Snake Venom Peptides: Tools of Biodiscovery
Abstract
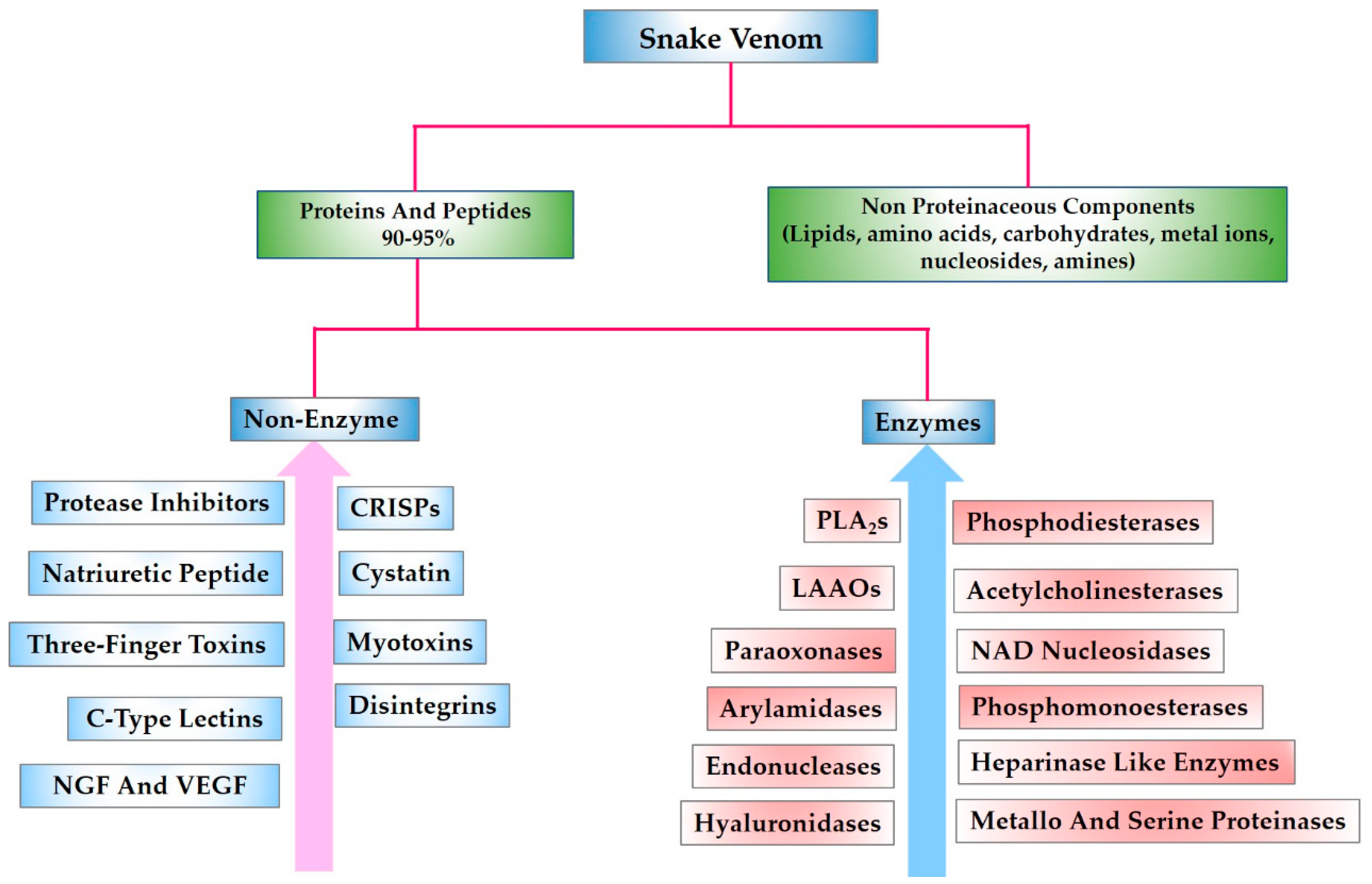
1. Introduction
2. Snake Venom Peptides and Their Potential Pharmacological Applications
2.1. Three-Finger Toxins (3FTxs)
2.1.1. Neurotoxins
Curaremimetic Toxins (α-Neurotoxins or Postsynaptic Neurotoxins)
Muscarinic Toxins
k-Neurotoxins
2.1.2. Cardiotoxins (CTXs)
2.1.3. Acetylcholinesterase Inhibitors
2.1.4. Non-Conventional 3FTxs
2.1.5. Ion Channel Blockers/Modulators
2.1.6. Platelet Aggregation Inhibitor
2.2. Kunitz-Type Serine Protease Inhibitor
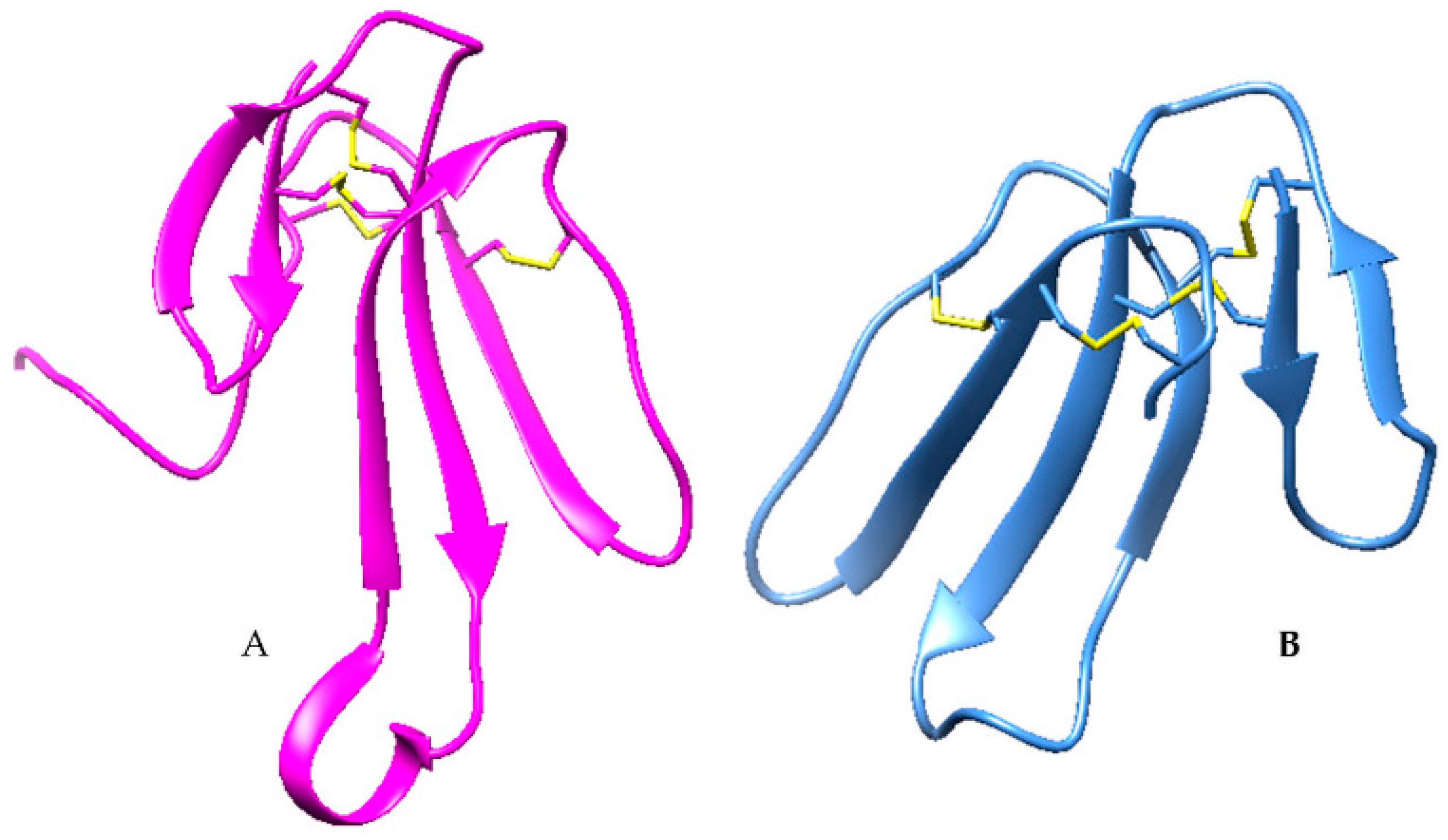
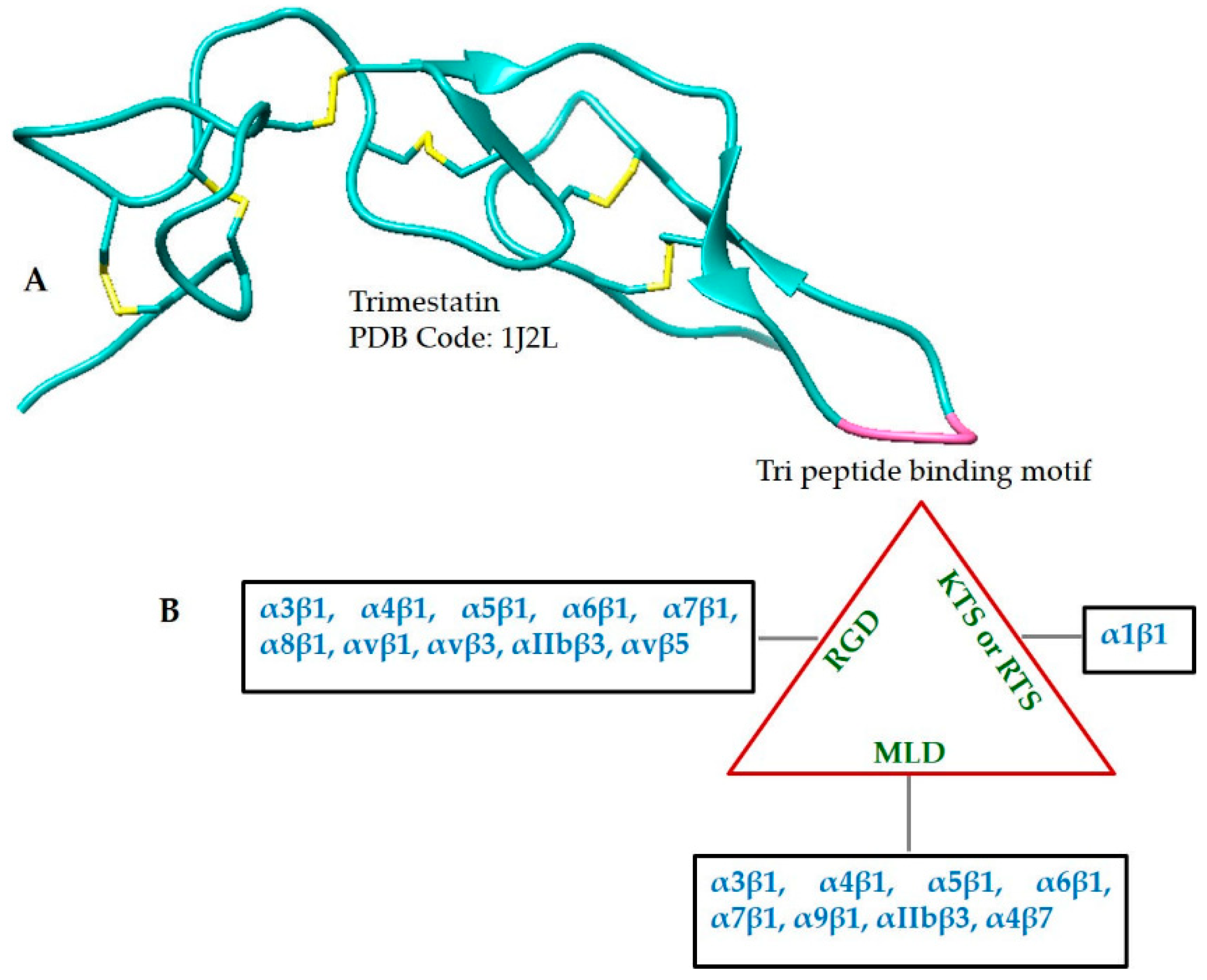
2.3. Disintegrins
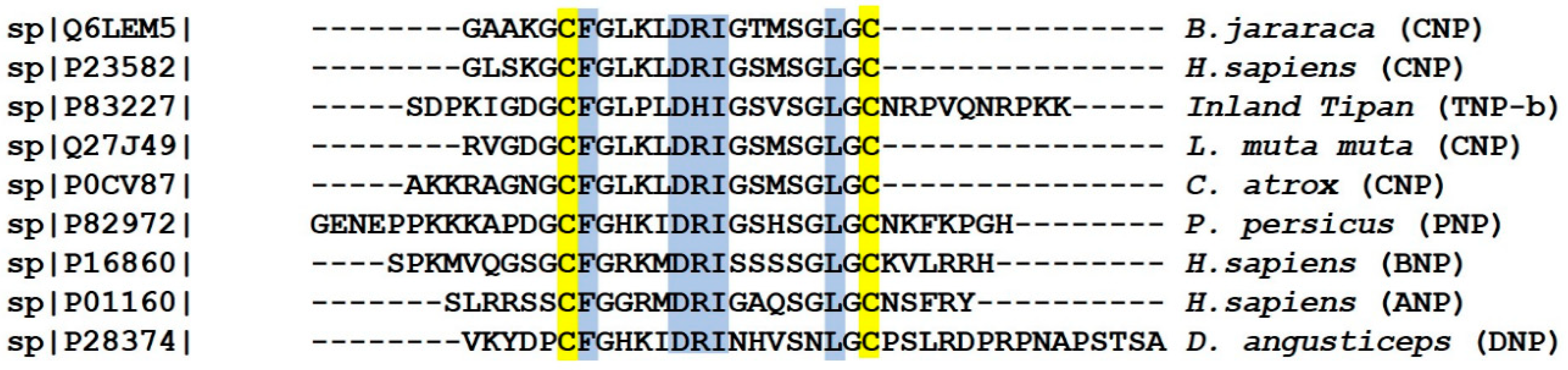
2.4. Natriuretic Peptides (NPs)
2.5. Bradykinin Potentiating Peptides (BPPs)
2.6. Sarafotoxins (SRTXs)
2.7. Tripeptides
2.8. Crotamine
2.9. Waprin Family
2.10. Waglerins
2.11. Antimicrobial Peptides
3. Research Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Utkin, Y.N. Animal venom studies: Current benefits and future developments. World J. Biol. Chem. 2015, 6, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.; Georgieva, D.N.; Thoen, H.H.; Norman, J.A.; Mork, J.; Betzel, C.; Fry, B.G. Venom on ice: First insights into antarctic octopus venoms. Toxicon 2010, 56, 897–913. [Google Scholar] [CrossRef] [PubMed]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Ruder, T.; Ali, S.A.; Ormerod, K.; Brust, A.; Roymanchadi, M.L.; Ventura, S.; Undheim, E.A.; Jackson, T.N.; Mercier, A.J.; King, G.F.; et al. Functional characterization on invertebrate and vertebrate tissues of tachykinin peptides from octopus venoms. Peptides 2013, 47, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.Y.; Kini, R.M. From snake venom toxins to therapeutics—Cardiovascular examples. Toxicon 2012, 59, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake venom: From deadly toxins to life-saving therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A. Venom therapy in multiple sclerosis. Neuropharmacology 2007, 53, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Otvos, R.A.; Heus, F.; Vonk, F.J.; Halff, J.; Bruyneel, B.; Paliukhovich, I.; Smit, A.B.; Niessen, W.M.; Kool, J. Analytical workflow for rapid screening and purification of bioactives from venom proteomes. Toxicon 2013, 76, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Simoes-Silva, R.; Alfonso, J.; Gomez, A.; Holanda, R.J.; Sobrinho, J.C.; Zaqueo, K.D.; Moreira-Dill, L.S.; Kayano, A.M.; Grabner, F.P.; da Silva, S.L.; et al. Snake venom, a natural library of new potential therapeutic molecules: Challenges and current perspectives. Curr. Pharm. Biotechnol. 2018, 19, 308–335. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Del Brutto, V.J. Neurological complications of venomous snake bites: A review. Acta Neurol. Scand. 2012, 125, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, F.; Zhang, Q.; Sun, M.Z.; Gao, Y.; Shao, S. “Anatomical” view of the protein composition and protein characteristics for gloydius shedaoensis snake venom via proteomics approach. Anat. Rec. 2011, 294, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Stocker, K.F. Biology and distributuion of venomous snakes of medical importance and the composition of snake venom. In Handbook of Clinical Toxicology of Animal Venoms and Proteins; Meier, J., White, J., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 367–412. [Google Scholar]
- Georgieva, D.; Arni, R.K.; Betzel, C. Proteome analysis of snake venom toxins: Pharmacological insights. Expert Rev. Proteom. 2008, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A. Analysis of the Low Molecular Weight Peptides of Selected Snake Venoms; University of Hamburg: Hamburg, Germany, 2012. [Google Scholar]
- Calvete, J.J.; Moreno-Murciano, M.P.; Theakston, R.D.; Kisiel, D.G.; Marcinkiewicz, C. Snake venom disintegrins: Novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem. J. 2003, 372, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Lucena, S.; Rodriguez-Acosta, A.; Grilli, E.; Alfonso, A.; Goins, A.; Ogbata, I.; Walls, R.; Suntravat, M.; Uzcategui, N.L.; Guerrero, B.; et al. The characterization of trans-pecos copperhead (agkistrodon contortrix pictigaster) venom and isolation of two new dimeric disintegrins. Biologicals 2016, 44, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Marcinkiewicz, C.; Sanz, L. Snake venomics of bitis gabonica gabonica. Protein family composition, subunit organization of venom toxins, and characterization of dimeric disintegrins bitisgabonin-1 and bitisgabonin-2. J. Proteome Res. 2007, 6, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Evolution of three-finger toxins—A versatile mini protein scaffold. Acta Chim. Slov. 2011, 58, 693–701. [Google Scholar] [PubMed]
- Osipov, A.V.; Rucktooa, P.; Kasheverov, I.E.; Filkin, S.Y.; Starkov, V.G.; Andreeva, T.V.; Sixma, T.K.; Bertrand, D.; Utkin, Y.N.; Tsetlin, V.I. Dimeric alpha-cobratoxin X-ray structure: Localization of intermolecular disulfides and possible mode of binding to nicotinic acetylcholine receptors. J. Biol. Chem. 2012, 287, 6725–6734. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.C.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell. Mol. Life Sci. 2006, 63, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Arruda Macedo, J.K.; Fox, J.W.; de Souza Castro, M. Disintegrins from snake venoms and their applications in cancer research and therapy. Curr. Prot. Pept. Sci. 2015, 16, 532–548. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, Y.; Yang, W.; Xie, Z.; Li, Z. Lx0702, a novel snake venom peptide derivative, inhibits thrombus formation via affecting the binding of fibrinogen with gpiib/iiia. J. Pharmacol. Sci. 2015, 127, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Atlas, N.; Malik, S.B.; Jamil, N.; Salaam, T.O.; Rehman, M.U.; Khan, B.A. Snake venom as an effective tool against colorectal cancer. Protein Pept. Lett. 2018, 25, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Mackessy, S.P. Pharmacological properties and pathophysiological significance of a kunitz-type protease inhibitor (rusvikunin-ii) and its protein complex (rusvikunin complex) purified from daboia russelii russelii venom. Toxicon 2014, 89, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Nicke, A.; Tsetlin, V.I. Nicotinic acetylcholine receptor inhibitors derived from snake and snail venoms. Neuropharmacology 2017, 127, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Araya-Maturana, R. Targeting metastasis with snake toxins: Molecular mechanisms. Toxins 2017, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of snake venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Utsintong, M.; Talley, T.T.; Taylor, P.W.; Olson, A.J.; Vajragupta, O. Virtual screening against alpha-cobratoxin. J. Biomol. Screen. 2009, 14, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, K.; Shirazi, F.H.; Mirakabadi, A.Z.; Vatanpour, H. Cobra venom cytotoxins; apoptotic or necrotic agents? Toxicon 2015, 108, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Cabassi, C.S.; Santospirito, D.; Polverini, E.; Flisi, S.; Cavirani, S.; Taddei, S. Novel naja atra cardiotoxin 1 (ctx-1) derived antimicrobial peptides with broad spectrum activity. PLoS ONE 2018, 13, e0190778. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.R.; Gould, R.J.; Jacobs, J.W.; Friedman, P.A.; Polokoff, M.A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, echis carinatus. J. Biol. Chem. 1988, 263, 19827–19832. [Google Scholar] [PubMed]
