Natural Products to Fight Cancer: A Focus on Juglans regia
Abstract
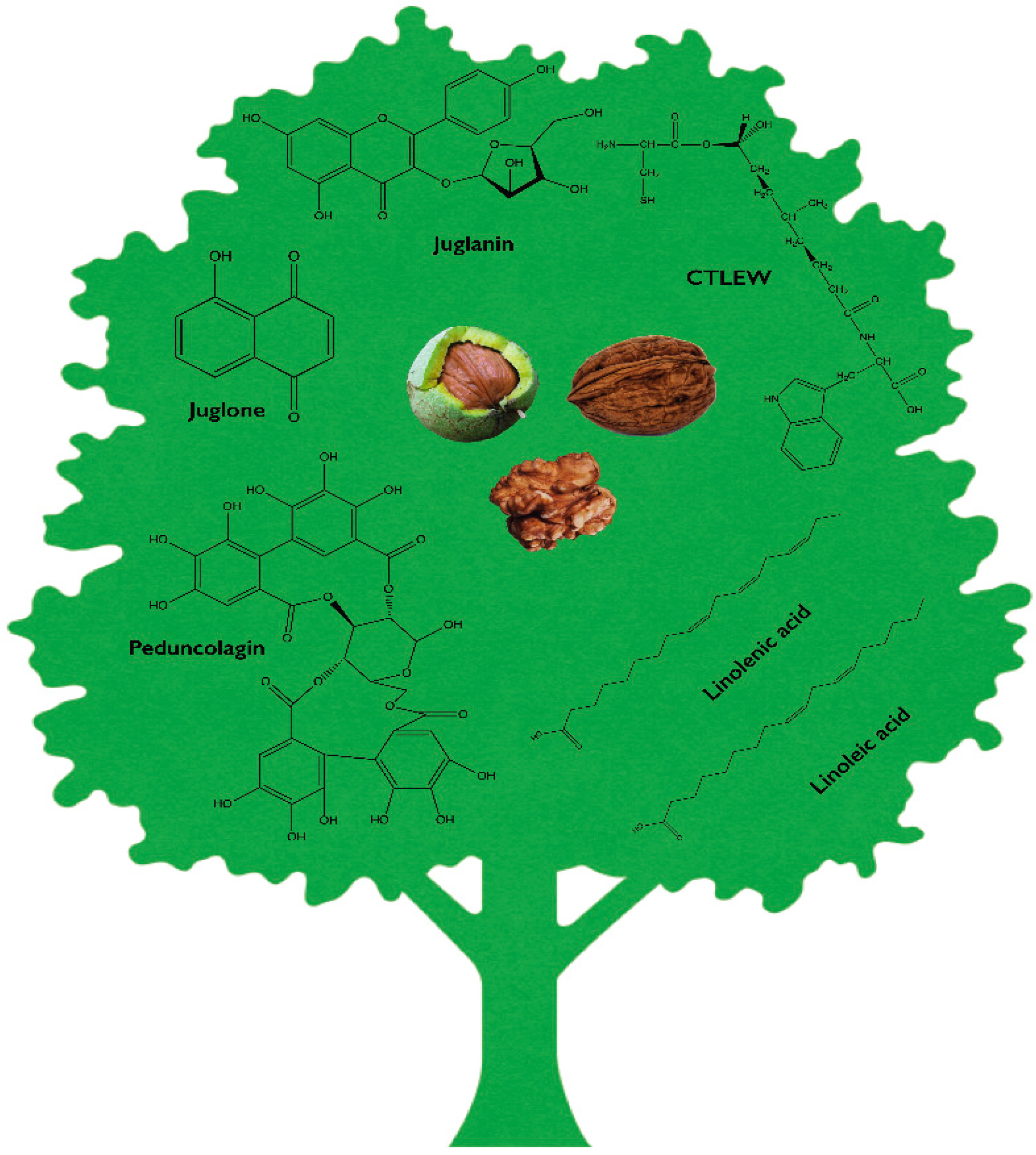
1. Introduction
2. Phytochemical Profile of Juglans regia
3. Anticancer Activity
3.1. In Vitro Studies
3.1.1. Uro-A
3.1.2. Juglanin
3.1.3. Juglone
3.1.4. Peptides
3.1.5. Extracts
3.2. Animal Studies
3.2.1. Uro-A
3.2.2. Juglanin
3.2.3. Juglone
3.2.4. Phenolic Extract
3.2.5. Walnut Intake in Animal Studies
4. Human Studies
5. Conclusions
Funding
Conflicts of Interest
References
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, W. Allelopathic effects of juglone on germination and growth of several herbaceous and woody species. J. Chem. Ecol. 1983, 9, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Lopez-Uriarte, P.; Ros, E.; Bullo, M.; Salas-Salvado, J. Nuts, hypertension and endothelial function. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 1), S21–S33. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Walnuts decrease risk of cardiovascular disease: A summary of efficacy and biologic mechanisms. J. Nutr. 2014, 144, 547s–554s. [Google Scholar] [CrossRef] [PubMed]
- Warrier, P.K.; Nambiar, V. Indian Medicinal Plants: A Compendium of 500 Species; Orient Blackswan: Hyderabad, India, 1993; Volume 5. [Google Scholar]
- Jackson, C.L.; Hu, F.B. Long-term associations of nut consumption with body weight and obesity. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 408s–411s. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Manson, J.E.; Willett, W.C.; Hu, F.B. Walnut consumption is associated with lower risk of type 2 diabetes in women. J. Nutr. 2013, 143, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Cai, Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012, 3, 134–140. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Botanical Drug Development Guidance for Industry; Pharmaceutical Quality/CMC, Ed.; U.S. Department of Health and Human Services FDA, Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2016.
- Khan, M. γ-Sitosterol, a cytotoxic sterol from Markhamia zanzibarica and Kigelia africana. Fitoterapia 1999, 70, 96–97. [Google Scholar] [CrossRef]
- Paur, I.; Balstad, T.R.; Kolberg, M.; Pedersen, M.K.; Austenaa, L.M.; Jacobs, D.R., Jr.; Blomhoff, R. Extract of oregano, coffee, thyme, clove, and walnuts inhibits NF-κB in monocytes and in transgenic reporter mice. Cancer Prev. Res. 2010, 3, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.E. The classification within the Juglandaceae. Ann. Mo. Bot. Gard. 1978, 65, 1058–1087. [Google Scholar] [CrossRef]
- Mohni, C.P.F.; Hemery, G.E. The modern silviculture of Juglans regia L.: A literature review. Die Bodenkult. 2009, 60, 19–32. [Google Scholar]
- Savill, P.S. The silviculture of trees used in British forestry. In The Silviculture of Trees Used in British Forestry, 2nd ed.; CABI: Wallingford, UK; Boston, MA, USA, 2013. [Google Scholar]
- Mitchell, A.F. A Field Guide to the Trees of Britain and Northern Europe; HarperCollins Distribution Services: Glasgow, UK, 1974. [Google Scholar]
- Hemery, G.E. Growing scattered broadleaved tree species in Europe in a changing climate: A review of risks and opportunities. Forestry 2010, 83, 65–81. [Google Scholar] [CrossRef]
- Ercisli, S.; Sayinci, B.; Kara, M.; Yildiz, C.; Ozturk, I. Determination of size and shape features of walnut (Juglans regia L.) cultivars using image processing. Sci. Hortic. 2012, 133, 47–55. [Google Scholar] [CrossRef]
- Martinez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Vavilov, N.I. The wild relatives of fruit trees of the Asian part of the USSR and Caucasus and problems of origin of fruit trees. Proc. Appl. Bot. Genet. Pl. Breed. 1931, 26, 85–107. [Google Scholar]
- Kaltenrieder, P.; Procacci, G.; Vannière, B.; Tinner, W. Postglacial vegetation and fire history of the Euganean Hills (Colli Euganei) as recorded by sedimentary pollen and charcoal series from Lago della Costa (northeastern Italy). Holocene 2010, 20, 679–695. [Google Scholar] [CrossRef]
- Clark, J.R.; Hemery, G.; Savill, P. Early growth and form of common walnut (Juglans regia L.) in mixture with tree and shrub nurse species in Southern England. Forestry 2008, 81, 631–644. [Google Scholar] [CrossRef]
- Savage, G.P. Chemical composition of walnuts (Juglans regia L.) grown in New Zealand. Plant Foods Hum. Nutr. 2001, 56, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six Walnut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Jamei, R.; Nojoomi, F.; Zamanian, Z. Persian Walnut Composition and its Importance in Human Health. Int. J. Enteric Pathog. 2018, 6, 3–9. [Google Scholar] [CrossRef]
- Sobajic, S.; Gajic-Krstajic, L. Determination of fatty acid and tocopherol compositions and the oxidative stability of walnut (Juglans regia L.) cultivars grown in Serbia. Czech. J. Food Sci. 2011, 29, 74–78. [Google Scholar]
- Calcabrini, C.; Mancini, U.; De Bellis, R.; Frati, A.; Mastrogiacomo, A.R.; Annibalini, G.; Sestili, P.; Cucchiarini, L.; Stocchi, V.; Potenza, L. Protective Role of Italian Juglans regia L. nut Ethanolic Extract in Human Keratinocytes under Oxidative and Inflammatory Stress. Curr. Pharm. Biotechnol. 2017, 18, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, C.; Yucel, S.; Erturk, U.; Korukluoglu, M. Proximate composition, minerals and fatty acid composition of Juglans Regia L. genotypes and cultivars grown in Turkey. Braz. Arch. Biol. Technol. 2012, 55, 677–683. [Google Scholar] [CrossRef]
- Buidosò, G.; Végvàri, G.; Hajnal, V.; Ficzec, G.; Tòth, M. Phenolic Profile of the Kernel of Selected Persian Walnut (Juglans regia L.) Cultivars. Not. Bot. Horti Agrobot. 2014, 42, 24–29. [Google Scholar]
- Shimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshida, T. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J. Agric. Food Chem. 2009, 57, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Esposito, D.; Grace, M.H.; Ha, D.; Pham, A.; Bortolazzo, A.; Bevens, Z.; Kim, J.; Okuda, R.; Komarnytsky, S.; et al. Cytotoxic effects of ellagitannins isolated from walnuts in human cancer cells. Nutr. Cancer 2014, 66, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Stanislawska, I.J.; Piwowarski, J.P.; Granica, S.; Kiss, A.K. The effects of urolithins on the response of prostate cancer cells to non-steroidal antiandrogen bicalutamide. Phytomedicine 2018, 46, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, C.; Ciudad, C.J.; Noe, V.; Izquierdo-Pulido, M. Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct. 2014, 5, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Kasmi, M.; Rama, P.; Hodaj, B.; Kukali, E.; Rabeta, A. Budding of walnut (Juglans regia L.). Albanian J. Agric. Sci. 2013, 12, 465–469. [Google Scholar]
