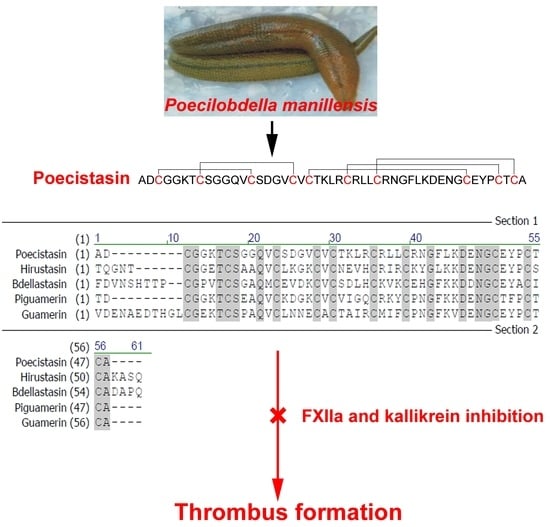
Isolation and Characterization of Poecistasin, an Anti-Thrombotic Antistasin-Type Serine Protease Inhibitor from Leech Poecilobdella manillensis
Abstract
1. Introduction
2. Results
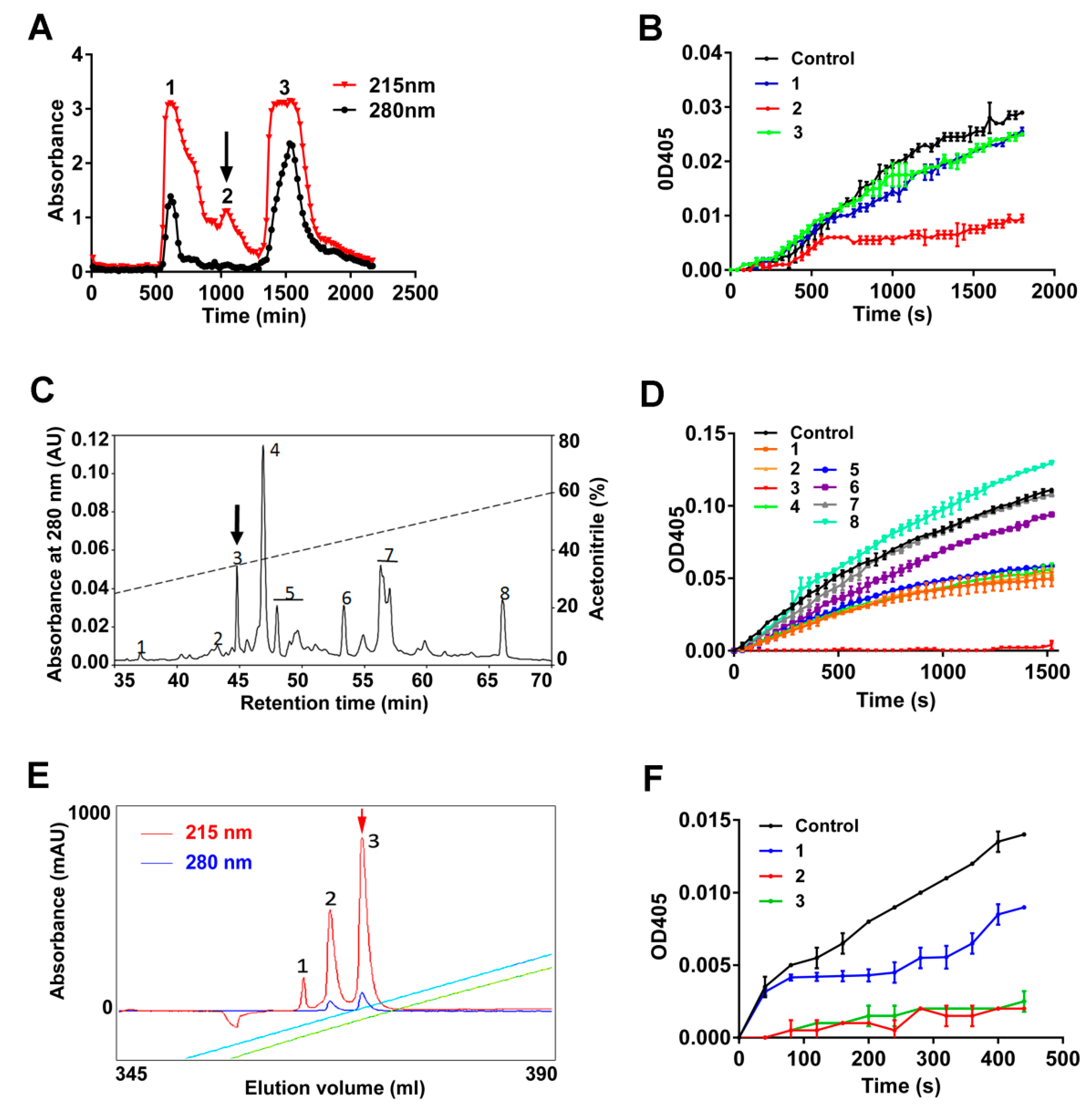
2.1. Purification of Poecistasin
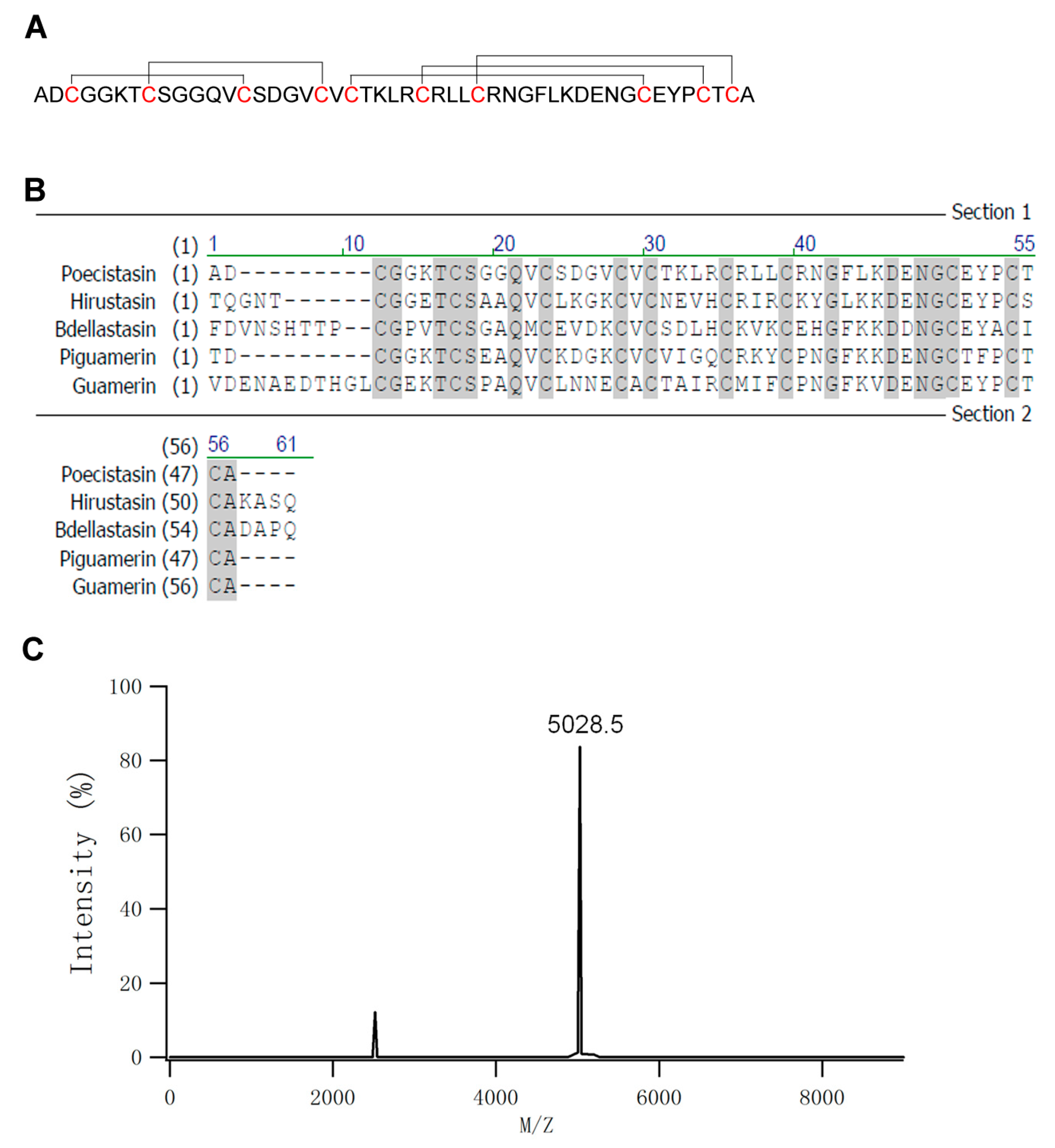
2.2. Primary Structure of Poecistasin
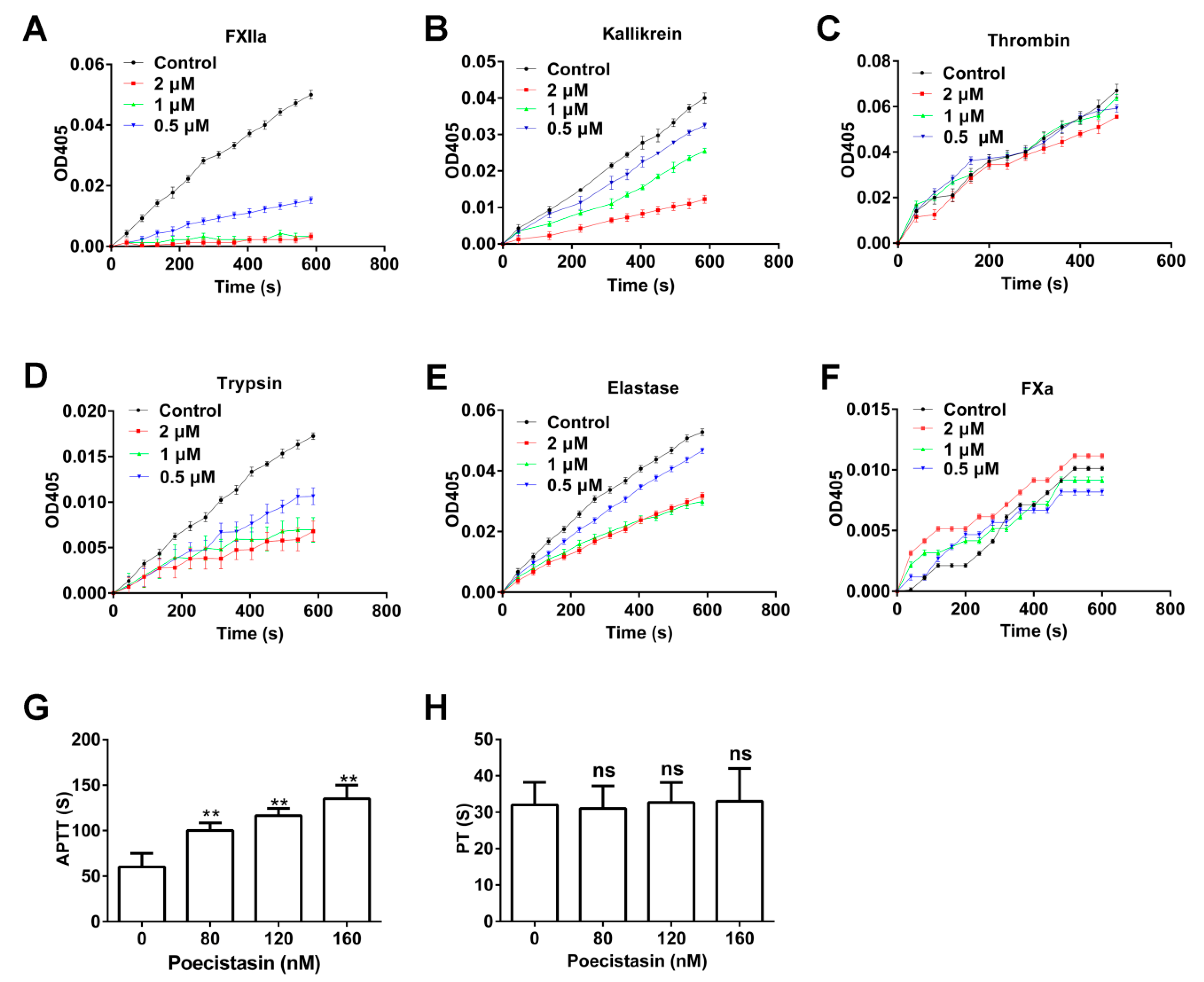
2.3. Effects of Poecistasin on Proteases and Coagulation
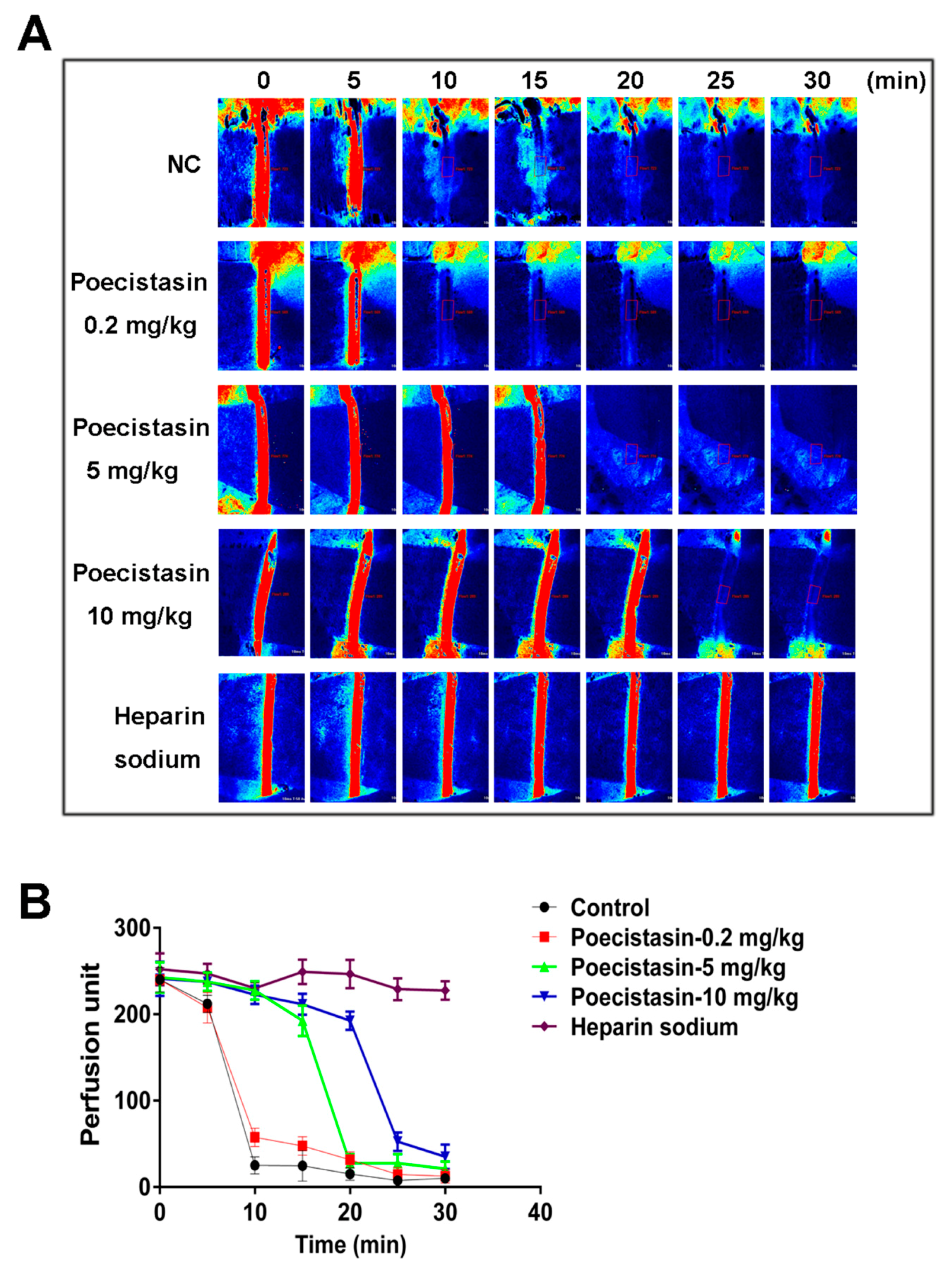
2.4. Effects of Poecistasin on FeCl3-Induced Carotid Artery Injury Model
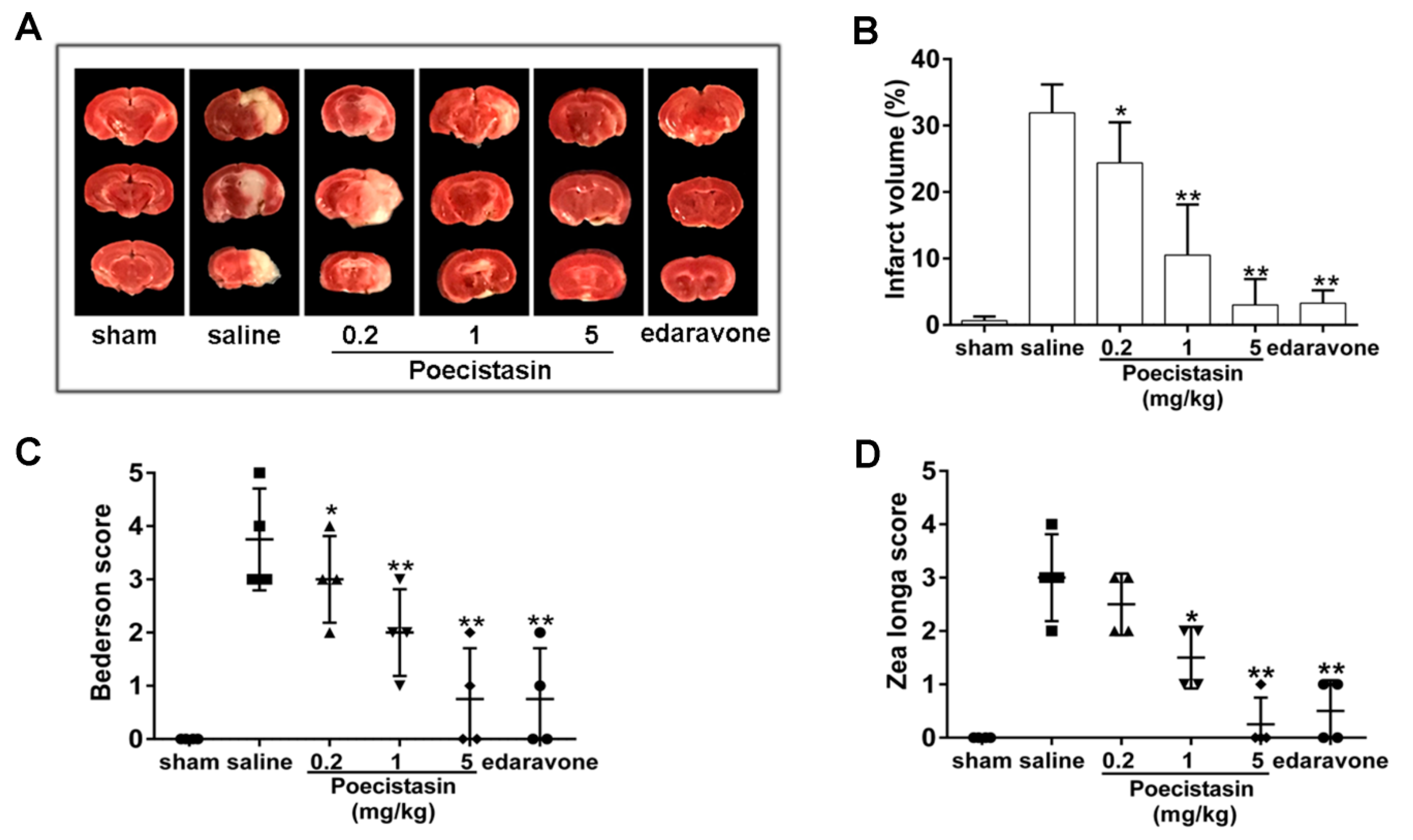
2.5. Effects of Poecistasin on Stroke Model
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Collection of Crude Secretions
5.2. Purification of Poecistasin
5.3. Mass Spectrometric Analysis and Sequencing of Poecistasin
5.4. Poecistasin Recombinant Expression and Purification
5.5. Effects of Poecistasin on Proteases
5.6. APTT and PT Assays
5.7. FeCl3-Induced Carotid Artery Injury Model
5.8. Stroke Model
5.9. Animals and Ethics Statement
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Birrell, G.W.; Earl, S.T.H.; Wallis, T.P.; Masci, P.P.; de Jersey, J.; Gorman, J.J.; Lavin, M.F. The diversity of bioactive proteins in Australian snake venoms. Mol. Cell. Proteom. 2007, 6, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Lips, K.R.; Knoop, F.C.; Fritzsch, B.; Miller, C.; Conlon, J.M. Antimicrobial peptides and protease inhibitors in the skin secretions of the crawfish frog, Rana areolata. Bba-Proteins Proteom. 