- Scarborough, R.M.; Rose, J.W.; Hsu, M.A.; Phillips, D.R.; Fried, V.A.; Campbell, A.M.; Nannizzi, L.; Charo, I.F. Barbourin. A gpiib-iiia-specific integrin antagonist from the venom of sistrurus m. Barbouri. J. Biol. Chem. 1991, 266, 9359–9362. [Google Scholar] [PubMed]
- Moiseeva, N.; Swenson, S.D.; Markland, F.S., Jr.; Bau, R. Purification, crystallization and preliminary X-ray analysis of the disintegrin contortrostatin from agkistrodon contortrix contortrix snake venom. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 2122–2124. [Google Scholar] [CrossRef] [PubMed]
- Juan-Rivera, M.C.; Martinez-Ferrer, M. Integrin inhibitors in prostate cancer. Cancers 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sherwin, R.P.; Parrish, C.; Richters, V.; Groshen, S.G.; Tsao-Wei, D.; Markland, F.S. Contortrostatin, a dimeric disintegrin from agkistrodon contortrix contortrix, inhibits breast cancer progression. Breast Cancer Res. Treat. 2000, 61, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Millers, E.K.; Johnson, L.A.; Birrell, G.W.; Masci, P.P.; Lavin, M.F.; de Jersey, J.; Guddat, L.W. The structure of human microplasmin in complex with textilinin-1, an aprotinin-like inhibitor from the Australian brown snake. PLoS ONE 2013, 8, e54104. [Google Scholar] [CrossRef] [PubMed]
- Flight, S.; Mirtschin, P.; Masci, P.P. Comparison of active venom components between eastern brown snakes collected from south australia and queensland. Ecotoxicology 2006, 15, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Huntley, B.K.; McCormick, D.J.; Ichiki, T.; Sangaralingham, S.J.; Lisy, O.; Burnett, J.C., Jr. Cenderitide: Structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [PubMed]
- St Pierre, L.; Flight, S.; Masci, P.P.; Hanchard, K.J.; Lewis, R.J.; Alewood, P.F.; de Jersey, J.; Lavin, M.F. Cloning and characterisation of natriuretic peptides from the venom glands of australian elapids. Biochimie 2006, 88, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Rocha e Silva, M. Potentiation of bradykinin and eledoisin by bpf (bradykinin potentiating factor) from bothrops jararaca venom. Experientia 1965, 21, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.C.; Porta, L.C.; Lima, A.D.C.; Campeiro, J.D.; Meurer, Y.; Teixeira, N.B.; Duarte, T.; Oliveira, E.B.; Picolo, G.; Godinho, R.O.; et al. Pharmacological characterization of crotamine effects on mice hind limb paralysis employing both ex vivo and in vivo assays: Insights into the involvement of voltage-gated ion channels in the crotamine action on skeletal muscles. PLoS Negl. Trop. Dis. 2018, 12, e0006700. [Google Scholar] [CrossRef] [PubMed]
- Kloog, Y.; Ambar, I.; Sokolovsky, M.; Kochva, E.; Wollberg, Z.; Bdolah, A. Sarafotoxin, a novel vasoconstrictor peptide: Phosphoinositide hydrolysis in rat heart and brain. Science 1988, 242, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.A.; Schmidt, J.J.; Bernheimer, A.W.; Smith, L.A. Characterization and amino acid sequences of two lethal peptides isolated from venom of wagler′s pit viper, trimeresurus wagleri. Toxicon 1991, 29, 227–236. [Google Scholar] [CrossRef]
- Nirthanan, S.; Gwee, M.C. Three-finger alpha-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J. Pharmacol. Sci. 2004, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.; Marchot, P.; Silva, M.; Servent, D. The three-finger toxin fold: A multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 2017, 142, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutierrez, J.M. Toxicovenomics and antivenom profiling of the eastern green mamba snake (dendroaspis angusticeps). J. Proteom. 2016, 136, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Pla, D.; Perez, A.; Rodriguez, Y.; Zavaleta, A.; Salas, M.; Lomonte, B.; Calvete, J.J. Venomic analysis of the poorly studied desert coral snake, micrurus tschudii tschudii, supports the 3ftx/pla(2) dichotomy across micrurus venoms. Toxins 2016, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Hus, K.K.; Buczkowicz, J.; Petrilla, V.; Petrillova, M.; Lyskowski, A.; Legath, J.; Bocian, A. First look at the venom of naja ashei. Molecules 2018, 23, 609. [Google Scholar] [CrossRef] [PubMed]
- Olamendi-Portugal, T.; Batista, C.V.F.; Pedraza-Escalona, M.; Restano-Cassulini, R.; Zamudio, F.Z.; Benard-Valle, M.; Rafael de Roodt, A.; Possani, L.D. New insights into the proteomic characterization of the coral snake micrurus pyrrhocryptus venom. Toxicon 2018, 153, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sharma, M.; Kumar Das, H.; Pratim Sahu, P.; Doley, R. Purification and characterization of nk-3ftx: A three finger toxin from the venom of north east indian monocled cobra. J. Biochem. Mol. Toxicol. 2016, 30, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.L.; Gao, J.F.; Zhang, Y.X.; Shen, S.S.; He, Y.; Wang, J.; Ma, X.M.; Ji, X. Proteomic characterization and comparison of venoms from two elapid snakes (bungarus multicinctus and naja atra) from china. J. Proteom. 2016, 138, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern india naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteom. 2017, 156, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Slagboom, J.; Otvos, R.A.; Cardoso, F.C.; Iyer, J.; Visser, J.C.; van Doodewaerd, B.R.; McCleary, R.J.R.; Niessen, W.M.A.; Somsen, G.W.; Lewis, R.J.; et al. Neurotoxicity fingerprinting of venoms using on-line microfluidic achbp profiling. Toxicon 2018, 148, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, R.H.; Kovalchuk, S.I.; Arapidi, G.P.; Starkov, V.G.; Hoang, A.N.; Thi Nguyen, T.T.; Nguyen, K.C.; Shoibonov, B.B.; Tsetlin, V.I.; Utkin, Y.N. Quantitative proteomic analysis of vietnamese krait venoms: Neurotoxins are the major components in bungarus multicinctus and phospholipases a2 in bungarus fasciatus. Toxicon 2015, 107, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Rusmili, M.R.; Yee, T.T.; Mustafa, M.R.; Hodgson, W.C.; Othman, I. Proteomic characterization and comparison of malaysian bungarus candidus and bungarus fasciatus venoms. J. Proteom. 2014, 110, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.M.F.; Tan, C.H.; Ariaranee, G.C.; Quraishi, N.; Tan, N.H. Venomics of bungarus caeruleus (indian krait): Comparable venom profiles, variable immunoreactivities among specimens from Sri Lanka, India and Pakistan. J. Proteom. 