- Cosmulescu, S.; Trandafir, I. Variation of phenols content in walnut (Juglans regia L.). South West. J. Hortic. Biol. Environ. 2011, 2, 25–33. [Google Scholar]
- Liu, J.; Meng, M.; Li, C.; Huang, X.; Di, D. Simultaneous determination of three diarylheptanoids and an alpha-tetralone derivative in the green walnut husks (Juglans regia L.) by high-performance liquid chromatography with photodiode array detector. J. Chromatogr. A 2008, 1190, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Trandafir, I.; Achim, G.; Baciu, A. Juglone Content in Leaf and Green Husk of Five Walnut (Juglans regia L.) Cultivars. Not. Bot. Horti Agrobot. 2011, 39, 237–240. [Google Scholar] [CrossRef]
- Thakur, A.; Cahalan, C. Geographical variation of Juglans regia L. in juglone content: Rapid analysis using micro-plate reader. Curr. Sci. 2011, 100, 1483–1485. [Google Scholar]
- Solar, A.; Colarič, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Sci. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Ion, T. Seasonal variation of total phenols in leaves of walnut (Juglans regia L.). J. Med. Plants Res. 2011, 5, 4938–4942. [Google Scholar]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Sanchez-Gonzalez, C.; Vallverdu-Queralt, A.; Simal-Gandara, J.; Lamuela-Raventos, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Landete, J. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Álvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Sanchez, M.A.; Garcia-Villalba, R.; Monedero-Saiz, T.; Garcia-Talavera, N.V.; Gomez-Sanchez, M.B.; Sanchez-Alvarez, C.; Garcia-Albert, A.M.; Rodriguez-Gil, F.J.; Ruiz-Marin, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, Z.; Zhou, B.; Liu, C.; Ruan, J.; Yan, Q.; Liao, J.; Zhu, F. In vitro antiproliferative and antioxidant effects of urolithin A, the colonic metabolite of ellagic acid, on hepatocellular carcinomas HepG2 cells. Toxicol. In Vitro 2015, 29, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Nunez-Sanchez, M.A.; Tome-Carneiro, J.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Comprehensive characterization of the effects of ellagic acid and urolithins on colorectal cancer and key-associated molecular hallmarks: MicroRNA cell specific induction of CDKN1A (p21) as a common mechanism involved. Mol. Nutr. Food Res. 2016, 60, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Cerda, B.; Espin, J.C.; Parra, S.; Martinez, P.; Tomas-Barberan, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T. Gene expression, cell cycle arrest and MAPK signalling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins. Mol. Nutr. Food Res. 2009, 53, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Kasimsetty, S.G.; Bialonska, D.; Reddy, M.K.; Ma, G.; Khan, S.I.; Ferreira, D. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J. Agric. Food Chem. 2010, 58, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Gimenez-Bastida, J.A.; Nunez-Sanchez, M.A.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 2014, 53, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Kreis, N.N.; Louwen, F.; Yuan, J. Less understood issues: p21(Cip1) in mitosis and its therapeutic potential. Oncogene 2015, 34, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Melino, G.; Knight, R.A. Cell death pathology: Cross-talk with autophagy and its clinical implications. Biochem. Biophys. Res. Commun. 2011, 414, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Polo, R.A.; Boya, P.; Pauleau, A.L.; Jalil, A.; Larochette, N.; Souquere, S.; Eskelinen, E.L.; Pierron, G.; Saftig, P.; Kroemer, G. The apoptosis/autophagy paradox: Autophagic vacuolization before apoptotic death. J. Cell Sci. 2005, 118, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shi, F.; Guo, Z.; Zhao, J.; Song, X.; Yang, H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol. Carcinog. 2018, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Tomé-Carneiro, J.; Bellesia, A.; Tomás-Barberán, F.A.; Espín, J.C. The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct. 2015, 6, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zhou, J.; Zhang, C.; Cheng, Y.; Hu, J.; Zheng, G. Antiproliferative effect of urolithin A, the ellagic acid-derived colonic metabolite, on hepatocellular carcinoma HepG2. 2.15 cells by targeting Lin28a/let-7a axis. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Ricci, R.; Kenner, L.; Zenz, R.; David, J.P.; Rath, M.; Wagner, E.F. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell 2003, 112, 181–192. [Google Scholar] [CrossRef]
- Sanchez-Prieto, R.; Rojas, J.M.; Taya, Y.; Gutkind, J.S. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000, 60, 2464–2472. [Google Scholar] [PubMed]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Dangi-Garimella, S.; Yun, J.; Eves, E.M.; Newman, M.; Erkeland, S.J.; Hammond, S.M.; Minn, A.J.; Rosner, M.R. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009, 28, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.A.; Thomson, J.M.; Hammond, S.M. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA 2008, 14, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.R.; Daley, G.Q.; Gregory, R.I. Selective blockade of microRNA processing by Lin28. Science 2008, 320, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Dutta, A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007, 21, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.; Hemann, M.T.; Bartel, D.P. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 2007, 315, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Thuault, S.; Valcourt, U.; Petersen, M.; Manfioletti, G.; Heldin, C.H.; Moustakas, A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J. Cell Biol. 2006, 174, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, K.; Wang, X.; Liu, X.; Zhang, Z. Expression of transcription factors snail, slug, and twist in human bladder carcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 119. [Google Scholar] [CrossRef] [PubMed]
- Liberal, J.; Carmo, A.; Gomes, C.; Cruz, M.T.; Batista, M.T. Urolithins impair cell proliferation, arrest the cell cycle and induce apoptosis in UMUC3 bladder cancer cells. Investig. New Drugs 2017, 35, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zhou, B.; Jin, L.; Yu, H.; Liu, L.; Liu, Y.; Qin, C.; Xie, S.; Zhu, F. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem. Toxicol. 2013, 59, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J. Hormones and cancer in humans. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1995, 333, 59–67. [Google Scholar] [CrossRef]
- National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/types/hormone-therapy (accessed on 22 August 2018).