2002, 1601, 55–63. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012, 40, D343–D350. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. Using the MEROPS Database for Proteolytic Enzymes and Their Inhibitors and Substrates. Curr. Protoc. Bioinform. 2014. [Google Scholar] [CrossRef]
- Bode, W.; Huber, R. Natural Protein Proteinase-Inhibitors and Their Interaction with Proteinases. Eur. J. Biochem. 1992, 204, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Kroger, B.; Bialojan, S.; Lemaire, H.G.; Hoffken, H.W.; Reuschenbach, P.; Otte, M.; Dodt, J. A Kazal-Type Inhibitor with Thrombin Specificity from Rhodnius-Prolixus. J. Biol. Chem. 1993, 268, 16216–16222. [Google Scholar] [PubMed]
- Lu, S.M.; Lu, W.Y.; Qasim, M.A.; Anderson, S.; Apostol, I.; Ardelt, W.; Bigler, T.; Chiang, Y.W.; Cook, J.; James, M.N.G.; et al. Predicting the reactivity of proteins from their sequence alone: Kazal family of protein inhibitors of serine proteinases. Proc. Natl. Acad. Sci. USA 2001, 98, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Derache, C.; Epinette, C.; Roussel, A.; Gabant, G.; Cadene, M.; Korkmaz, B.; Gauthier, F.; Kellenberger, C. Crystal structure of greglin, a novel non-classical Kazal inhibitor, in complex with subtilisin. FEBS J. 2012, 279, 4466–4478. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.S.; Nunes, V.A.; Gozzo, A.J.; Sampaio, M.U.; Auerswald, E.; Ura, N.; Shimamoto, K.; Sampaio, C.A.M. Preliminary characterization of a Kazal-type serine protease inhibitor from Caiman crocodilus yacare plasma. Immunopharmacology 1999, 45, 179–183. [Google Scholar] [CrossRef]