2017, 164, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Wong, K.Y.; Tan, K.Y.; Tan, N.H. Venom proteome of the yellow-lipped sea krait, laticauda colubrina from Bali: Insights into subvenomic diversity, venom antigenicity and cross-neutralization by antivenom. J. Proteom. 2017, 166, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, S.; Petras, D.; Engmark, M.; Sussmuth, R.D.; Whiteley, G.; Albulescu, L.O.; Kazandjian, T.D.; Wagstaff, S.C.; Rowley, P.; Wuster, W.; et al. The medical threat of mamba envenoming in sub-Saharan Africa revealed by genus-wide analysis of venom composition, toxicity and antivenomics profiling of available antivenoms. J. Proteom. 2017, 172, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Heiss, P.; Harrison, R.A.; Sussmuth, R.D.; Calvete, J.J. Top-down venomics of the east african green mamba, dendroaspis angusticeps, and the black mamba, dendroaspis polylepis, highlight the complexity of their toxin arsenals. J. Proteom. 2016, 146, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernandez, J.; Gutierrez, J.M. Unveiling the nature of black mamba (dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Mackessy, S.P.; Sixberry, N.M.; Stura, E.A.; Le Du, M.H.; Menez, R.; Foo, C.S.; Menez, A.; Nirthanan, S.; Kini, R.M. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009, 23, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Pahari, S.; Mackessy, S.P.; Kini, R.M. The venom gland transcriptome of the desert massasauga rattlesnake (sistrurus catenatus edwardsii): Towards an understanding of venom composition among advanced snakes (superfamily colubroidea). BMC Mol. Biol. 2007, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Sasa, M.; Rey-Suarez, P.; Bryan, W.; Gutierrez, J.M. Venom of the coral snake micrurus clarki: Proteomic profile, toxicity, immunological cross-neutralization, and characterization of a three-finger toxin. Toxins 2016, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G. From genome to “venome”: Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Jackson, T.N.; Undheim, E.A.; Ali, S.A.; Antunes, A.; Fry, B.G. Three-fingered ravers: Rapid accumulation of variations in exposed residues of snake venom toxins. Toxins 2013, 5, 2172–2208. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom--milestones of discovery. Toxicon 2013, 62, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.I.; Reeks, T.; Vetter, I.; Vergara, I.; Kovtun, O.; Lewis, R.J.; Alewood, P.F.; Durek, T. Isolation and structural and pharmacological characterization of alpha-elapitoxin-dpp2d, an amidated three finger toxin from black mamba venom. Biochemistry 2014, 53, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lee, C.Y. Isolation of neurotoxins from the venom of bungarus multicinctus and their modes of neuromuscular blocking action. Arch. Int. Pharmacodyn. Ther. 1963, 144, 241–257. [Google Scholar] [PubMed]
- Changeux, J.P.; Kasai, M.; Lee, C.Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Nat. Acad. Sci. USA 1970, 67, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.S. Contribution of a snake venom toxin to myasthenia gravis: The discovery of alpha-bungarotoxin in Taiwan. J. Hist. Neurosci. 2005, 14, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.M.; Brengman, J.; Neubauer, D.; Sine, S.M.; Engel, A.G. Investigation of congenital myasthenia reveals functional asymmetry of invariant acetylcholine receptor (achr) cys-loop aspartates. J. Biol. Chem. 2016, 291, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Kryukova, E.V.; Shelukhina, I.V.; Kolacheva, A.A.; Alieva, A.K.; Shadrina, M.I.; Slominsky, P.A.; Kasheverov, I.E.; Utkin, Y.N.; Ugrumov, M.V.; Tsetlin, V.I. Possible involvement of neuronal nicotinic acetylcholine receptors in compensatory brain mechanisms at early stages of Parkinson’s disease. Biomeditsinskaia Khimiia 2017, 63, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Kou, J.Q.; Wang, S.Z.; Chen, C.X.; Qin, Z.H. Neurotoxin from naja naja atra venom inhibits skin allograft rejection in rats. Int. Immunopharmacol. 2015, 28, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Puttaswamy, V.; Adams, D.J.; Kini, R.M. A distinct functional site in omega-neurotoxins: Novel antagonists of nicotinic acetylcholine receptors from snake venom. ACS Chem. Biol. 2015, 10, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Jerusalinsky, D.; Kornisiuk, E.; Alfaro, P.; Quillfeldt, J.; Ferreira, A.; Rial, V.E.; Duran, R.; Cervenansky, C. Muscarinic toxins: Novel pharmacological tools for the muscarinic cholinergic system. Toxicon 2000, 38, 747–761. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Paramonov, A.S.; Chugunov, A.O.; Janickova, H.; Dolejsi, E.; Dolezal, V.; Utkin, Y.N.; Tsetlin, V.I.; et al. Structural insight into specificity of interactions between nonconventional three-finger weak toxin from naja kaouthia (wtx) and muscarinic acetylcholine receptors. J. Biol. Chem. 2015, 290, 23616–23630. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Zhou, X.; Chong, M.Z.; D′Hoedt, D.; Foo, C.S.; Rajagopalan, N.; Nirthanan, S.; Bertrand, D.; Sivaraman, J.; Kini, R.M. Structural and functional characterization of a novel homodimeric three-finger neurotoxin from the venom of ophiophagus hannah (king cobra). J. Biol. Chem. 2010, 285, 8302–8315. [Google Scholar] [CrossRef] [PubMed]
- Antil-Delbeke, S.; Gaillard, C.; Tamiya, T.; Corringer, P.J.; Changeux, J.P.; Servent, D.; Menez, A. Molecular determinants by which a long chain toxin from snake venom interacts with the neuronal alpha 7-nicotinic acetylcholine receptor. J. Biol. Chem. 2000, 275, 29594–29601. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.Y.; Chiang, C.M.; Hseu, Y.C.; Vyas, A.A.; Rule, G.S.; Wu, W. Two distinct types of cardiotoxin as revealed by the structure and activity relationship of their interaction with zwitterionic phospholipid dispersions. J. Biol. Chem. 1994, 269, 14473–14483. [Google Scholar] [PubMed]
- Dubovskii, P.V.; Konshina, A.G.; Efremov, R.G. Cobra cardiotoxins: Membrane interactions and pharmacological potential. Curr. Med. Chem. 2014, 21, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Lesovoy, D.M.; Dubinnyi, M.A.; Konshina, A.G.; Utkin, Y.N.; Efremov, R.G.; Arseniev, A.S. Interaction of three-finger toxins with phospholipid membranes: Comparison of s- and p-type cytotoxins. Biochem. J. 2005, 387, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Konshina, A.G.; Dubovskii, P.V.; Efremov, R.G. Structure and dynamics of cardiotoxins. Curr. Prot. Pept. Sci. 2012, 13, 570–584. [Google Scholar] [CrossRef]
- Munawar, A.; Akrem, A.; Hussain, A.; Spencer, P.; Betzel, C. Molecular model of cytotoxin-1 from naja mossambica mossambica venom in complex with chymotrypsin. Theor. Biol. Forum 2015, 108, 89–99. [Google Scholar] [PubMed]