- Sanchez-Gonzalez, C.; Ciudad, C.J.; Izquierdo-Pulido, M.; Noe, V. Urolithin A causes p21 up-regulation in prostate cancer cells. Eur. J. Nutr. 2016, 55, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, N.R.; Chandrasekaran, B.; Kolluru, V.; Ankem, M.; Damodaran, C.; Vadhanam, M.V. A natural molecule, urolithin A, downregulates androgen receptor activation and suppresses growth of prostate cancer. Mol. Carcinog. 2018, 57, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Altuwaijri, S. Role of prostate specific antigen (PSA) in pathogenesis of prostate cancer. J. Cancer Ther. 2012, 3, 331. [Google Scholar] [CrossRef]
- Espin, J.C.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: The evidence so far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Gonzalez-Sarrias, A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J. Agric. Food Chem. 2006, 54, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Avila-Galvez, M.A.; Espin, J.C.; Gonzalez-Sarrias, A. Physiological Relevance of the Antiproliferative and Estrogenic Effects of Dietary Polyphenol Aglycones versus Their Phase-II Metabolites on Breast Cancer Cells: A Call of Caution. J. Agric. Food Chem. 2018, 66, 8547–8555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.H.; Aguilera-Barrantes, I.; Shiau, C.W.; Sheng, X.; Wang, L.S.; Stoner, G.D.; Huang, Y.W. Urolithin A suppresses the proliferation of endometrial cancer cells by mediating estrogen receptor-α-dependent gene expression. Mol. Nutr. Food Res. 2016, 60, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L.; Dong, J.L.; Wu, J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed. Pharmacother. 2017, 85, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, Y.Q.; Xu, J.; Wang, J.P.; Meng, Z.L.; Hong, Y.Q. Juglanin inhibits lung cancer by regulation of apoptosis, ROS and autophagy induction. Oncotarget 2017, 8, 93878–93898. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.R.; Zeng, K.; Lan, H.M.; Wang, Q. Juglanin ameliorates UVBinduced skin carcinogenesis via antiinflammatory and proapoptotic effects in vivo and in vitro. Int. J. Mol. Med. 2018, 42, 41–52. [Google Scholar] [PubMed]
- Ji, Y.; Xin, G.; Qu, Z.; Zou, X.; Yu, M. Mechanism of juglone-induced apoptosis of MCF-7 cells by the mitochondrial pathway. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Yazdanparast, R.; Roshanzamir, F. Augmentation of oxidative stress-induced apoptosis in MCF7 cells by ascorbate–tamoxifen and/or ascorbate–juglone treatments. In Vitro Cell. Dev. Biol.-Anim. 2016, 52, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.G.; Shen, Y.F.; Li, Y. Effect of Pin1 inhibitor juglone on proliferation, migration and angiogenic ability of breast cancer cell line MCF7Adr. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Yazdanparast, R. Sensitizing effect of juglone is mediated by down regulation of Notch1 signaling pathway in trastuzumab-resistant SKBR3 cells. Apoptosis 2017, 22, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, Y.; Zhang, Z.; Che, D.; Lv, H. Juglone loaded poloxamer 188/phospholipid mixed micelles evaluated in vitro and in vivo in breast cancer. Int. J. Pharm. 2016, 515, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Avci, E.; Arikoglu, H.; Erkoc Kaya, D. Investigation of juglone effects on metastasis and angiogenesis in pancreatic cancer cells. Gene 2016, 588, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.B.; Qu, Z.Y.; Zou, X. Juglone-induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway. Exp. Toxicol. Pathol. 2011, 63, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Aithal, B.K.; Kumar, M.R.; Rao, B.N.; Udupa, N.; Rao, B.S. Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol. Int. 2009, 33, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Zhang, Y.; Du, J.; Lv, Y.; Mo, S.; Liu, Y.; Ding, F.; Wu, J.; Li, J. Juglone potentiates TRAILinduced apoptosis in human melanoma cells via activating the ROSp38p53 pathway. Mol. Med. Rep. 2017, 16, 9645–9651. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.I.; Cho, J.H.; Kim, D.J.; Lee, K.A.; Cho, M.K.; Nam, H.S.; Woo, K.M.; Lee, S.H.; Shim, J.H. Phosphoinositol 3-kinase, a novel target molecule for the inhibitory effects of juglone on TPA-induced cell transformation. Int. J. Mol. Med. 2012, 30, 8–14. [Google Scholar] [PubMed]
- Lu, Z.; Chen, H.; Zheng, X.M.; Chen, M.L. Experimental study on the apoptosis of cervical cancer Hela cells induced by juglone through c-Jun N-terminal kinase/c-Jun pathway. Asian Pac. J. Trop. Med. 2017, 10, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, A.; Li, Y.; Zhao, X.; Lv, S.; Zhu, W.; Jin, Y. Anticancer activity and mechanism of juglone on human cervical carcinoma HeLa cells. Can. J. Physiol. Pharmacol. 2012, 90, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Qin, Y.; Qi, L.; Fang, Q.; Zhao, L.; Chen, S.; Li, Q.; Zhang, D.; Wang, L. Juglone exerts antitumor effect in ovarian cancer cells. Iran J. Basic Med. Sci. 2015, 18, 544–548. [Google Scholar] [PubMed]
- Xu, H.; Yu, X.; Qu, S.; Sui, D. Juglone, isolated from Juglans mandshurica Maxim, induces apoptosis via down-regulation of AR expression in human prostate cancer LNCaP cells. Bioorg. Med. Chem. Lett. 2013, 23, 3631–3634. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, R.; Kushiyama, A.; Seno, Y.; Nakatsu, Y.; Matsunaga, Y.; Fukushima, T.; Tsuchiya, Y.; Sakoda, H.; Fujishiro, M.; Yamamotoya, T.; et al. Pin1 Inhibitor Juglone Exerts Anti-Oncogenic Effects on LNCaP and DU145 Cells despite the Patterns of Gene Regulation by Pin1 Differing between These Cell Lines. PLoS ONE 2015, 10, e0127467. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chen, S.; Ma, J.; Cui, J.; Li, Q.; Meng, G.; Wang, L. Juglone suppresses epithelial-mesenchymal transition in prostate cancer cells via the protein kinase B/glycogen synthase kinase-3β/Snail signaling pathway. Oncol. Lett. 2018, 16, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Kviecinski, M.R.; Pedrosa, R.C.; Felipe, K.B.; Farias, M.S.; Glorieux, C.; Valenzuela, M.; Sid, B.; Benites, J.; Valderrama, J.A.; Verrax, J.; et al. Inhibition of cell proliferation and migration by oxidative stress from ascorbate-driven juglone redox cycling in human bladder-derived T24 cells. Biochem. Biophys. Res. Commun. 2012, 421, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Meskelevicius, D.; Sidlauskas, K.; Bagdonaviciute, R.; Liobikas, J.; Majiene, D. Juglone Exerts Cytotoxic, Anti-proliferative and Anti-invasive Effects on Glioblastoma Multiforme in a Cell Culture Model. Anti-Cancer Agents Med. Chem. 2016, 16, 1190–1197. [Google Scholar] [CrossRef]
