphatase-1 and phosphatase-2a from both mammals and higher-plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Gurbuz, F.; Uzunmehmetoglu, O.Y.; Diler, O.; Metcalf, J.S.; Codd, G.A. Occurrence of microcystins in water, bloom, sediment and fish from a public water supply. Sci. Total Environ. 2016, 562, 860–868. [Google Scholar] [CrossRef]
- Humpage, A.R.; Hardy, S.J.; Moore, E.J.; Froscio, S.M.; Falconer, I.R. Microcystins (cyanobacterial toxins) in drinking water enhance the growth of aberrant crypt foci in the mouse colon. J. Toxicol. Environ. Health A 2000, 61, 155–165. [Google Scholar]
- Trout-Haney, J.V.; Wood, Z.T.; Cottingham, K.L. Presence of the cyanotoxin microcystin in arctic lakes of southwestern greenland. Toxins 2016, 8, 256. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, P.; Liu, Y.; Qiu, T. Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in lake taihu, china, with potential risks to human health. Sci. Total Environ. 2009, 407, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Shen, L.; Zhao, M.; Li, W.; Jia, C.; Zhu, H.; Zhang, Q. Determination of the role of microcystis aeruginosa in toxin generation based on phosphoproteomic profiles. Toxins 2018, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, S.; Kato, H.; Mizuno, M.; Tsuji, K.; Harada, K. Bacterial degradation of microcystins and nodularin. Chem. Res. Toxicol. 2005, 18, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Sano, T.; Kubota, R.; Kobayashi, N.; Tahara, M.; Obama, T.; Sugimoto, N.; Nishimura, T.; Ikarashi, Y. Effects of the amino acid constituents of microcystin variants on cytotoxicity to primary cultured rat hepatocytes. Toxins 2013, 6, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Massey, I.Y.; Zhang, X.; Yang, F. Importance of bacterial biodegradation and detoxification processes of microcystins for environmental health. J. Toxicol. Environ. Health B Crit. Rev. 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Lisznianska, M.; Wladyka, B. Bioreactor study employing bacteria with enhanced activity toward cyanobacterial toxins microcystins. Toxins 2014, 6, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Hegde, K.; Brar, S.K.; Cledon, M.; Kermanshahi-Pour, A.; Roy-Lachapelle, A.; Galvez-Cloutier, R. Biodegradation of microcystin-lr using acclimatized bacteria isolated from different units of the drinking water treatment plant. Environ. Pollut. 2018, 242, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, G. Simultaneous removal of harmful algal blooms and microcystins using microorganism- and chitosan-modified local soil. Environ. Sci. Technol. 2015, 49, 6249–6256. [Google Scholar] [CrossRef]
- Dziga, D.; Wasylewski, M.; Wladyka, B.; Nybom, S.; Meriluoto, J. Microbial degradation of microcystins. Chem. Res. Toxicol. 2013, 26, 841–852. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Q.; Ding, Q.; Pu, Y. A novel and native microcystin-degrading bacterium of sphingopyxis sp. Isolated from lake taihu. Inter. J. Environ. Res. Public Health 2017, 14, 1187. [Google Scholar] [CrossRef] [PubMed]