- Girish, V.M.; Kumar, S.; Joseph, L.; Jobichen, C.; Kini, R.M.; Sivaraman, J. Identification and structural characterization of a new three-finger toxin hemachatoxin from hemachatus haemachatus venom. PLoS ONE 2012, 7, e48112. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, N.; Pung, Y.F.; Zhu, Y.Z.; Wong, P.T.; Kumar, P.P.; Kini, R.M. Beta-cardiotoxin: A new three-finger toxin from ophiophagus hannah (king cobra) venom with beta-blocker activity. FASEB J. 2007, 21, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.C.; Fu, Y.S.; Chang, L.S.; Lin, S.R. Cardiotoxin iii inhibits hepatocyte growth factor-induced epithelial-mesenchymal transition and suppresses invasion of mda-mb-231 cells. J. Biochem. Mol. Toxicol. 2016, 30, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, T.; Saha, P.P.; Sarkar, A.; Gomes, A. Evaluation of cytotoxicity of a purified venom protein from naja kaouthia (nkct1) using gold nanoparticles for targeted delivery to cancer cell. Chem.-Biol. Interact. 2017, 261, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Charpantier, E.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Cheah, L.S.; Kini, R.M.; Bertrand, D. Neuromuscular effects of candoxin, a novel toxin from the venom of the malayan krait (bungarus candidus). Br. J. Pharmacol. 2003, 139, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Diochot, S.; Baron, A.; Salinas, M.; Douguet, D.; Scarzello, S.; Dabert-Gay, A.S.; Debayle, D.; Friend, V.; Alloui, A.; Lazdunski, M.; et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012, 490, 552–555. [Google Scholar] [CrossRef] [PubMed]
- De Weille, J.R.; Schweitz, H.; Maes, P.; Tartar, A.; Lazdunski, M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the l-type calcium channel. Proc. Nat. Acad. Sci. USA 1991, 88, 2437–2440. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, T.; Matsushima, K.; Kawaguchi, A.; Wada, T.; Yoshida, S.; Ichida, S. Increased response to high kcl-induced elevation in the intracellular-ca(2+) concentration in differentiated ng108-15 cell and the inhibitory effect of the l-type ca(2+) channel blocker, calciseptine. Neurochem. Res. 2006, 31, 33–40. [Google Scholar] [PubMed]
- Albrand, J.P.; Blackledge, M.J.; Pascaud, F.; Hollecker, M.; Marion, D. Nmr and restrained molecular dynamics study of the three-dimensional solution structure of toxin fs2, a specific blocker of the l-type calcium channel, isolated from black mamba venom. Biochemistry 1995, 34, 5923–5937. [Google Scholar] [CrossRef] [PubMed]
- Mourier, G.; Salinas, M.; Kessler, P.; Stura, E.A.; Leblanc, M.; Tepshi, L.; Besson, T.; Diochot, S.; Baron, A.; Douguet, D.; et al. Mambalgin-1 pain-relieving peptide, stepwise solid-phase synthesis, crystal structure, and functional domain for acid-sensing ion channel 1a inhibition. J. Biol. Chem. 2016, 291, 2616–2629. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Deuis, J.R.; Dashevsky, D.; Dobson, J.; Jackson, T.N.; Brust, A.; Xie, B.; Koludarov, I.; Debono, J.; Hendrikx, I.; et al. The snake with the scorpion′s sting: Novel three-finger toxin sodium channel activators from the venom of the long-glanded blue coral snake (calliophis bivirgatus). Toxins 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Torres, I.O.; Jin, T.B.; Cadene, M.; Chait, B.T.; Poget, S.F. Discovery and characterisation of a novel toxin from dendroaspis angusticeps, named tx7335, that activates the potassium channel kcsa. Sci. Rep. 2016, 6, 23904. [Google Scholar] [CrossRef] [PubMed]
- McDowell, R.S.; Dennis, M.S.; Louie, A.; Shuster, M.; Mulkerrin, M.G.; Lazarus, R.A. Mambin, a potent glycoprotein iib-iiia antagonist and platelet aggregation inhibitor structurally related to the short neurotoxins. Biochemistry 1992, 31, 4766–4772. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Davies, J.; Lu, D.; Xia, M.; Wattam, B.; Shang, D.; Sun, Y.; Scully, M.; Kakkar, V. The effect of the single substitution of arginine within the rgd tripeptide motif of a modified neurotoxin dendroaspin on its activity of platelet aggregation and cell adhesion. Cell Commun. Adhes. 2006, 13, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Kalita, B.; Mackessy, S.P. A proteomic analysis of pakistan daboia russelii russelii venom and assessment of potency of indian polyvalent and monovalent antivenom. J. Proteom. 2016, 144, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Trusch, M.; Georgieva, D.; Spencer, P.; Frochaux, V.; Harder, S.; Arni, R.K.; Duhalov, D.; Genov, N.; Schluter, H.; et al. Venom peptide analysis of vipera ammodytes meridionalis (viperinae) and bothrops jararacussu (crotalinae) demonstrates subfamily-specificity of the peptidome in the family viperidae. Mol. Biosyst. 2011, 7, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Trusch, M.; Georgieva, D.; Hildebrand, D.; Kwiatkowski, M.; Behnken, H.; Harder, S.; Arni, R.; Spencer, P.; Schluter, H.; et al. Elapid snake venom analyses show the specificity of the peptide composition at the level of genera naja and notechis. Toxins 2014, 6, 850–868. [Google Scholar] [CrossRef] [PubMed]
- Zupunski, V.; Kordis, D.; Gubensek, F. Adaptive evolution in the snake venom kunitz/bpti protein family. FEBS Lett. 2003, 547, 131–136. [Google Scholar] [CrossRef]
- Millers, E.K.; Trabi, M.; Masci, P.P.; Lavin, M.F.; de Jersey, J.; Guddat, L.W. Crystal structure of textilinin-1, a kunitz-type serine protease inhibitor from the venom of the australian common brown snake (pseudonaja textilis). FEBS J. 2009, 276, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.S.; Chandy, K.G. Venom-derived peptide inhibitors of voltage-gated potassium channels. Neuropharmacology 2017, 127, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Vanzolini, K.L.; Ainsworth, S.; Bruyneel, B.; Herzig, V.; Seraus, M.G.L.; Somsen, G.W.; Casewell, N.R.; Cass, Q.B.; Kool, J. Rapid ligand fishing for identification of acetylcholinesterase-binding peptides in snake venom reveals new properties of dendrotoxins. Toxicon 2018, 152, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, T. Crystal structure of alpha-dendrotoxin from the green mamba venom and its comparison with the structure of bovine pancreatic trypsin inhibitor. J. Mol. Biol. 1992, 224, 671–683. [Google Scholar] [CrossRef]