- Macedo, M.L.; Garcia, V.A.; Freire, M.; Richardson, M. Characterization of a Kunitz trypsin inhibitor with a single disulfide bridge from seeds of Inga laurina (SW.) Willd. Phytochemistry 2007, 68, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Marlor, C.W.; Delaria, K.A.; Davis, G.; Muller, D.K.; Greve, J.M.; Tamburini, P.P. Identification and cloning of human placental bikunin, a novel serine protease inhibitor containing two Kunitz domains. J. Biol. Chem. 1997, 272, 12202–12208. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Denda, K.; Kitamura, A.; Kawaguchi, T.; Kito, M.; Kondo, J.; Kagaya, S.; Qin, L.; Takata, H.; Miyazawa, K.; et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J. Biol. Chem. 1997, 272, 6370–6376. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y. The Bowman-Birk Inhibitor—Trypsin-Inhibitor and Chymotrypsin-Inhibitor from Soybeans. Int. J. Pept. Protein Res. 1985, 25, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R. The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am. J. Clin. Nutr. 1998, 68, 1406s–1412s. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Selvaraj, S.; Murthy, M.R.N.; Sreerama, Y.N.; Rao, D.R.; Gowda, L.R. Analysis of the amino acid sequences of plant Bowman-Birk inhibitors. J. Mol. Evol. 1996, 42, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Inouye, K.; Tonomura, B.I.; Hiromi, K. Interactions between Streptomyces Subtilisin Inhibitor (SSI) and α-Chymotrypsin. Agric. Biol. Chem. 2014, 39, 1159–1161. [Google Scholar] [CrossRef]