- Somdee, T.; Thunders, M.; Ruck, J.; Lys, I.; Allison, M.; Page, R. Degradation of [dha(7)]mc-lr by a microcystin degrading bacterium isolated from lake rotoiti, new zealand. ISRN Microbiol. 2013, 2013, 596429. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhang, Y.; Xu, H.; Zhu, G.; Qin, B.; Huang, C.; Liu, X.; Zhou, Y.; Lv, H. Long-term satellite observations of microcystin concentrations in lake taihu during cyanobacterial bloom periods. Environ. Sci. Technol. 2015, 49, 6448–6456. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, L.; Peng, L.; Wan, N.; Zhang, X.; Gan, N. Reduction in microcystin concentrations in large and shallow lakes: Water and sediment-interface contributions. Water Res. 2008, 42, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Major, Y.; Kifle, D.; Spoof, L.; Meriluoto, J. Cyanobacteria and microcystins in koka reservoir (ethiopia). Environ. Sci. Pollut. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.G.; Jones, G.J.; Blakeley, R.L.; Jones, A.; Negri, A.P.; Riddles, P. Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin lr. Appl. Environ. Microbiol. 1996, 62, 4086–4094. [Google Scholar]
- Edwards, C.; Graham, D.; Fowler, N.; Lawton, L.A. Biodegradation of microcystins and nodularin in freshwaters. Chemosphere 2008, 73, 1315–1321. [Google Scholar] [CrossRef]
- Valeria, A.M.; Ricardo, E.J.; Stephan, P.; Alberto, W.D. Degradation of microcystin-rr by sphingomonas sp. Cba4 isolated from san roque reservoir (cordoba—argentina). Biodegradation 2006, 17, 447–455. [Google Scholar] [CrossRef]
- Dziga, D.; Maksylewicz, A.; Maroszek, M.; Budzynska, A.; Napiorkowska-Krzebietke, A.; Toporowska, M.; Grabowska, M.; Kozak, A.; Rosinska, J.; Meriluoto, J. The biodegradation of microcystins in temperate freshwater bodies with previous cyanobacterial history. Ecotoxicol. Environ. Saf. 2017, 145, 420–430. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, G.; Yan, H. Microbial biodegradation of microcystin-rr by bacterium sphingopyxis sp. Ustb-05. J. Environ. Sci. 2010, 22, 168–175. [Google Scholar] [CrossRef]
- Dziga, D.; Zielinska, G.; Wladyka, B.; Bochenska, O.; Maksylewicz, A.; Strzalka, W.; Meriluoto, J. Characterization of enzymatic activity of mlrb and mlrc proteins involved in bacterial degradation of cyanotoxins microcystins. Toxins 2016, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, E.H.; Kato, H.; Kawasaki, Y.; Nozawa, Y.; Tsuji, K.; Hirooka, E.Y.; Harada, K.-i. Further investigation of microbial degradation of microcystin using the advanced marfey method. Chem. Res. Toxicol. 