- Jin, L.; Wu, Y. Molecular mechanism of delta-dendrotoxin-potassium channel recognition explored by docking and molecular dynamic simulations. J. Mol. Recognit. 2011, 24, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Katoh, E.; Nishio, H.; Inui, T.; Nishiuchi, Y.; Kimura, T.; Sakakibara, S.; Yamazaki, T. Structural basis for the biological activity of dendrotoxin-i, a potent potassium channel blocker. Biopolymers 2000, 54, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.; Bell, N.; Reid, P.; Smith, L.A.; McIntosh, P.; Robertson, B.; Dolly, J.O. Identification of residues in dendrotoxin k responsible for its discrimination between neuronal k+ channels containing kv1.1 and 1.2 alpha subunits. Eur. J. Biochem. 1999, 263, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, J.; Wang, B.; Cao, Z.; Li, W.; Wu, Y.; Chen, Z. Bf9, the first functionally characterized snake toxin peptide with kunitz-type protease and potassium channel inhibiting properties. J. Biochem. Mol. Toxicol. 2014, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Wang, S.; Zhang, M.; Gao, F.; Yin, C.; Li, H.; Zhang, Y.; Hu, S.J.; Duan, J.H. Alpha-dendrotoxin-sensitive kv1 channels contribute to conduction failure of polymodal nociceptive c-fibers from rat coccygeal nerve. J. Neurophysiol. 2016, 115, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Mukherjee, A.K. Pathophysiological significance and therapeutic applications of snake venom protease inhibitors. Toxicon 2017, 131, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Earl, S.T.; Masci, P.P.; de Jersey, J.; Lavin, M.F.; Dixon, J. Drug development from australian elapid snake venoms and the venomics pipeline of candidates for haemostasis: Textilinin-1 (q8008), haempatch (q8009) and covase (v0801). Toxicon 2012, 59, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Flight, S.; Johnson, L.; Trabi, M.; Gaffney, P.; Lavin, M.; de Jersey, J.; Masci, P. Comparison of textilinin-1 with aprotinin as serine protease inhibitors and as antifibrinolytic agents. Pathophysiol. Haemost. Thromb. 2005, 34, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.P.; Whitaker, A.N.; Sparrow, L.G.; de Jersey, J.; Winzor, D.J.; Watters, D.J.; Lavin, M.F.; Gaffney, P.J. Textilinins from pseudonaja textilis textilis. Characterization of two plasmin inhibitors that reduce bleeding in an animal model. Blood Coagul. Fibrinol. 2000, 11, 385–393. [Google Scholar] [CrossRef]
- Flight, S.M.; Johnson, L.A.; Du, Q.S.; Warner, R.L.; Trabi, M.; Gaffney, P.J.; Lavin, M.F.; de Jersey, J.; Masci, P.P. Textilinin-1, an alternative anti-bleeding agent to aprotinin: Importance of plasmin inhibition in controlling blood loss. Br. J. Haematol. 2009, 145, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Vivas, J.; Ibarra, C.; Salazar, A.M.; Neves-Ferreira, A.G.; Sanchez, E.E.; Perales, J.; Rodriguez-Acosta, A.; Guerrero, B. Purification and characterization of tenerplasminin-1, a serine peptidase inhibitor with antiplasmin activity from the coral snake (micrurus tener tener) venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 179, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Honore, S.; Bazaa, A.; Abdelkafi-Koubaa, Z.; Ellafi, A.; Mabrouk, K.; Kovacic, H.; El Ayeb, M.; Marrakchi, N.; Luis, J. Pivl, a snake venom kunitz-type serine protease inhibitor, inhibits in vitro and in vivo angiogenesis. Microvasc. Res. 2014, 95, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Kallech-Ziri, O.; Bazaa, A.; Othman, H.; Mabrouk, K.; Zouari-Kessentini, R.; Sanz, L.; Calvete, J.J.; Srairi-Abid, N.; El Ayeb, M.; et al. Pivl, a new serine protease inhibitor from macrovipera lebetina transmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol. 2013, 32, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Earl, S.T.; Richards, R.; Johnson, L.A.; Flight, S.; Anderson, S.; Liao, A.; de Jersey, J.; Masci, P.P.; Lavin, M.F. Identification and characterisation of kunitz-type plasma kallikrein inhibitors unique to oxyuranus sp. Snake venoms. Biochimie 2012, 94, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Saviola, A.J.; Modahl, C.M.; Mackessy, S.P. Disintegrins of crotalus simus tzabcan venom: Isolation, characterization and evaluation of the cytotoxic and anti-adhesion activities of tzabcanin, a new rgd disintegrin. Biochimie 2015, 116, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. The continuing saga of snake venom disintegrins. Toxicon 2013, 62, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Accelerated evolution of toxin genes: Exonization and intronization in snake venom disintegrin/metalloprotease genes. Toxicon 2018, 148, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bilgrami, S.; Yadav, S.; Kaur, P.; Sharma, S.; Perbandt, M.; Betzel, C.; Singh, T.P. Crystal structure of the disintegrin heterodimer from saw-scaled viper (echis carinatus) at 1.9 a resolution. Biochemistry 2005, 44, 11058–11066. [Google Scholar] [CrossRef] [PubMed]
- Juarez, P.; Comas, I.; Gonzalez-Candelas, F.; Calvete, J.J. Evolution of snake venom disintegrins by positive darwinian selection. Mol. Biol. Evol. 2008, 25, 2391–2407. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka-Patynowski, I.; Niewiarowski, S.; Marcinkiewicz, C.; Calvete, J.J.; Marcinkiewicz, M.M.; McLane, M.A. Structural requirements of echistatin for the recognition of alpha(v)beta(3) and alpha(5)beta(1) integrins. J. Biol. Chem. 1999, 274, 37809–37814. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Marcinkiewicz, C. Non-rgd-containing snake venom disintegrins, functional and structural relations. Toxicon 2011, 58, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Hong, S.Y.; Kim, Y.D.; Nam, H.S.; Kang, S.; Yang, S.H.; Heo, J.H. Thrombolytic effects of the snake venom disintegrin saxatilin determined by novel assessment methods: A fecl3-induced thrombosis model in mice. PLoS ONE 2013, 8, e81165. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Succar, B.B.; de Moraes, J.A.; Saldanha-Gama, R.F.G.; Barja-Fidalgo, C.; Zingali, R.B. Recombinant and chimeric disintegrins in preclinical research. Toxins 2018, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Peerlinck, K.; De Lepeleire, I.; Goldberg, M.; Farrell, D.; Barrett, J.; Hand, E.; Panebianco, D.; Deckmyn, H.; Vermylen, J.; Arnout, J. Mk-383 (l-700,462), a selective nonpeptide platelet glycoprotein iib/iiia antagonist, is active in man. Circulation 1993, 88, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, R.M.; Naughton, M.A.; Teng, W.; Rose, J.W.; Phillips, D.R.; Nannizzi, L.; Arfsten, A.; Campbell, A.M.; Charo, I.F. Design of potent and specific integrin antagonists. Peptide antagonists with high specificity for glycoprotein iib-iiia. J. Biol. Chem. 1993, 268, 1066–1073. [Google Scholar] [PubMed]
- Vink, S.; Jin, A.H.; Poth, K.J.; Head, G.A.; Alewood, P.F. Natriuretic peptide drug leads from snake venom. Toxicon 2012, 59, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Yasue, H.; Morita, E.; Sakaino, N.; Jougasaki, M.; Kurose, M.; Mukoyama, M.; Saito, Y.; Nakao, K.; Imura, H. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation 1991, 84, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, P.; Vuolteenaho, O.; Ruskoaho, H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: Effect of stretching. Endocrinology 1993, 132, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Suga, S.; Itoh, H.; Komatsu, Y.; Ogawa, Y.; Hama, N.; Yoshimasa, T.; Nakao, K. Cytokine-induced c-type natriuretic peptide (cnp) secretion from vascular endothelial cells--evidence for cnp as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology 1993, 133, 3038–3041. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 191, 341–366. [Google Scholar]