- Sidlauskas, K.; Sidlauskiene, R.; Li, N.; Liobikas, J. 5-Hydroxy-1,4-naphthalenedione exerts anticancer effects on glioma cells through interaction with the mitochondrial electron transport chain. Neurosci. Lett. 2017, 639, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Wang, X.F.; Sun, D.J. Juglone reduces growth and migration of U251 glioblastoma cells and disrupts angiogenesis. Oncol. Rep. 2017, 38, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Xu, Y.; Zhang, J.; Zhu, W.; Zhang, Y.; Chen, L.; Hua, W.; Mao, Y. Juglone induces apoptosis of tumor stem-like cells through ROS-p38 pathway in glioblastoma. BMC Neurol. 2017, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Qu, X.R.; Jiang, Y.F. Juglone, from Juglans mandshruica Maxim, inhibits growth and induces apoptosis in human leukemia cell HL-60 through a reactive oxygen species-dependent mechanism. Food Chem. Toxicol. 2012, 50, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Segura-Aguilar, J.; Jonsson, K.; Tidefelt, U.; Paul, C. The cytotoxic effects of 5-OH-1,4-naphthoquinone and 5,8-diOH-1,4-naphthoquinone on doxorubicin-resistant human leukemia cells (HL-60). Leuk. Res. 1992, 16, 631–637. [Google Scholar] [CrossRef]
- Ourique, F.; Kviecinski, M.R.; Felipe, K.B.; Correia, J.F.; Farias, M.S.; Castro, L.S.; Grinevicius, V.M.; Valderrama, J.; Rios, D.; Benites, J.; et al. DNA damage and inhibition of akt pathway in mcf-7 cells and ehrlich tumor in mice treated with 1,4-naphthoquinones in combination with ascorbate. Oxid. Med. Cell. Longev. 2015, 2015, 495305. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huang, D.; Zhai, M.; Yang, L.; Peng, S.; Chen, C.; Feng, X.; Weng, Q.; Zhang, B.; Xu, M. Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells. BMC Complement. Altern. Med. 2015, 15, 413. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; Gomez-Ruiz, J.A.; Ruiz, A.L.; Carvalho, J.E. Antiproliferative Activity of Walnut (Juglans regia L.) Proteins and Walnut Protein Hydrolysates. J. Med. Food 2017, 20, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani, R.; Ghaffari, S.M.; Salami, M.; Vahdati, K.; Sepehri, H.; Sarvestani, N.N.; Sheibani, N.; Moosavi-Movahedi, A.A. Antioxidant and Anticancer Activities of Walnut (Juglans regia L.) Protein Hydrolysates Using Different Proteases. Plant Foods Hum. Nutr. 2016, 71, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Hasan, T.N.; Shafi, G.; Syed, N.A.; Al-Assaf, A.H.; Alamri, M.S.; Al-Khalifa, A.S. Validation of the Antiproliferative Effects of Organic Extracts from the Green Husk of Juglans regia L. on PC-3 Human Prostate Cancer Cells by Assessment of Apoptosis-Related Genes. Evid.-Based Complement. Altern. Med. 2012, 2012, 103026. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jeronimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.N.; Leena Grace, B.; Shafi, G.; Al-Hazzani, A.A.; Alshatwi, A.A. Anti-proliferative effects of organic extracts from root bark of Juglans regia L. (RBJR) on MDA-MB-231 human breast cancer cells: Role of Bcl-2/Bax, caspases and Tp53. Asian Pac. J. Cancer Prev. 2011, 12, 525–530. [Google Scholar] [PubMed]
- Salimi, M.; Majd, A.; Sepahdar, Z.; Azadmanesh, K.; Irian, S.; Ardestaniyan, M.H.; Hedayati, M.H.; Rastkari, N. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm. Biol. 2012, 50, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.S.; Lee, J.; Heo, S.C.; Lee, K.L.; Choi, S.W.; Kim, Y. Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients 2016, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kim, Y.S.; Lee, J.; Lee, J.H.; Choi, S.W.; Kim, Y. Compositional analysis of walnut lipid extracts and properties as an anti-cancer stem cell regulator via suppression of the self-renewal capacity. Food Sci. Biotechnol. 2016, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.S.; Luqman, S.; Srivastava, S.; Krishna, V.; Gupta, N.; Darokar, M.P. Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm. Biol. 2011, 49, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Di, D.L.; Wei, X.N.; Han, Y. Cytotoxic diarylheptanoids from the pericarps of walnuts (Juglans regia). Planta Med. 2008, 74, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.-X.; Zhao, L.; Di, D.-L.; Meng, M.; Jiang, S.-X. Capillary zone electrophoresis for separation and analysis of four diarylheptanoids and an α-tetralone derivative in the green walnut husks (Juglans regia L.). J. Pharm. Biomed. Anal. 2008, 48, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Hwangbo, K.; Zheng, M.S.; Son, J.K.; Kim, H.Y.; Baek, S.H.; Choi, H.C.; Park, S.Y.; Kim, J.R. Inhibitory effects of juglanin on cellular senescence in human dermal fibroblasts. J. Nat. Med. 2014, 68, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-Y.; Yi, Y.-X.; Jin, L.-X.; Lin, W.; Fang, P.-P.; Lin, X.-Z.; Zheng, Y.; Pan, C.-W. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed. Pharmacother. 2016, 81, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Dibling, B. The true face of JNK activation in apoptosis. Aging Cell 2002, 1, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.M.; Jie, J.; Zhang, Y.; Liu, H.; Peng, L.P. A self-assembled polyjuglanin nanoparticle loaded with doxorubicin and anti-Kras siRNA for attenuating multidrug resistance in human lung cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P. Anti-cancer activities of 1,4-naphthoquinones: A QSAR study. Anti-Cancer Agents Med. Chem. 2006, 6, 489–499. [Google Scholar] [CrossRef]
- Pavan, V.; Ribaudo, G.; Zorzan, M.; Redaelli, M.; Pezzani, R.; Mucignat-Caretta, C.; Zagotto, G. Antiproliferative activity of Juglone derivatives on rat glioma. Nat. Prod. Res. 2017, 31, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.S.; Aithal, K.; Rao, B.N.; Udupa, N.; Rao, B.S.S. Cytotoxic, genotoxic and oxidative stress induced by 1,4-naphthoquinone in B16F1 melanoma tumor cells. Toxicol. In Vitro 2009, 23, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Zou, C.L.; Duan, Y.X.; Wu, F.; Li, G. Activity guided isolation and modification of juglone from Juglans regia as potent cytotoxic agent against lung cancer cell lines. BMC Complement. Altern. Med. 2015, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Genovese, S.; Taddeo, V.A.; Mathieu, V.; Kiss, R.; Epifano, F. Novel juglone and plumbagin 5-O derivatives and their in vitro growth inhibitory activity against apoptosis-resistant cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Luo, J.; Lu, X.; Chen, M.; Zhu, W.; Jiang, Y. Juglone inhibits proliferation and induces apoptosis of human cervical squamous cancer SiHa cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015, 31, 186–189. [Google Scholar] [PubMed]