2009, 22, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.G.; Blakeley, R.L.; Riddles, P.; Jones, G.J. Biodegradation of the cyanobacterial toxin microcystin lr in natural water and biologically active slow sand filters. Water Res. 2006, 40, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Watanabe, M.F. Microcystin lr degradation by pseudomonas aeruginosa alkaline protease. Chemosphere 1997, 34, 749–757. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Azam, S.; Lakshman, S.A.; Narasimha, R.V.; Gudla, R.; Parapatla, H.; Yakkala, H.; Ghanta, V.S.; Siddavattam, D. Genome-guided insights reveal organophosphate-degrading brevundimonas diminuta as sphingopyxis wildii and define its versatile metabolic capabilities and environmental adaptations. Genome Biol. Evol. 2017, 9, 77–81. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, I.; Pérez-Pulido, A.J.; González-Flores, Y.E.; Reyes-Ramírez, F.; Santero, E.; Floriano, B. Genomic analysis of the nitrate-respiring sphingopyxis granuli (formerly sphingomonas macrogoltabida) strain tfa. BMC Genomics 2016, 17. [Google Scholar] [CrossRef]
- Oelschlagel, M.; Zimmerling, J.; Schlomann, M.; Tischler, D. Styrene oxide isomerase of sphingopyxis sp. Kp5.2. Microbiology 2014, 160, 2481–2491. [Google Scholar] [CrossRef]
- Williams, T.J.; Ertan, H.; Ting, L.; Cavicchioli, R. Carbon and nitrogen substrate utilization in the marine bacterium sphingopyxis alaskensis strain rb2256. ISME J. 2009, 3, 1036–1052. [Google Scholar] [CrossRef]
- Jones, G.J.; Orr, P.T. Release and degradation of microcystin following algicide treatment of a microcystis-aeruginosa bloom in a recreational lake, as determined by hplc and protein phosphatase inhibition assay. Water Res. 1994, 28, 871–876. [Google Scholar] [CrossRef]
- Ho, L.; Meyn, T.; Keegan, A.; Hoefel, D.; Brookes, J.; Saint, C.P.; Newcombe, G. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res. 2006, 40, 768–774. [Google Scholar] [CrossRef]
- Yan, H.; Pan, G.; Zou, H.; Li, X.L.; Chen, H. Effective removal of microcystins using carbon nanotubes embedded with bacteria. Chin. Sci. Bull. 2004, 49, 1694–1698. [Google Scholar] [CrossRef]
- Wang, R.; Tai, Y.; Wan, X.; Ruan, W.; Man, Y.; Wang, J.; Yang, Y.; Yang, Y. Enhanced removal of microcystis bloom and microcystin-lr using microcosm constructed wetlands with bioaugmentation of degrading bacteria. Chemosphere 2018, 210, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Pei, H.; Hu, W.; Xu, X.; Ma, C.; Li, X. The removal of cyanobacteria and their metabolites through anoxic biodegradation in drinking water sludge. Bioresour. Technol. 2014, 165, 191–198. [Google Scholar] [CrossRef]
- Yang, F.; Guo, J.; Huang, F.; Massey, I.Y.; Huang, R.; Li, Y.; Wen, C.; Ding, P.; Zeng, W.; Liang, G. Removal of microcystin-lr by a novel native effective bacterial community designated as yfmcd4 isolated from lake taihu. Toxins 2018, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Ramani, A.; Rein, K.; Shetty, K.G.; Jayachandran, K. Microbial degradation of microcystin in florida's freshwaters. Biodegradation 2012, 23, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Sasaki, Y.; Maruyama, T.; Yanagisawa, E.; Hiraishi, A.; Kato, K. Degradation of the cyanobacterial hepatotoxin microcystin by a new bacterium isolated from a hypertrophic lake. Environ. Toxicol. 2001, 16, 337–343. [Google Scholar] [CrossRef]