- Volpe, M. Natriuretic peptides and cardio-renal disease. Int. J. Cardiol. 2014, 176, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Kini, R.M. Decoding the molecular switches of natriuretic peptides which differentiate its vascular and renal functions. Biochem. J. 2018, 475, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Kini, R.M. Tail wags the dog: Activity of krait natriuretic peptide is determined by its c-terminal tail in a natriuretic peptide receptor-independent manner. Biochem. J. 2015, 469, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Reeks, T.; Jones, A.; Brust, A.; Sridharan, S.; Corcilius, L.; Wilkinson, B.L.; Thaysen-Andersen, M.; Payne, R.J.; Kini, R.M.; Daly, N.L.; et al. A defined alpha-helix in the bifunctional o-glycosylated natriuretic peptide tcnpa from the venom of tropidechis carinatus. Angew. Chem. Int. Ed. Engl. 2015, 54, 4828–4831. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Lomonte, B.; Leon, G.; Rucavado, A.; Chaves, F.; Angulo, Y. Trends in snakebite envenomation therapy: Scientific, technological and public health considerations. Curr. Pharm. Des. 2007, 13, 2935–2950. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W. Assembling an arsenal: Origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Mol. Biol. Evol. 2004, 21, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lainchbury, J.G.; Burnett, J.C., Jr. Natriuretic peptide receptors and neutral endopeptidase in mediating the renal actions of a new therapeutic synthetic natriuretic peptide dendroaspis natriuretic peptide. J. Am. Coll. Cardiol. 2002, 40, 1186–1191. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Yu, G.; Chen, Z.; Zhou, X.; Zhu, S.; Li, R.; Zhang, Y.; Lu, Q. A novel natriuretic peptide from the cobra venom. Toxicon 2011, 57, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tourki, B.; Mateo, P.; Morand, J.; Elayeb, M.; Godin-Ribuot, D.; Marrakchi, N.; Belaidi, E.; Messadi, E. Lebetin 2, a snake venom-derived natriuretic peptide, attenuates acute myocardial ischemic injury through the modulation of mitochondrial permeability transition pore at the time of reperfusion. PLoS ONE 2016, 11, e0162632. [Google Scholar] [CrossRef] [PubMed]
- Ondetti, M.A.; Williams, N.J.; Sabo, E.F.; Pluscec, J.; Weaver, E.R.; Kocy, O. Angiotensin-converting enzyme inhibitors from the venom of bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry 1971, 10, 4033–4039. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Ondetti, M.A. Design of angiotensin converting enzyme inhibitors. Nat. Med. 1999, 5, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Van Vark, L.C.; Bertrand, M.; Akkerhuis, K.M.; Brugts, J.J.; Fox, K.; Mourad, J.J.; Boersma, E. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: A meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158 998 patients. Eur. Heart J. 2012, 33, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Zahid, A.; Negm, A.; Akrem, A.; Spencer, P.; Betzel, C. Isolation and characterization of bradykinin potentiating peptides from agkistrodon bilineatus venom. Proteome Sci. 2016, 14, 1. [Google Scholar] [CrossRef]
- Morais, K.L.; Ianzer, D.; Miranda, J.R.; Melo, R.L.; Guerreiro, J.R.; Santos, R.A.; Ulrich, H.; Lameu, C. Proline rich-oligopeptides: Diverse mechanisms for antihypertensive action. Peptides 2013, 48, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Resende, L.M.; Watanabe, R.K.; Carregari, V.C.; Huancahuire-Vega, S.; Coutinho-Neto, A.; Soares, A.M.; Vale, N.; Marangoni, S.; Da, S.S. Snake venom peptides and low mass proteins: Molecular tools and therapeutic agents. Curr. Med. Chem. 2017, 24, 3254–3282. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.; Ligny-Lemaire, C.; Wollberg, Z.; Wery, M.; Galat, A.; Ogawa, T.; Muller, B.H.; Lamthanh, H.; Doljansky, Y.; Bdolah, A.; et al. Long-sarafotoxins: Characterization of a new family of endothelin-like peptides. Peptides 2004, 25, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Ducancel, F. Endothelin-like peptides. Cell. Mol. Life Sci. 2005, 62, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Quinton, L.; Le Caer, J.P.; Phan, G.; Ligny-Lemaire, C.; Bourdais-Jomaron, J.; Ducancel, F.; Chamot-Rooke, J. Characterization of toxins within crude venoms by combined use of fourier transform mass spectrometry and cloning. Anal. Chem. 2005, 77, 6630–6639. [Google Scholar] [CrossRef] [PubMed]
- Mourier, G.; Hajj, M.; Cordier, F.; Zorba, A.; Gao, X.; Coskun, T.; Herbet, A.; Marcon, E.; Beau, F.; Delepierre, M.; et al. Pharmacological and structural characterization of long-sarafotoxins, a new family of endothelin-like peptides: Role of the c-terminus extension. Biochimie 2012, 94, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kloog, Y.; Sokolovsky, M. Similarities in mode and sites of action of sarafotoxins and endothelins. Trends Pharmacol. Sci. 1989, 10, 212–214. [Google Scholar] [CrossRef]
- Malaquin, S.; Bayat, S.; Abou Arab, O.; Mourier, G.; Lorne, E.; Kamel, S.; Dupont, H.; Ducancel, F.; Mahjoub, Y. Respiratory effects of sarafotoxins from the venom of different atractaspis genus snake species. Toxins 2016, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, Y.; Malaquin, S.; Mourier, G.; Lorne, E.; Abou Arab, O.; Massy, Z.A.; Dupont, H.; Ducancel, F. Short- versus long-sarafotoxins: Two structurally related snake toxins with very different in vivo haemodynamic effects. PLoS ONE 2015, 10, e0132864. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, B.; Zhu, S.; Rong, R. Hypotensive peptides from snake venoms: Structure, function and mechanism. Curr. Top. Med. Chem. 2015, 15, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Woodward, B.; Williams, K.I. Actions of endothelins and sarafotoxin 6c in the rat isolated perfused lung. Br. J. Pharmacol. 1995, 115, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Woodward, B.; Williams, K.I. Investigation of the contributions of nitric oxide and prostaglandins to the actions of endothelins and sarafotoxin 6c in rat isolated perfused lungs. Br. J. Pharmacol. 1996, 118, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.; Kaiser, I.I. Inhibition of metalloproteinases in bothrops asper venom by endogenous peptides. Toxicon 1993, 31, 889–899. [Google Scholar] [CrossRef]