- Zhang, W.; Li, Y.; Luo, J.; Zhao, X.; Xu, J.; Zhu, W.; Jiang, Y.; Fang, F. Proliferation inhibition and apoptosis induction of Juglone on human cervical cancer Caski cells. Wei Sheng Yan Jiu 2014, 43, 959–961. [Google Scholar] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Itsumi, M.; Takeuchi, A.; Imada, K.; Yokomizo, A.; Kuruma, H.; Inokuchi, J.; Tatsugami, K.; Uchiumi, T.; Oda, Y.; et al. Crosstalk between epithelial-mesenchymal transition and castration resistance mediated by Twist1/AR signaling in prostate cancer. Endocr.-Relat. Cancer 2015, 22, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H.; Moore, W.G.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Antonaci, S. Gelatinases and their inhibitors in tumor metastasis: From biological research to medical applications. Histol. Histopathol. 2002, 17, 339–345. [Google Scholar] [PubMed]
- Mook, O.R.; Frederiks, W.M.; Van Noorden, C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: http://www.who.int/cancer/ (accessed on 12 September 2018).
- Lenaz, G.; Genova, M.L. Structure and organization of mitochondrial respiratory complexes: A new understanding of an old subject. Antioxid. Redox Signal. 2010, 12, 961–1008. [Google Scholar] [CrossRef] [PubMed]
- Lemarie, A.; Grimm, S. Mitochondrial respiratory chain complexes: Apoptosis sensors mutated in cancer? Oncogene 2011, 30, 3985–4003. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Takamiya, M.; Takahashi, M.; Miyashita, H.; Ikeda, H.; Terada, T.; Matsuo, Y.; Shirouzu, M.; Yokoyama, S.; Fujimori, F.; et al. Pin1 and Par14 peptidyl prolyl isomerase inhibitors block cell proliferation. Chem. Biol. 2003, 10, 15–24. [Google Scholar] [CrossRef]
- Wang, H.Y.; Fu, J.C.; Lee, Y.C.; Lu, P.J. Hyperthermia stress activates heat shock protein expression via propyl isomerase 1 regulation with heat shock factor 1. Mol. Cell. Biol. 2013, 33, 4889–4899. [Google Scholar] [CrossRef] [PubMed]
- Atchison, F.W.; Means, A.R. Spermatogonial depletion in adult Pin1-deficient mice. Biol. Reprod. 2003, 69, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, Y.; Otani, Y.; Sakoda, H.; Zhang, J.; Guo, Y.; Okubo, H.; Kushiyama, A.; Fujishiro, M.; Kikuch, T.; Fukushima, T.; et al. Role of Pin1 protein in the pathogenesis of nonalcoholic steatohepatitis in a rodent model. J. Biol. Chem. 2012, 287, 44526–44535. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.C.; Yang, C.H.; Lin, C.H.; Ch’ang, H.J.; Chang, V.H.S.; Yu, W.C.Y. Destabilization of KLF10, a tumor suppressor, relies on thr93 phosphorylation and isomerase association. Biochim. Biophys. Acta 2013, 1833, 3035–3045. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, F.; Shao, K.; Hang, J.; Wang, H.; Rayburn, E.; Xiao, Z.X.; Lee, S.W.; Xue, Q.; Feng, X.L.; et al. Overexpression of Pin1 in non-small cell lung cancer (NSCLC) and its correlation with lymph node metastases. Lung Cancer 2007, 56, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Dalerba, P.; Cho, R.W.; Clarke, M.F. Cancer stem cells: Models and concepts. Annu. Rev. Med. 2007, 58, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, M.T.; Ljungman, M. The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblasts. Toxicol. Appl. Pharmacol. 2005, 209, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M. Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava). Food Sci. Biotechnol. 2011, 20, 1075. [Google Scholar]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Jan, S.; Fazili, K.M. Bioefficacy potential of different genotypes of walnut Juglans regia L. J. Food Sci. Technol. 2018, 55, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.R.; Favarin, J.L.; Leandro, R.A.; Lima Filho, O.F. Total phenol concentrations in coffee tree leaves during fruit development. Sci. Agric. 2008, 65, 354–359. [Google Scholar] [CrossRef]
- Kiss, I.Z.; Mándi, G.; Beck, M.T. Artificial neural network approach to predict the solubility of C60 in various solvents. J. Phys. Chem. A 2000, 104, 8081–8088. [Google Scholar] [CrossRef]
- Berquin, I.M.; Edwards, I.J.; Chen, Y.Q. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008, 269, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Comba, A.; Maestri, D.M.; Berra, M.A.; Garcia, C.P.; Das, U.N.; Eynard, A.R.; Pasqualini, M.E. Effect of ω-3 and ω-9 fatty acid rich oils on lipoxygenases and cyclooxygenases enzymes and on the growth of a mammary adenocarcinoma model. Lipids Health Dis. 2010, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Vanden Heuvel, J.P.; Belda, B.J.; Hannon, D.B.; Kris-Etherton, P.M.; Grieger, J.A.; Zhang, J.; Thompson, J.T. Mechanistic examination of walnuts in prevention of breast cancer. Nutr. Cancer 2012, 64, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Ardestaniyan, M.H.; Mostafapour Kandelous, H.; Saeidnia, S.; Gohari, A.R.; Amanzadeh, A.; Sanati, H.; Sepahdar, Z.; Ghorbani, S.; Salimi, M. Anti-proliferative and apoptotic activities of constituents of chloroform extract of Juglans regia leaves. Cell Prolif. 2014, 47, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Mumm, J.S.; Kopan, R. Notch signaling: From the outside in. Dev. Biol. 2000, 228, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Groden, J.; Joslyn, G.; Samowitz, W.; Jones, D.; Bhattacharyya, N.; Spirio, L.; Thliveris, A.; Robertson, M.; Egan, S.; Meuth, M.; et al. Response of colon cancer cell lines to the introduction of APC, a colon-specific tumor suppressor gene. Cancer Res. 1995, 55, 1531–1539. [Google Scholar] [PubMed]
- Metcalfe, C.; Bienz, M. Inhibition of GSK3 by Wnt signalling—Two contrasting models. J. Cell Sci. 2011, 124, 3537–3544. [Google Scholar] [CrossRef] [PubMed]
- Swales, K.E.; Korbonits, M.; Carpenter, R.; Walsh, D.T.; Warner, T.D.; Bishop-Bailey, D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006, 66, 10120–10126. [Google Scholar] [CrossRef] [PubMed]
- Catalano, S.; Malivindi, R.; Giordano, C.; Gu, G.; Panza, S.; Bonofiglio, D.; Lanzino, M.; Sisci, D.; Panno, M.L.; Ando, S. Farnesoid X receptor, through the binding with steroidogenic factor 1-responsive element, inhibits aromatase expression in tumor Leydig cells. J. Biol. Chem. 2010, 285, 5581–5593. [Google Scholar] [CrossRef] [PubMed]
- Journe, F.; Durbecq, V.; Chaboteaux, C.; Rouas, G.; Laurent, G.; Nonclercq, D.; Sotiriou, C.; Body, J.J.; Larsimont, D. Association between farnesoid X receptor expression and cell proliferation in estrogen receptor-positive luminal-like breast cancer from postmenopausal patients. Breast Cancer Res. Treat. 2009, 115, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, Q.; Shen, D. The Function of Retinoid X Receptor α in Cancer Cells. Biol. Syst. Open Access 2016, 5, 2. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Heilman, J.; Andreux, P.; Tran, N.; Rinsch, C.; Blanco-Bose, W. Safety assessment of Urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem. Toxicol. 2017, 108, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sugie, S.; Okamoto, K.; Rahman, K.M.; Tanaka, T.; Kawai, K.; Yamahara, J.; Mori, H. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998, 127, 177–183. [Google Scholar] [CrossRef]