- Ye, R.; Shan, K.; Gao, H.; Zhang, R.; Xiong, W.; Wang, Y.; Qian, X. Spatio-temporal distribution patterns in environmental factors, chlorophyll-a and microcystins in a large shallow lake, lake taihu, china. Int. J. Environ. Res. Public Health 2014, 11, 5155–5169. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Liu, A.; Cao, Z.; Hao, J.; Gong, R. Identification of a new microcystin-degrading bacterium isolated from lake chaohu, china. Bull. Environ. Contam. Toxicol. 2015, 94, 661–666. [Google Scholar] [CrossRef]
- Okano, K.; Shimizu, K.; Kawauchi, Y.; Maseda, H.; Utsumi, M.; Zhang, Z.; Neilan, B.A.; Sugiura, N. Characteristics of a microcystin-degrading bacterium under alkaline environmental conditions. J. Toxicol. 2009, 2009, 954291. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Jiang, Y.; Lu, Z.; Li, R.; Li, J. Heterologous expression of mlra gene originated from novosphingobium sp. Thn1 to degrade microcystin-rr and identify the first step involved in degradation pathway. Chemosphere 2017, 184, 159–167. [Google Scholar] [CrossRef]
- Shimizu, K.; Maseda, H.; Okano, K.; Kurashima, T.; Kawauchi, Y.; Xue, Q.; Utsumi, M.; Zhang, Z.; Sugiura, N. Enzymatic pathway for biodegrading microcystin lr in sphingopyxis sp. C-1. J. Biosci. Bioeng. 2012, 114, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Kormas, K.A.; Lymperopoulou, D.S. Cyanobacterial toxin degrading bacteria: Who are they? BioMed. Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.G.; Riddles, P.; Jones, G.J.; Smith, W.; Blakeley, R.L. Characterisation of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin lr. Environ. Toxicol. 2001, 16, 523–534. [Google Scholar] [CrossRef]
- Duan, X.; Sanan, T.; de la Cruz, A.; He, X.; Kong, M.; Dionysiou, D.D. Susceptibility of the algal toxin microcystin-lr to uv/chlorine process: Comparison with chlorination. Environ. Sci. Technol. 2018, 52, 8252–8262. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ai, H.; Kang, L.; Sun, X.; He, Q. Simultaneousmicrocystis algicidal and microcystin degrading capability by a singleacinetobacter bacterial strain. Environ. Sci. Technol. 2016, 50, 11903–11911. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, Y.; Sun, R.; Wei, H.; Li, Y.; Yin, L.; Pu, Y. Biodegradation of microcystin-lr and-rr by a novel microcystin-degrading bacterium isolated from lake taihu. Biodegradation 2014, 25, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. Mega4: Molecular evolutionary genetics analysis (mega) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Phujomjai, Y.; Somdee, A.; Somdee, T. Biodegradation of microcystin [dha(7)]mc-lr by a novel microcystin-degrading bacterium in an internal airlift loop bioreactor. Water Sci. Technol. 2016, 73, 267–274. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Xu, Q.; Lv, L.; Yin, C.; Liu, X.; Du, H.; Yan, H. Pathway for biodegrading microcystin-yr by sphingopyxis sp. Ustb-05. PLoS ONE 2015, 10, e0124425. [Google Scholar] [CrossRef]