- Taguchi, S.; Kojima, S.; Kumagai, I.; Ogawara, H.; Miura, K.; Momose, H. Isolation and Partial Characterization of Ssi-Like Protease Inhibitors from Streptomyces. FEMS Microbiol. Lett. 1992, 99, 293–297. [Google Scholar] [CrossRef]
- Taguchi, S.; Kikuchi, H.; Kojima, S.; Kumagai, I.; Nakase, T.; Miura, K.; Momose, H. High-Frequency of Ssi-Like Protease Inhibitors among Streptomyces. Biosci. Biotechnol. Biochem. 1993, 57, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Fujimura, K.; Kumagai, I.; Miura, K. Contribution of Salt Bridge in the Protease Inhibitor Ssi (Streptomyces Subtilisin Inhibitor) to Its Inhibitory-Action. FEBS Lett. 1994, 337, 195–199. [Google Scholar] [CrossRef]
- Moreau, T.; Baranger, K.; Dade, S.; Dallet-Choisy, S.; Guyot, N.; Zani, M.L. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie 2008, 90, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Francart, C.; Dauchez, M.; Alix, A.J.P.; Lippens, G. Solution structure of r-elafin, a specific inhibitor of elastase. J. Mol. Biol. 1997, 268, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, G.P.; Gasic, T.B.; Gasic, G.J. Isolation and Characterization of Antistasin—An Inhibitor of Metastasis and Coagulation. J. Biol. Chem. 1987, 262, 9718–9723. [Google Scholar] [PubMed]