- Okada, T.A.; Roberts, E.; Brodie, A.F. Mitotic abnormalities produced by juglone in Ehrlich ascites tumor cells. Proc. Soc. Exp. Biol. Med. 1967, 126, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Ourique, F.; Kviecinski, M.R.; Zirbel, G.; Castro, L.S.; Castro, A.J.G.; Silva, F.R.M.B.; Valderrama, J.A.; Rios, D.; Benites, J.; Calderon, P.B. In vivo inhibition of tumor progression by 5 hydroxy-1,4-naphthoquinone (juglone) and 2-(4-hydroxyanilino)-1,4-naphthoquinone (Q7) in combination with ascorbate. Biochem. Biophys. Res. Commun. 2016, 477, 640–646. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. Practical strategies for suppressing hypoxia-inducible factor activity in cancer therapy. Med Hypotheses 2010, 74, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Targeting glucose metabolism for cancer therapy. J. Exp. Med. 2012, 209, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Choi, Y.I.; Kim, Y.; Kim, Y.S.; Choi, S.W.; Kim, J.W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor κB signaling in intestinal epithelial cells, and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Vasu, V.T.; Gohil, K.; Kim, H.; Khan, I.H.; Cross, C.E.; Yokoyama, W. A high-fat diet containing whole walnuts (Juglans regia) reduces tumour size and growth along with plasma insulin-like growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. Br. J. Nutr. 2012, 108, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.P.; Lamarque, A.L.; Comba, A.; Berra, M.A.; Silva, R.A.; Labuckas, D.O.; Das, U.N.; Eynard, A.R.; Pasqualini, M.E. Synergistic anti-tumor effects of melatonin and PUFAs from walnuts in a murine mammary adenocarcinoma model. Nutrition 2015, 31, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yokoyama, W.; Davis, P.A. TRAMP prostate tumor growth is slowed by walnut diets through altered IGF-1 levels, energy pathways, and cholesterol metabolism. J. Med. Food 2014, 17, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, J.; Blackburn, G.; Mantzoros, C.S. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 2012, 28, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Korkmaz, A.; Fuentes-Broto, L.; Hardman, W.E.; Rosales-Corral, S.A.; Qi, W. A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Investig 2013, 31, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Ion, G.; Akinsete, J.A.; Witte, T.R. Dietary walnut suppressed mammary gland tumorigenesis in the C(3)1 TAg mouse. Nutr. Cancer 2011, 63, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J. Diet, insulin-like growth factor-1 and cancer risk. Proc. Nutr. Soc. 2011, 70, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Aithal, B.K.; Sunil Kumar, M.R.; Rao, B.N.; Upadhya, R.; Prabhu, V.; Shavi, G.; Arumugam, K.; Sajankila, S.P.; Udupa, N.; Satyamoorthy, K.; et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J. Pharm. Sci. 2011, 100, 3517–3528. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Ion, G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr. Cancer 2008, 60, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Smith, D.S.; Ornstein, D.K. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA 1997, 277, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Spaccarotella, K.J.; Kris-Etherton, P.M.; Stone, W.L.; Bagshaw, D.M.; Fishell, V.K.; West, S.G.; Lawrence, F.R.; Hartman, T.J. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr. J. 2008, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Tanzman, J.S.; Sabate, J. Lack of effect of walnuts on serum levels of prostate specific antigen: A brief report. J. Am. Coll. Nutr. 2007, 26, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Fimognari, C. Antileukemic Activity of Sulforaphane. In Glucosinolates; Springer International Publishing: Cham, Switzerland, 2017; pp. 301–317. [Google Scholar]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef] [PubMed]
| Compound or Extract | Cell Line | IC50 or Concentration Range (μM) a | Cell-cycle Inhibition Phase and Markers | Apoptosis Markers | Inhibition of Tumour Invasion and Metastasis Markers | Other Mechanisms and Markers | Reference |
|---|---|---|---|---|---|---|---|
| Juglanin | MCF-7 | IC50 24 h: 26.35 | G2/M | ⬇ Bcl2, ⬆ Bad | Autophagy: formation of autophagosome, ⬆ LC3B-II | [89] | |
| IC50 48 h: 14.38 | |||||||
| SKBR3 | IC50 24 h: 20.07 | ⬆ Bax | |||||
| IC50 48 h: 17.69 | ⬆ Caspase 3, 8 and 9 | ||||||
| ⬆ ROS | |||||||
| Chromatin condensation | |||||||
| MDA-MB231 | IC50 24 h: 29.13 | ||||||
| IC50 48 h: 23.25 | |||||||
| BT474 | IC50 24 h: 24.17 | ||||||
| IC50 48 h: 19.85 | |||||||
| A549 | 0–80 | Sub-G1 cells | Autophagy: autophagic vacuoles, ⬆ LC3, ATG7 and ATG3 | [90] | |||
| Chromatin condensation and DNA fragmentation | |||||||
| ⬆ PARP | |||||||
| ⬆ Caspase 3, 8 and 9 | |||||||
| ⬇ Bcl-2 and Bcl-xl, ⬆ Bax and Bad | |||||||
| ⬇ TRAIL, DR4, DR5 and FADD | |||||||
| H1975 | ⬆ p53 | ||||||
| ⬆ ROS | |||||||
| ⬇ NF-kB | |||||||
| ⬇ PI3K/Akt | |||||||
| ⬇ MAPK and ERK1/2, ⬆ p38 and JNK | |||||||
| ⬆ C-Jun | |||||||
| ⬆ C-Abl | |||||||
| ⬆ p73 | |||||||
| HCC827 | |||||||
| B16F10 | 0–30 | ⬆ PARP | [91] | ||||
| ⬇ p38/JNK | |||||||
| ⬇ PI3K/Akt | |||||||
| ⬇ NF-kB | |||||||
| ⬆ Caspase 3 | |||||||
| ⬆ p53, p21 and p27 | |||||||
| Juglone | MCF-7 | 0–50 | ⬆ Caspase 3 | [92,93] | |||
| IC50 24 h: 11.99 | ⬇ Bcl-2, ⬆ Bax | ||||||
| ⬇ ΔΨ, ⬆ [Ca2+], MOMP and Cyt-c | |||||||
| ⬆ p53 | |||||||
| ⬇ p-Akt | |||||||
| ⬆ ROS | |||||||
| ⬇ GSH, catalase, superoxide dismutase and glutathione peroxidase | |||||||
| ⬆ Lipid peroxidation | |||||||
| MCF-7 Adr | The concentrations tested were not reported. | G2/M | ⬇ Migration | [94] | |||
| ⬇ Cyclin E | ⬇ VEGF-A, -B and -C | ||||||
| SKBR3 | 0–50 | G0/G1 | ⬇ Colony formation | [95] | |||
| MDA-MB231 | IC50 24 h: 10.35 | G2/M | ⬇ Migration | [96] | |||
| BxPC-3 | IC50 24 h: 21.05 | ⬇ Adhesion and cell invasion | [97] | ||||
| ⬇ MMP-2 and -9 | |||||||
| ⬇ VEGF | |||||||
| ⬇ Phactr-1 | |||||||
| PANC-1 | IC50 24 h: 21.25 | ⬇ Adhesion and cell invasion | |||||
| ⬇ MMP-9 | |||||||
| ⬇ VEGF | |||||||
| ⬇ Phactr-1 | |||||||
| SGC-7901 | IC50 24 h: 36.52 | ⬆ Caspase 3 | [98] | ||||
| IC50 48 h: 25.38 | ⬇ Bcl-2, ⬆ Bax | ||||||
| ⬇ ΔΨ, ⬆ Cyt-c | |||||||
| ⬆ ROS | |||||||
| B16F1 | IC50 24 h: 7.69 | ⬆ Sub-G1 | ⬇ Colony formation | Necrosis | [99] | ||
| Membrane blebbing | Mutagenic activity: ⬆ Micronuclei frequency | ||||||
| Chromatin condensation | |||||||
| DNA fragmentation | |||||||
| ⬆ ROS | |||||||
| ⬆ LDH | |||||||
| A2058 | 0–20 | ⬆ ROS | [100] | ||||
| ⬆ p53 | |||||||
| ⬆ p38 | |||||||
| MEWO | 0–20 | ⬆ TRAIL | |||||
| ⬆ ROS | |||||||
| ⬆ p53 | |||||||
| ⬆ p38 | |||||||
| JB6 CI41 | 0–5 | ⬇ PI3K | ⬇ TPA- or EGF-induced cell transformation | ⬇ TPA- or EGF-induced AP-1 and COX-2 | [101] | ||
| ⬇ TPA-induced activation of AKT | |||||||
| ⬇ TPA-induced c-Jun and c-fos activation | |||||||
| HeLa | 0–100 | ⬆ Caspase 3, 8 and 9 | [102,103] | ||||
| IC50 24 h: 33 | ⬆ PARP | ||||||
| ⬇ Bcl-2, ⬆ Bax | |||||||
| ⬆ Cyt-C | |||||||