- Saito, T.; Okano, K.; Park, H.D.; Itayama, T.; Inamori, Y.; Neilan, B.A.; Burns, B.P.; Sugiura, N. Detection and sequencing of the microcystin lr-degrading gene, mlra, from new bacteria isolated from japanese lakes. FEMS Microbiol. Lett. 2003, 229, 271–276. [Google Scholar] [CrossRef]

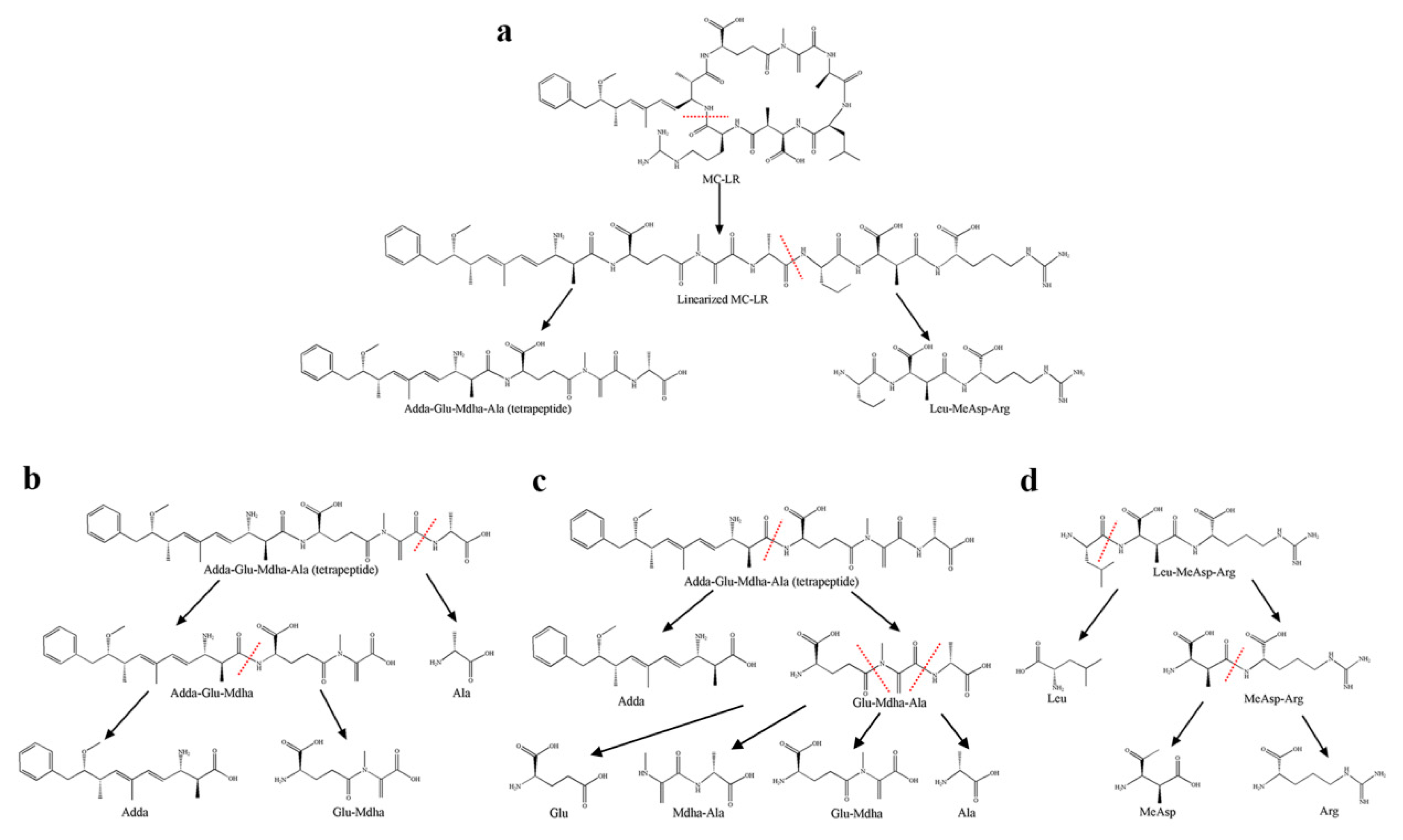
| Compound | Retention Time/min | Mass-to-Charge Ratio (m/z) | Predicted Structure | |
|---|---|---|---|---|
| Predicted | Detected | |||
| MC-LR | 8.6 | 995.5561 | 995.5545 | cyclo(Ala-Leu-MeAsp-Arg-Adda-Glu-Mdha-H) |
| 498.2817 | 498.2815 | cyclo(Ala-Leu-MeAsp-Arg-Adda-Glu-Mdha-2H) | ||
| linear MC-LR | 8.4 | 1013.5666 | 1013.5666 | Ala-Leu-MeAsp-Arg-Adda-Glu-Mdha-H |
| 507.2869 | 507.2853 | Ala-Leu-MeAsp-Arg-Adda-Glu-Mdha-2H | ||
| tetrapeptide | 8.3 | 615.3388 | 615.3405 | Adda-Glu-Mdha-Ala-H |
| Adda-Glu-Mdha | 11.8 | 544.3017 | 544.3400 | Adda-Glu-Mdha-H |
| Glu-Mdha-Ala | 4.9 | 302.1347 | 302.1354 | Glu-Mdha-Ala-H |
| Leu-MeAsp-Arg | 5.1 | 417.2456 | 417.2458 | Leu-MeAsp-Arg-H |
| Glu-Mdha | 7.4 | 231.0976 | 231.1057 | Glu-Mdha-H |
| Mdha-Ala | 4.9 | 173.0921 | 173.0925 | Mdha-Ala-H |
| MeAsp-Arg | 1.3 | 304.1616 | 304.1619 | MeAsp-Arg-H |
| Adda | 10.4 | 332.2220 | 332.2088 | Adda-H |
| Leu | 3.4 | 132.1019 | 132.1023 | Leu-H |
| Arg | 1.3 | 175.1190 | 175.1202 | Arg-H |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Liu, K.; Xu, K.; Sun, R.; Zhang, J.; Yin, L.; Pu, Y. Further Understanding of Degradation Pathways of Microcystin-LR by an Indigenous Sphingopyxis sp. in Environmentally Relevant Pollution Concentrations. Toxins 2018, 10, 536. https://doi.org/10.3390/toxins10120536
Ding Q, Liu K, Xu K, Sun R, Zhang J, Yin L, Pu Y. Further Understanding of Degradation Pathways of Microcystin-LR by an Indigenous Sphingopyxis sp. in Environmentally Relevant Pollution Concentrations. Toxins. 2018; 10(12):536. https://doi.org/10.3390/toxins10120536
Chicago/Turabian StyleDing, Qin, Kaiyan Liu, Kai Xu, Rongli Sun, Juan Zhang, Lihong Yin, and Yuepu Pu. 2018. "Further Understanding of Degradation Pathways of Microcystin-LR by an Indigenous Sphingopyxis sp. in Environmentally Relevant Pollution Concentrations" Toxins 10, no. 12: 536. https://doi.org/10.3390/toxins10120536
APA StyleDing, Q., Liu, K., Xu, K., Sun, R., Zhang, J., Yin, L., & Pu, Y. (2018). Further Understanding of Degradation Pathways of Microcystin-LR by an Indigenous Sphingopyxis sp. in Environmentally Relevant Pollution Concentrations. Toxins, 10(12), 536. https://doi.org/10.3390/toxins10120536