- Dunwiddie, C.; Thornberry, N.A.; Bull, H.G.; Sardana, M.; Friedman, P.A.; Jacobs, J.W.; Simpson, E. Antistasin, a Leech-Derived Inhibitor of Factor-Xa—Kinetic-Analysis of Enzyme-Inhibition and Identification of the Reactive Site. J. Biol. Chem. 1989, 264, 16694–16699. [Google Scholar] [PubMed]
- Theunissen, H.J.M.; Dijkema, R.; Swinkels, J.C.; Depoorter, T.L.; Vink, P.M.F.; Vondinther, T.G. Mutational Analysis of Antistasin, an Inhibitor of Blood-Coagulation Factor-Xa Derived from the Mexican Leech Haementeria-Officinalis. Thromb. Res. 1994, 75, 41–50. [Google Scholar] [CrossRef]
- Nikapitiya, C.; De Zoysa, M.; Oh, C.; Lee, Y.; Ekanayake, P.M.; Whang, I.; Choi, C.Y.; Lee, J.S.; Lee, J. Disk abalone (Haliotis discus discus) expresses a novel antistasin-like serine protease inhibitor: Molecular cloning and immune response against bacterial infection. Fish Shellfish Immunol. 2010, 28, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Tak, E.; Park, S.; Cho, S.; Hahn, Y.; Joo, S.; Lee, D.; Ahn, C.; Park, S. Eisenstasin, new antistasin family inhibitor from the earthworm. Biologia 2010. [Google Scholar] [CrossRef]
- Przysiecki, C.T.; Joyce, J.G.; Keller, P.M.; Markus, H.Z.; Carty, C.E.; Hagopian, A.; Sardana, M.K.; Dunwiddie, C.T.; Ellis, R.W.; Miller, W.J.; et al. Characterization of Recombinant Antistasin Secreted by Saccharomyces-Cerevisiae. Protein Expr. Purif. 1992, 3, 185–195. [Google Scholar] [CrossRef]