| ⬆ Fas and FasL | |||||||
| ⬆ p-JNK | |||||||
| SKOV3 | IC50 24 h: 30.13 | G0/G1 | ⬆ Caspase 3, ⬇ Procaspase 9 | ⬇ MMP-2 | [104] | ||
| ⬇ Cyclin D1 | ⬇ Bcl-2, ⬆ Bax | ||||||
| ⬆ Cyt-c | |||||||
| LNCap | IC50 24 h: 13.8–32.2 | Chromatin condensation, cell shrinkage and membrane blebbing | ⬇ EMT | ⬇ PSA | [105,106,107] | ||
| IC50 48 h: ≃15 | ⬆ Caspase 3 and 9 | ⬆ E-cadherin, ⬇ N-caderin and vimentin | ⬇ AR | ||||
| ⬇ ΔΨ | ⬇ Akt/GSK-3β/Snail | ||||||
| LNCap-AI | IC50 24 h: 43.1 | [107] | |||||
| DU145 | IC50 48 h: ≃10 | [106] | |||||
| T24 | IC50 24 h: ≃28.5 | ⬆ Caspase 3 | ⬇ Cell motility | DNA damage: ⬆ γ-H2AX | [108] | ||
| ⬆ PARP | ER stress: ⬆ PERK and Eif2-α | ||||||
| C6 | 0–64 | G0/G1 | ⬆ ROS | ⬇ Cell spheroid invasiveness | [109,110] | ||
| IC50 24 h: ≃10.4 | ⬇ MRC complex 1 | ⬇ Metastasis formation | |||||
| U251 | 0–20 | Chromatin condensation | ⬇ Migration | [111] | |||
| ⬆ Caspase 3 | ⬇ Angiogenesis | ||||||
| ⬇ TGFβ1/Smad/miR-21 | |||||||
| ⬇ Pin1 | |||||||
| U87 | 0–40 | ⬆ Caspase 9 | [112] | ||||
| SHG62 | ⬆ ROS | ||||||
| SHG66 | ⬆ p38/MAPK | ||||||
| HL-60 | IC50 24 h: ≃8 | ⬆ Caspase 3 and 8 | [113] | ||||
| ⬆ PARP | |||||||
| cc Cyt-c | |||||||
| ⬆ ROS, ⬇ GSH | |||||||
| ⬆ Smac | |||||||
| ⬇ Akt/mTor | |||||||
| HL-60 doxo-resistant | ⬆ Oxygen consuption | [114] | |||||
| ⬆ Quinone reductase activities | |||||||
| ⬆ Superoxide dismutase and glutathione S-transferase | |||||||
| Juglone + Ascorbate (1 mM) | MCF-7 | 0–50 | DNA fragmentation | DNA damage: ⬆ γ-H2AX | [93,115] | ||
| IC50 24 h: 28 | ⬇ Bcl-2, ⬆ Bax | Necrosis | |||||
| ⬆ ROS | |||||||
| ⬇ Catalase and glutathione peroxidase | |||||||
| ⬆ Foxo3a and Foxo1 | |||||||
| ⬇ p-Akt | |||||||
| T24 | IC50 24 h: 6.3 | ⬆ ROS, ⬇ GSH | ⬇ Cell motility | DNA damage: ⬆ γ-H2AX | [108] | ||
| ER stress: ⬆ PERK, and eif2-α | |||||||
| Uro-A | MCF-7 | IC50 48 h: 95.56 | [78] | ||||
| A549 | IC50 48 h: 17.81 | ||||||
| HepG2 | 0–200 | G2/M | ⬆ Caspase 3 | [51,78] | |||
| IC50 24 h: 137 | ⬇ Cyclin D1 | ⬆ Bax | |||||
| IC50 48 h: 40.53 | ⬆ p53 | ||||||
| ⬇ c-Myc | |||||||
| ⬆ p38/MAPK | |||||||
| ⬇ TCF/LEF | |||||||
| ⬇ β-catenin | |||||||
| ⬇ IL6 and IL1β | |||||||
| ⬇ NF-kB, | |||||||
| ⬇ COX-2 and iNOS | |||||||
| ⬆ PUMA and NOXA | |||||||
| HepG2.2.15 | IC50 24 h: >120 | ⬆ Caspase 3 | [67] | ||||
| ⬇ Bcl-2, ⬆ Bax | |||||||
| ⬇ Lin28a, ⬆ Let-7a | |||||||
| ⬇ HMGA2 and K-Ras | |||||||
| ⬇ Sp-1 and Zcchc11 | |||||||
| ECC-1 | 0–50 | G2/M | [88] | ||||
| HEC1A | ⬆ Cyclin B1 and E2 | ||||||
| ⬆ p-cdc2 and cdc25B | |||||||
| ⬆ Myt1 | |||||||
| Ishikawa | ⬆ p21 | ⬇ ER-α ans GRIP1, ⬆ ER-β, PGR, pS2 and GREB1 | |||||
| LNCap | 0–40 | G0/G1 | ⬆ Caspase 3 and 7 | ⬇ PSA | [36,37,82] | ||
| IC50 24 h: 13.8 | ⬇ Bcl-2 | ⬇ AR | |||||
| ⬆ CDKN1A | |||||||
| ⬇ Fibronectin-1 | |||||||
| ⬆ p21 | |||||||
| PC3 | 0–200 | ⬆ Caspase 3 | [37,83] | ||||
| IC50 24 h: 70 | ⬆ PARP | ||||||
| C4-2B | 0–200 | ⬆ PARP | ⬇ PSA | [83] | |||
| IC50 24 h: 35 | ⬆ Caspase 3 | ⬇ AR | |||||
| ⬇ p-Akt | |||||||
| DU145 | IC50 24 h: 33.4 | [36] | |||||
| UMUC3 | IC50 48 h: 23.92 | G2/M | ⬇ PI3K/Akt | [78] | |||
| ⬇ ERK 1/2 | |||||||
| ⬇ SAPK/JNK | |||||||
| T24 | IC50 48 h: 43.90 | Chromatin condensation ⬆ Caspase 3 ⬆ PPAR-γ ⬆ p38/MAPK ⬇ MEKK1 and c-Jun | [79] | ||||
| Caco-2 | 0–100 | ⬆ C-Myc | [52,55,58,66] | ||||
| IC50 24 h: 87 | ⬆ DUSP6 | ||||||
| IC50 48 h: 42.80–81 | S | ⬆ Fos | |||||
| SW480 | 0–100 | G2/M | ⬆ C-Myc | [52,58,66] | |||
| IC50 48 h: 59.45 | ⬆ Cyclin A and B1 | ⬆ CDKN1A | |||||
| ⬆ CTMNBI | |||||||
| ⬆ EGF3 | |||||||
| HT-29 | 0–100 | S | |||||
| IC50 48 h: 46.01 | G2/M | ||||||
| SW620 | IC50 24 h: ≥15 | G2/M | ⬆ Caspase 3 | ⬇ Cell migration | Autophagy: ⬆ LC3 | [64] | |
| ⬇ MMP-9 activity | |||||||
| Uro-A + Uro-B | LNCaP | 20 + 20 | ⬇ Bcl-2 | ⬇ PSA | [37] | ||
| PC3 | ⬇ AR | ||||||
| Uro-A + Uro-C + EA | Caco-2 | 85 + 10 + 5 | S | ⬆ p53 | [52] | ||
| Iso-Uro-A + Uro-A + Uro-B +Uro-C + AE | 50 + 30 + 10 + 5 + 5 | G2/M | ⬆ K-ras | ||||
| CTLEW | MCF-7 | IC50 48 h: 1.620 μg/mL | ⬆ Sub-G1 | Autophagy: ⬇ LC3-I and ⬆ LC3-II | [116] | ||
| Phosphatidilserine externalisation | |||||||
| Caco-2 | IC50 48 h: 650 μg/mL | ||||||
| HeLa | IC50 48 h: 600 μg/mL | ||||||
| Peptide from walnut pepsine-colorase pp hydrolysis | UACC-62 | IC50 24 h: 0.25 μg/mL | [117] | ||||
| U251 | IC50 24 h: >250 μg/mL | ||||||
| MCF-7 | |||||||
| NCI-adriamycin resistant | |||||||
| 786-O | |||||||
| NCI-H460 | |||||||
| PC3 | |||||||
| OVCAR-03 | |||||||
| HT-29 | |||||||
| K562 | |||||||
| Peptide from walnut pepsine hydrolysis | UACC-62 | IC50 24 h: 710 μg/mL | |||||
| U251 | IC50 24 h: > 250 μg/mL | ||||||
| MCF-7 | |||||||
| NCI-adriamycin resistant | |||||||
| 786-O | |||||||
| NCI-H460 | |||||||
| PC3 | |||||||
| OVCAR-03 | |||||||
| HT-29 | |||||||
| K562 | |||||||
| Peptide from walnut neutrase hydrolysis | UACC-62 | IC50 24 h: 25 μg/mL | |||||
| U251 | IC50 24 h: >250 μg/mL | ||||||
| MCF-7 | |||||||
| NCI-adriamycin resistant | |||||||
| 786-O | |||||||
| NCI-460 | |||||||
| PC3 | |||||||
| OVCAR-03 | |||||||
| HT-29 | |||||||
| K562 | |||||||
| Peptide from walnut chymotrypsin hydrolysis | MDA-MB231 | IC50 24 h: 650 μg/mL | [118] | ||||
| Chloroform green husk extract | PC3 | IC50 24 h: 91.14 μg/mL | ⬆ Caspase 3 ⬇ Bcl-2, ⬆ Bax ⬆ p53 | [119] | |||
| N-hexane green husk extract | IC50 24 h: 27.29 μg/mL | ||||||
| Methanol green husk extract | IC50 24 h: 66.72 μg/mL | ||||||
| A-498 | IC50 24 h: 285 μg/mL | [120] | |||||
| 769-P | IC50 24 h: 496 μg/mL | ||||||
| Caco-2 | IC50 24 h: > 500 μg/mL | ||||||
| Chloroform root bark extract | MDA-MB231 | 0–50 μg/mL | ⬆ Caspase 3 and 8 | [121] | |||
| ⬇ Bcl-2, ⬆ Bax | |||||||
| N-hexane root bark extract | ⬆ p53 | ||||||
| ⬆ TNF-α | |||||||
| Methanol root bark extract | ⬇ Mdm-2 | ||||||
| Methanol leaf extract | A-498 | IC50 24 h: 226 μg/mL | [120] | ||||
| 769-P | IC50 24 h: 352 μg/mL | ||||||
| Caco-2 | IC50 24 h: >500 μg/mL | ||||||
| Chloroform fraction of aqueous-ethanol leaf extract | MCF-7 | IC50 24 h: 500 μg/mL | G0/G1 | ⬆ Sub-G1 | [122] | ||
| IC50 48 h: 360 μg/mL | |||||||
| HT-29 | IC50 24 h: 810 μg/mL | ||||||
| IC50 48 h: 530 μg/mL | |||||||
| BHY | IC50 24 h: 590 μg/mL | ||||||
| IC50 48 h: 450 μg/mL | |||||||
| N-hexane fraction of aqueous-ethanol leaf extract | MCF-7 | IC50 24 h: >1500 μg/mL | |||||
| HT-29 | IC50 48 h: >1500 μg/mL | ||||||
| BHY | |||||||
| Methanol fraction of aqueous-ethanol leaf extract | MCF-7 | ||||||
| HT-29 | |||||||
| BHY | |||||||
| Ethyl acetate fraction of aqueous-ethanol leaf extract | MCF-7 | IC50 24 h: 1060 μg/mL | |||||
| IC50 48 h: 520 μg/mL | |||||||
| HT-29 | IC50 24 h: 1490 μg/mL | ||||||
| IC50 48 h: 1060 μg/mL | |||||||
| BHY | IC50 24 h: 1410 μg/mL | ||||||
| IC50 48 h: 820 μg/mL | |||||||
| Methanol fruit extract | CSCs | 0–40 μg/mL | ⬇ Formation of colonies and spheres | ⬆ CK20 | [123] | ||
| ⬇ Notch 1 | |||||||