- Huang, K.F.; Hung, C.C.; Wu, S.H.; Chiou, S.H. Characterization of three endogenous peptide inhibitors for multiple metalloproteinases with fibrinogenolytic activity from the venom of taiwan habu (trimeresurus mucrosquamatus). Biochem. Biophys. Res. Commun. 1998, 248, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, S.C.; Favreau, P.; Cheneval, O.; Laing, G.D.; Wilkinson, M.C.; Miller, R.L.; Stocklin, R.; Harrison, R.A. Molecular characterisation of endogenous snake venom metalloproteinase inhibitors. Biochem. Biophys. Res. Commun. 2008, 365, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.T.; Pitts, M.; Tongyoo, P.; Rojnuckarin, P.; Wilkinson, M.C. Snake venom metalloproteinases and their peptide inhibitors from myanmar russell′s viper venom. Toxins 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.P.; Santos, N.A.G.; Sisti, F.M.; Ferreira, R.S.; Santos-Filho, N.A.; Cintra, A.C.O.; Cilli, E.M.; Sampaio, S.V.; Santos, A.C. A synthetic snake-venom-based tripeptide (glu-val-trp) protects pc12 cells from mpp(+) toxicity by activating the ngf-signaling pathway. Peptides 2018, 104, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Xu, Z.; Qian, C.; Jiang, F.; Ding, X.; Ruan, Y.; Ding, Z.; Fan, Y. Antiplatelet aggregation and antithrombosis efficiency of peptides in the snake venom of deinagkistrodon acutus: Isolation, identification, and evaluation. Evid. Based Complement. Altern. Med. 2015, 2015, 412841. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Hayashi, M.A.; Prieto da Silva, A.R.; Pereira, A.; De Sa Junior, P.L.; Zaharenko, A.J.; Radis-Baptista, G.; Kerkis, A.; Yamane, T. State of the art in the studies on crotamine, a cell penetrating peptide from south american rattlesnake. Biomed. Res. Int. 2014, 2014, 675985. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.A.; Gabdulkhakov, A.; Georgieva, D.; Sankaran, B.; Murakami, M.T.; Arni, R.K.; Betzel, C. Structure of the polypeptide crotamine from the brazilian rattlesnake crotalus durissus terrificus. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Kerkis, A.; Hayashi, M.A.; Pereira, A.S.; Silva, F.S.; Oliveira, E.B.; Prieto da Silva, A.R.; Yamane, T.; Radis-Baptista, G.; Kerkis, I. Crotamine toxicity and efficacy in mouse models of melanoma. Expert Opin. Investig. Drugs 2011, 20, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. Host defense peptides as new weapons in cancer treatment. Cell. Mol. Life Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, R.; Bernardi, M.M.; Cury, Y. Analgesic effect evoked by low molecular weight substances extracted from crotalus durissus terrificus venom. Toxicon 1993, 31, 1257–1265. [Google Scholar] [CrossRef]
- Batista da Cunha, D.; Pupo Silvestrini, A.V.; Gomes da Silva, A.C.; Maria de Paula Estevam, D.; Pollettini, F.L.; de Oliveira Navarro, J.; Alves, A.A.; Remedio Zeni Beretta, A.L.; Annichino Bizzacchi, J.M.; Pereira, L.C.; et al. Mechanistic insights into functional characteristics of native crotamine. Toxicon 2018, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mambelli-Lisboa, N.C.; Mozer Sciani, J.; Rossan Brandao Prieto da Silva, A.; Kerkis, I. Co-localization of crotamine with internal membranes and accentuated accumulation in tumor cells. Molecules 2018, 23, 968. [Google Scholar] [CrossRef] [PubMed]
- Banigan, J.R.; Mandal, K.; Sawaya, M.R.; Thammavongsa, V.; Hendrickx, A.P.; Schneewind, O.; Yeates, T.O.; Kent, S.B. Determination of the X-ray structure of the snake venom protein omwaprin by total chemical synthesis and racemic protein crystallography. Prot. Sci. 2010, 19, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Wong, H.Y.; Desai, M.; Moochhala, S.; Kuchel, P.W.; Kini, R.M. Identification of a novel family of proteins in snake venoms. Purification and structural characterization of nawaprin from naja nigricollis snake venom. J. Biol. Chem. 2003, 278, 40097–40104. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.G.; Fry, B.G.; Alewood, P.; Kumar, P.P.; Kini, R.M. Antimicrobial activity of omwaprin, a new member of the waprin family of snake venom proteins. Biochem. J. 2007, 402, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, V.; Alewood, P.F.; Mobli, M.; King, G.F. The structural universe of disulfide-rich venom peptides. In Venoms to Drugs: Venom as a Source for the Development of Human Therapeutics; King, G., Ed.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 57–58. [Google Scholar]
- St Pierre, L.; Earl, S.T.; Filippovich, I.; Sorokina, N.; Masci, P.P.; De Jersey, J.; Lavin, M.F. Common evolution of waprin and kunitz-like toxin families in australian venomous snakes. Cell. Mol. Life Sci. 2008, 65, 4039–4054. [Google Scholar] [CrossRef] [PubMed]
- Viala, V.L.; Hildebrand, D.; Trusch, M.; Fucase, T.M.; Sciani, J.M.; Pimenta, D.C.; Arni, R.K.; Schluter, H.; Betzel, C.; Mirtschin, P.; et al. Venomics of the australian eastern brown snake (pseudonaja textilis): Detection of new venom proteins and splicing variants. Toxicon 2015, 107, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.F.; Andrade-Silva, D.; Zelanis, A.; Paes Leme, A.F.; Rocha, M.M.; Menezes, M.C.; Serrano, S.M.; Junqueira-de-Azevedo Ide, L. Trends in the evolution of snake toxins underscored by an integrative omics approach to profile the venom of the colubrid phalotris mertensi. Genome Biol. Evol. 2016, 8, 2266–2287. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.J.; Weinstein, S.A.; Smith, L.A. Molecular properties and structure-function relationships of lethal peptides from venom of wagler′s pit viper, trimeresurus wagleri. Toxicon 1992, 30, 1027–1036. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Yap, M.K.; Tan, N.H. Venomics of tropidolaemus wagleri, the sexually dimorphic temple pit viper: Unveiling a deeply conserved atypical toxin arsenal. Sci. Rep. 2017, 7, 43237. [Google Scholar] [CrossRef] [PubMed]
- Zainal Abidin, S.A.; Rajadurai, P.; Chowdhury, M.E.; Ahmad Rusmili, M.R.; Othman, I.; Naidu, R. Proteomic characterization and comparison of malaysian tropidolaemus wagleri and cryptelytrops purpureomaculatus venom using shotgun-proteomics. Toxins 2016, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Debono, J.; Xie, B.; Violette, A.; Fourmy, R.; Jaeger, M.; Fry, B.G. Viper venom botox: The molecular origin and evolution of the waglerin peptides used in anti-wrinkle skin cream. J. Mol. Evol. 2017, 84, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Molles, B.E.; Rezai, P.; Kline, E.F.; McArdle, J.J.; Sine, S.M.; Taylor, P. Identification of residues at the alpha and epsilon subunit interfaces mediating species selectivity of waglerin-1 for nicotinic acetylcholine receptors. J. Biol. Chem. 2002, 277, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, M.; Akhavan, M.M.; Fallah, F.; Karimi, A. A recombinant snake cathelicidin derivative peptide: Antibiofilm properties and expression in escherichia coli. Biomolecules 2018, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Perumal Samy, R.; Stiles, B.G.; Franco, O.L.; Sethi, G.; Lim, L.H.K. Animal venoms as antimicrobial agents. Biochem. Pharmacol. 2017, 134, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Falcao, C.B.; de La Torre, B.G.; Perez-Peinado, C.; Barron, A.E.; Andreu, D.; Radis-Baptista, G. Vipericidins: A novel family of cathelicidin-related peptides from the venom gland of south american pit vipers. Amino Acids 2014, 46, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Jesupret, C.; Baumann, K.; Jackson, T.N.; Ali, S.A.; Yang, D.C.; Greisman, L.; Kern, L.; Steuten, J.; Jouiaei, M.; Casewell, N.R.; et al. Vintage venoms: Proteomic and pharmacological stability of snake venoms stored for up to eight decades. J. Proteom. 2014, 105, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Undheim, E.A.B.; Pineda, S.S.; Jin, A.H.; Lavergne, V.; Fry, B.G.; Lewis, R.J.; Alewood, P.F.; King, G.F. Venoms-based drug discovery: Proteomic and transcriptomic approaches. RSC Drug Discov. 2015, 11, 80–96. [Google Scholar]