- Blankenship, D.T.; Brankamp, R.G.; Manley, G.D.; Cardin, A.D. Amino-Acid-Sequence of Ghilanten—Anticoagulant-Antimetastatic Principle of the South-American Leech, Haementeria-Ghilianii. Biochem. Biophys. Res. Commun. 1990, 166, 1384–1389. [Google Scholar] [CrossRef]
- Sollner, C.; Mentele, R.; Eckerskorn, C.; Fritz, H.; Sommerhoff, C.P. Isolation and Characterization of Hirustasin, an Antistasin-Type Serine-Proteinase Inhibitor from the Medical Leech Hirudo-Medicinalis. Eur. J. Biochem. 1994, 219, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Auerswald, E.; Mentele, R.; Eckerskorn, C.; Fritz, H.; Fink, E. Bdellastasin, a serine protease inhibitor of the antistasin family from the medical leech (Hirudo medicinalis)—Primary structure, expression in yeast, and characterisation of native and recombinant inhibitor. Eur. J. Biochem. 1998, 253, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.R.; Kang, K.W. Amino acid sequence of piguamerin, an antistasin-type protease inhibitor from the blood sucking leech Hirudo nipponia. Eur. J. Biochem. 1998, 254, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.I.; Kim, S.I.; Ha, K.S.; Joe, C.O.; Kang, K.W. Isolation and Characterization of Guamerin, a New Human-Leukocyte Elastase Inhibitor from Hirudo-Nipponia. J. Biol. Chem. 1995, 270, 13879–13884. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.R.; Hong, S.J.; Ha, K.S.; Joe, C.O.; Kang, K.W. A cysteine-rich serine protease inhibitor (Guamerin II) from the non-blood sucking leech Whitmania edentula: Biochemical characterization and amino acid sequence analysis. J. Enzym. Inhibition Med. Chem. 1996, 10, 81–91. [Google Scholar] [CrossRef]