| ⬇ DLK1 | |||||||
| ⬇ β-catenin | |||||||
| ⬇ p-GSK3β | |||||||
| Primary human colorectal cancer cells | ⬇ Notch 1 | ||||||
| ⬇ DLK1 | |||||||
| Chloroform-methanol fruit extract | CSCs | 0–1000 μg/mL | ⬇ Colony formation | ⬇ β-catenin | [124] | ||
| ⬇ p-GSK3β | |||||||
| ⬇ Notch 1 | |||||||
| Methanol fruit extract | MCF-7 | IC50 24 h: 348 μg/mL | [125] | ||||
| WRL-68 | IC50 24 h: 301 μg/mL | ||||||
| HepG2 | IC50 24 h: 405 μg/mL | ||||||
| Caco-2 | IC50 24 h: 305 μg/mL | ||||||
| KB | IC50 24 h: 403 μg/mL | ||||||
| Aqueous methanol fruit extract | MCF-7 | IC50 24 h: >500 μg/mL | |||||
| HepG2 | IC50 24 h: 66 μg/mL | ||||||
| WRL-68 | IC50 24 h: 55 μg/mL | ||||||
| Caco-2 | IC50 24 h: >500 μg/mL | ||||||
| KB | IC50 24 h: 251.6 μg/mL | ||||||
| Chloroform fraction of aqueous-methanol fruit extract | MCF-7 | IC50 24 h: >500 μg/mL | |||||
| WRL-68 | IC50 24 h: 60.6 μg/mL | ||||||
| HepG2 | IC50 24 h: 9 μg/mL | ||||||
| Caco-2 | IC50 24 h: 35.66 μg/mL | ||||||
| KB | IC50 24 h: 40 μg/mL | ||||||
| Methanol-soluble fraction of aqueous-methanol fruit extract | MCF-7 | IC50 24 h: 350 μg/mL | |||||
| HepG2 | IC50 24 h: 351.6 μg/mL | ||||||
| WRL-68 | IC50 24 h: 455μg/mL | ||||||
| Caco-2 | IC50 24 h: 301 μg/mL | ||||||
| KB | IC50 24 h: 351.6 μg/mL | ||||||
| Methanol-insoluble fraction of aqueous-methanol fruit extract | MCF-7 | IC50 24 h: 500 μg/mL | |||||
| HepG2 | IC50 24 h: 298.3 μg/mL | ||||||
| WRL-68 | IC50 24 h: 351 μg/mL | ||||||
| Caco-2 | IC50 24 h: 356.6 μg/mL | ||||||
| KB | IC50 24 h: 353 μg/mL | ||||||
| N-hexane fraction of aqueous-methanol fresh fruit extract | MCF-7 | IC50 24 h: 403 μg/mL | |||||
| HepG2 | IC50 24 h: 301.6 μg/mL | ||||||
| WRL-68 | IC50 24 h: 255 μg/mL | ||||||
| Caco-2 | IC50 24 h: 301.6 μg/mL | ||||||
| KB | IC50 24 h: 201.6 μg/mL | ||||||
| Ethyl acetate fraction of aqueous-methanol fresh fruit extract | MCF-7 | IC50 24 h: 448.3 μg/mL | |||||
| HepG2 | IC50 24 h: 15.3 μg/mL | ||||||
| WRL-68 | IC50 24 h: 70 μg/mL | ||||||
| Caco-2 | IC50 24 h: 200 μg/mL | ||||||
| KB | IC50 24 h: 50.3 μg/mL | ||||||
| Methanol seed extract | A-498 | IC50 24 h: 291 μg/mL | [120] | ||||
| 769-P | IC50 24 h: >500 μg/mL | ||||||
| Caco-2 | |||||||
| Compound/Diet | Experimental Model | Treatment Doses | Anticancer Effects | Molecular Targets | References |
|---|---|---|---|---|---|
| Juglanin | MCF-7-xenografted male BALB/c-nude mice | 0–10 mg/kg/day (7 days) | ⬇ Tumour growth | ⬆ Caspase 3, 9 | [89] |
| ⬆ LC3B | |||||
| ⬆ p-JNK | |||||
| A549-xenografted athymic nude mice | 0–30 mg/kg/day (28 days) | ⬇ Tumour volume | ⬆ Caspase 3 | [90] | |
| ⬇ Tumour weight | ⬆ PARP | ||||
| ⬇ Bcl-2, Bcl-xl, ⬆ Bax, Bad | |||||
| ⬆ p53 | |||||
| ⬆ TRAIL, DR4, DR5 and FADD | |||||
| ⬆ PI3K, Akt, and p-ERK1/2 | |||||
| ⬆ p-p38 | |||||
| ⬆ LC3BI/II, ATG7, Beclin1 and PIK3C3 | |||||
| Hairless mice subjected to UVB radiation | 0–20 mg/kg/2 days per week (10 weeks) | Suppression of epidermal hyperplasia and inflammatory cell infiltration | ⬇ Ki67 | [91] | |
| ⬇ p38/JNK | |||||
| ⬇ PI3K/AKT | |||||
| ⬇ IL-1β, TNF-α, IL-6 | |||||
| ⬇ Cyclin D1, CDK1, PCNA | |||||
| ⬆ p53, p27, p21 | |||||
| ⬆ PARP | |||||
| ⬆ Caspases 3 and 8 | |||||
| Juglone | Female BALB/c-nu mice implanted with U87 stem-like cells | 1 mg/kg/ day per 3 days (5 administrations) | ⬇ Tumour growth | [112] | |
| ⬆ Survival | |||||
| MDA-MB231-xenografted nude mouse | 10–40 mg/kg/day every 3 days (5 administrations) | ⬇ Tumour growth | [96] | ||
| Inbred C57BL/6J mice implanted with B16F1 | 1 mg/kg/day 1, 3 and 5 (3 administrations) | ⬇ Tumour growth | [193] | ||
| ⬆ Survival | |||||
| Weanling male F344 rats treated subcutaneously injections of azoxymethane | 200 ppm/once per week (3 weeks) | ⬇ Incidence and multiplicity of intestine tumours | [179] | ||
| Ehrlich ascites tumour xenografted swiss/HaICR mice | 0–2 mg (single injection) | Mitotic abnormalities | [180] | ||
| ⬇ Amount of ascitic fluid | |||||
| Juglone + Ascorbate | Ehrlich carcinoma- xenografted male BALB/c inbred mice | (1 mg/kg + 100 mg/kg)/day (9 days) | ⬇ Tumour growth | ⬆ G0/G1 cell-cycle arrest | [115,181] |
| ⬆ Survival | ⬆ p53, p16 | ||||
| ⬇ Cyclin A | |||||
| ⬆ PARP | |||||
| ⬆ Bax | |||||
| ⬇ Bcl-xL | |||||
| ⬇ HIF-α | |||||
| ⬇ GLUT1 | |||||
| ⬇ GSH, ⬆ SOD | |||||
| ⬇ p-Akt | |||||
| ⬆ Protein carboxylation | |||||
| ⬆ MDA | |||||
| ⬆ γ-H2AX | |||||
| Uro A | C4-2B-xenografted male BALB/c athymic mice (nu/nu) | 50 mg/kg/5 days per week (4–5 weeks) | ⬇ Tumour growth | ⬇ Ki67 | [83] |
| ⬇ Akt | |||||
| PC-3-xenografted male BALB/c athymic mice (nu/nu) | ⬇ Ki67 | ||||
| Walnut diet | TRAMP mice | 100 g whole walnut/kg of diet ad libitum (18 weeks) | ⬇ Tumour size | ⬇ IGF-1 | [188] |
| ⬇ High density lipoprotein, total cholesterol | |||||
| ⬆ Insulin sensitivity | |||||
| ⬇ Glucose-6-phosphate | |||||
| ⬇ Succinylcarnitine | |||||
| ⬇ 4-hydroxybutyrate | |||||
| ⬆ PCK1 and CIDEC | |||||
| 155 g of whole walnut/kg of diet ad libitum (9, 18, 24 weeks) | ⬇ Tumour growth and size. | ⬇ Plasma IGF-1 | [185] | ||
| ⬇ Resistin | |||||
| ⬇ Low density lipoprotein | |||||
| LNCaP xenografted nude mice | 113 g of whole walnut/kg of diet ad libitum (126 days) | ⬇ Number of tumours | [190] | ||
| ⬇ Xenografts growth | |||||
| HT-29 xenografted female nude (nu/nu) mice | 110 g of whole walnut/kg of diet (25 days) | ⬇ Tumour weight | ⬇ VEGF | [189] | |
| 111 g of walnut/kg of diet ad libitum (optional 2 weeks before breeding + 21 days of weaning + 110, 130 or 145 days) | ⬇ Tumour incidence | Altered expression of 84 genes associated with proliferation and differentiation | [191] | |
| ⬇ Tumour Multiplicity | |||||
| ⬇ Tumour size | |||||
| MDA-MB231-xenografted nude mice | 113 g of whole walnut/kg of diet (35 days) | ⬇ Tumour growth | [194] | ||
| HT-29-xenografted athymic nude (nu/nu) mice | 111 g of whole walnut/kg of diet ad libitum (25 days) | ⬇ Tumour growth | ⬆ ALN, eicosapentaenoic, DHA and total ω-3 fatty acids | [186] | |
| ⬇ Arachidonic acid | |||||
| ⬇ miRNAs 1903, 467c and 3068, ⬆ miRNA 297a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural Products to Fight Cancer: A Focus on Juglans regia. Toxins 2018, 10, 469. https://doi.org/10.3390/toxins10110469
Catanzaro E, Greco G, Potenza L, Calcabrini C, Fimognari C. Natural Products to Fight Cancer: A Focus on Juglans regia. Toxins. 2018; 10(11):469. https://doi.org/10.3390/toxins10110469
Chicago/Turabian StyleCatanzaro, Elena, Giulia Greco, Lucia Potenza, Cinzia Calcabrini, and Carmela Fimognari. 2018. "Natural Products to Fight Cancer: A Focus on Juglans regia" Toxins 10, no. 11: 469. https://doi.org/10.3390/toxins10110469
APA StyleCatanzaro, E., Greco, G., Potenza, L., Calcabrini, C., & Fimognari, C. (2018). Natural Products to Fight Cancer: A Focus on Juglans regia. Toxins, 10(11), 469. https://doi.org/10.3390/toxins10110469