- Meyer, A.; Dierks, K.; Hilterhaus, D.; Klupsch, T.; Muhlig, P.; Kleesiek, J.; Schopflin, R.; Einspahr, H.; Hilgenfeld, R.; Betzel, C. Single-drop optimization of protein crystallization. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Oberthuer, D.; Melero-Garcia, E.; Dierks, K.; Meyer, A.; Betzel, C.; Garcia-Caballero, A.; Gavira, J.A. Monitoring and scoring counter-diffusion protein crystallization experiments in capillaries by in situ dynamic light scattering. PLoS ONE 2012, 7, e33545. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Z.; Sun, L.H.; Oberthuer, D.; Zhang, C.Y.; Shi, J.Y.; Di, J.L.; Zhang, B.L.; Cao, H.L.; Liu, Y.M.; Li, J.; et al. Utilisation of adsorption and desorption for simultaneously improving protein crystallisation success rate and crystal quality. Sci. Rep. 2014, 4, 7308. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Undheim, E.A.B.; Jakson, T.N.W.; Roelants, K.; Grorgieva, D.; Vetter, I.; Calvete, J.J.; Scheib, H.; Cribb, B.W.; Yang, D.C.; et al. Research methods. In Venomous Reptiles and their Toxins: Evolution, Pathophysiology, and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; p. 169. [Google Scholar]
- Sequeira, A.F.; Turchetto, J.; Saez, N.J.; Peysson, F.; Ramond, L.; Duhoo, Y.; Blemont, M.; Fernandes, V.O.; Gama, L.T.; Ferreira, L.M.; et al. Gene design, fusion technology and tev cleavage conditions influence the purification of oxidized disulphide-rich venom peptides in escherichia coli. Microb. Cell Fact. 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Turchetto, J.; Sequeira, A.F.; Ramond, L.; Peysson, F.; Bras, J.L.; Saez, N.J.; Duhoo, Y.; Blemont, M.; Guerreiro, C.I.; Quinton, L.; et al. High-throughput expression of animal venom toxins in escherichia coli to generate a large library of oxidized disulphide-reticulated peptides for drug discovery. Microb. Cell Fact. 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Klupczynska, A.; Pawlak, M.; Kokot, Z.J.; Matysiak, J. Application of metabolomic tools for studying low molecular-weight fraction of animal venoms and poisons. Toxins 2018, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Gorai, B.; Sivaraman, T. Delineating residues for haemolytic activities of snake venom cardiotoxin 1 from naja naja as probed by molecular dynamics simulations and in vitro validations. Int. J. Biol. Macromol. 2017, 95, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Niazi, Z.R.; Khan, N.R.; Shah, K.; Rehman, K.; Wahab, A.; Khan, S. Simulation studies and dynamic interaction of venom peptides with ion channels. Prot. Pept. Lett. 2018, 25, 652–662. [Google Scholar] [CrossRef] [PubMed]


| Peptide | Mechanism of Action | Biological Significance/Therapeutics | Reference |
|---|---|---|---|
| 3FTX (neurotoxin) | Selective inhibition of nAChRs at neuromuscular junction and interfere with nerve transmission. | Tool to decipher structural and functional details of nAChRs. α-cobratoxin is under clinical trial for drug-resistant HIV strains, treatment of multiple sclerosis, muscular dystrophy, myasthenia gravis and amyotrophic lateral sclerosis. | [31] |
| 3FTX (cardiotoxin) | Membrane perturbation by electrostatic and hydrophobic interactions with the cell membranes. | Under scientific investigation for cancer inhibitory studies and potential use as anti-microbial agent. | [32,33] |
| Disintegrin | Selectively bind to integrin receptors present at the surface of platelet and other cells. | Tirofiban and Eptifibatide are under clinical use as antithrombotic agents. These compounds were developed from the snake venom disintegrns echistatin and barbourin. Contortrostatin is in preclinical studies for the inhibition of platelet aggregation and prostate cancer. | [34,35,36,37,38] |
| Kunitz-type inhibitor | Inhibition of serine proteases (e.g., plasmin, kallikrein, trypsin). Interferes with the blood coagulation cascade and fibrinolysis. | A plasmin inhibitor Textilinin-1 is in preclinical studies as antibleeding agent. | [39,40] |
| Natriuretic peptide | Interaction of Nps with guanylyl cyclase receptors leads to an increase of cylic guanosine monophosphate (cGMP), and affects subsequent signalling cascade. Nps can interfere the renin-angiotensin system by inhibiting the angiotensin converting enzyme. | These peptides serve as tool to understand NP biology. Cenderitide was under clinic studies for cardiovascular disease. However, its clinical development was terminated by Capiricor (US pharmaceutical company) in 2017. | [41,42,43] |
| BPPs | Inhibit the function of angiotensin converting enzyme, and raise the level of bradykinin. | Captopril and its analogue are under clinical use for the treatment of hypotension. These compounds were developed from the snake venom BPP. | [44] |
| Crotamine | Interacts electrostatically with DNA. Penetrates membranes via heparan sulphate proteoglycans binding. | Carrier for biomolecules, tool for cancer studies. | [45] |
| Sarafotoxin | Vasoconstriction via endothelin receptors. | Molecular probe to better understand endothelial system and related diseases. | [46] |
| Waglerin | nAChR antagonist. | Anti-wrinkle cosmetic cream SYN-AKE is available in the market. The active ingredient of this cream is a peptide mimic, which was designed using waglerin as a template. | [47] |
| Toxin Family | Representative Structure | Representative Sequences |
|---|---|---|
| 3FTX | 1UG4 |  |
| Kunitz-type Inhibitor | 3BYB |  |
| Disintegrin | 1J2L |  |
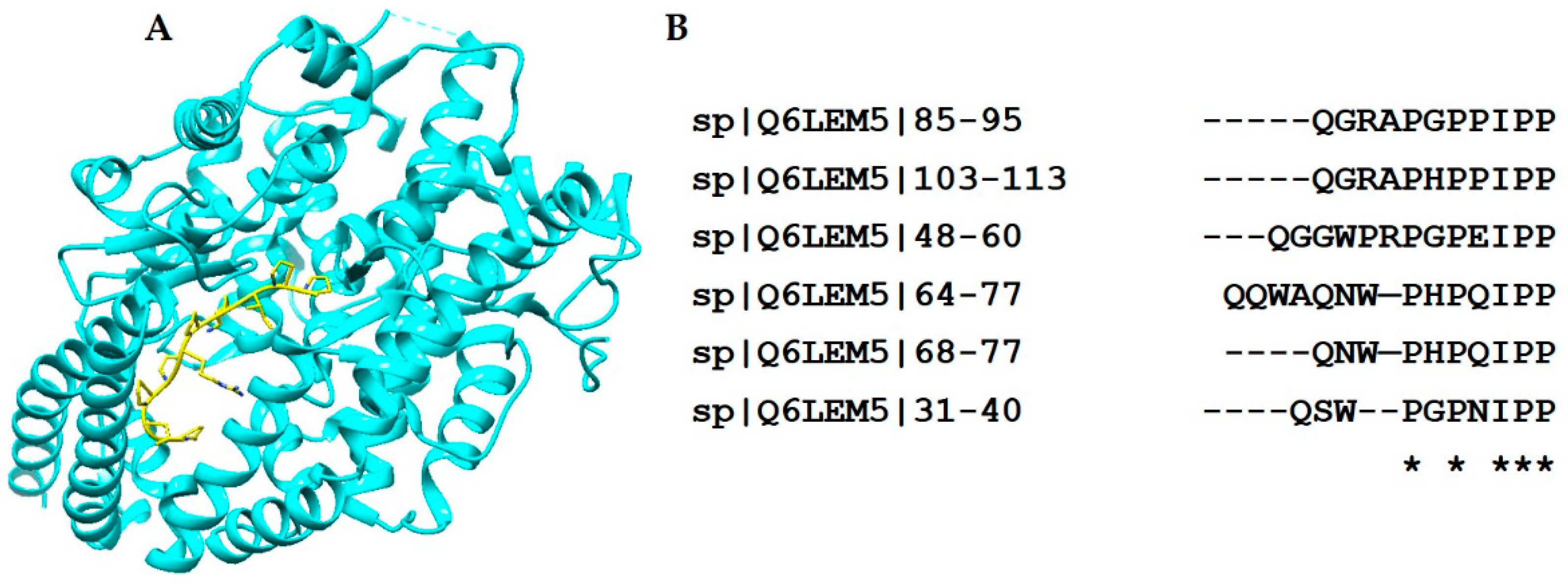
| Crotamine | 4GV5 | 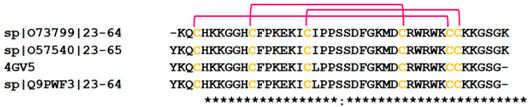 |
| Sarafotoxin | 2LDE | 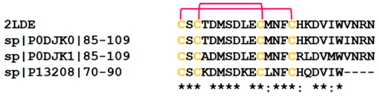 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake Venom Peptides: Tools of Biodiscovery. Toxins 2018, 10, 474. https://doi.org/10.3390/toxins10110474
Munawar A, Ali SA, Akrem A, Betzel C. Snake Venom Peptides: Tools of Biodiscovery. Toxins. 2018; 10(11):474. https://doi.org/10.3390/toxins10110474
Chicago/Turabian StyleMunawar, Aisha, Syed Abid Ali, Ahmed Akrem, and Christian Betzel. 2018. "Snake Venom Peptides: Tools of Biodiscovery" Toxins 10, no. 11: 474. https://doi.org/10.3390/toxins10110474
APA StyleMunawar, A., Ali, S. A., Akrem, A., & Betzel, C. (2018). Snake Venom Peptides: Tools of Biodiscovery. Toxins, 10(11), 474. https://doi.org/10.3390/toxins10110474