- de Marco, R.; Lovato, D.V.; Torquato, R.J.; Clara, R.O.; Buarque, D.S.; Tanaka, A.S. The first pacifastin elastase inhibitor characterized from a blood sucking animal. Peptides 2010, 31, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Markwardt, F. State-of-the-Art Review: Antithrombotic Agents from Hematophagous Animals. Clin. Appl. Thromb/Hemost. 2016, 2, 75–82. [Google Scholar] [CrossRef]
- Mende, K.; Petoukhova, O.; Koulitchkova, V.; Schaub, G.A.; Lange, U.; Kaufmann, R.; Nowak, G. Dipetalogastin, a potent thrombin inhibitor from the blood-sucking insect Dipetalogaster maximus—cDNA cloning, expression and characterization. Eur. J. Biochem. 1999, 266, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Dodt, J. Anticoagulatory Substances of Bloodsucking Animals—From Hirudin to Hirudin Mimetics. Angew. Chem. Int. Ed. 1995, 34, 867–880. [Google Scholar] [CrossRef]
- Nicastro, G.; Baumer, L.; Bolis, G.; Tato, M. NMR solution structure of a novel hirudin variant HM2, N-terminal 1-47 and N64->V+G mutant. Biopolymers 1997, 41, 731–749. [Google Scholar] [CrossRef]
- Magalhaes, A.; Magalhaes, H.P.B.; Richardson, M.; Gontijo, S.; Ferreira, R.N.; Almeida, A.P.; Sanchez, E.F. Purification and properties of a coagulant thrombin-like enzyme from the venom of Bothrops leucurus. Comp. Biochem. Physiol. Part A 2007, 146, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.F.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.N.; Li, L.; Fu, X.M.; Wu, Y.P.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Gob, E.; Reymann, S.; Langhauser, F.; Schuhmann, M.K.; Kraft, P.; Thielmann, I.; Gobel, K.; Brede, M.; Homola, G.; Solymosi, L.; et al. Blocking of Plasma Kallikrein Ameliorates Stroke by Reducing Thromboinflammation. Ann. Neurol. 2015, 77, 784–803. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Chen, M.; Duan, Z.; Mwangi, J.; Li, P.; Lai, R. Isolation and Characterization of Poecistasin, an Anti-Thrombotic Antistasin-Type Serine Protease Inhibitor from Leech Poecilobdella manillensis. Toxins 2018, 10, 429. https://doi.org/10.3390/toxins10110429
Tang X, Chen M, Duan Z, Mwangi J, Li P, Lai R. Isolation and Characterization of Poecistasin, an Anti-Thrombotic Antistasin-Type Serine Protease Inhibitor from Leech Poecilobdella manillensis. Toxins. 2018; 10(11):429. https://doi.org/10.3390/toxins10110429
Chicago/Turabian StyleTang, Xiaopeng, Mengrou Chen, Zilei Duan, James Mwangi, Pengpeng Li, and Ren Lai. 2018. "Isolation and Characterization of Poecistasin, an Anti-Thrombotic Antistasin-Type Serine Protease Inhibitor from Leech Poecilobdella manillensis" Toxins 10, no. 11: 429. https://doi.org/10.3390/toxins10110429
APA StyleTang, X., Chen, M., Duan, Z., Mwangi, J., Li, P., & Lai, R. (2018). Isolation and Characterization of Poecistasin, an Anti-Thrombotic Antistasin-Type Serine Protease Inhibitor from Leech Poecilobdella manillensis. Toxins, 10(11), 429. https://doi.org/10.3390/toxins10110429