Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Indepdendent Manner When Associated with Outer Membrane Vesicles
Abstract
1. Introduction
2. Results
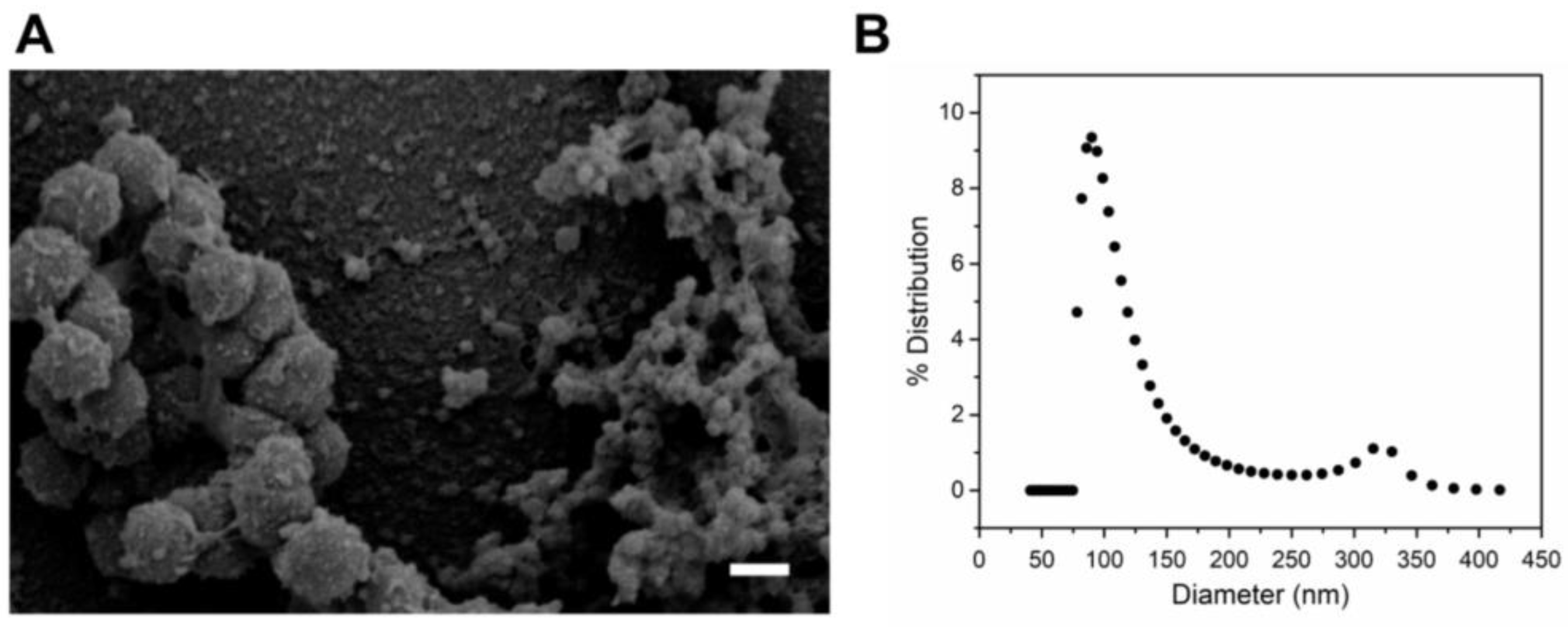
2.1. Purification and Characterization of A. actinomycetemcomitans JP2 Outer Membrane Vesicles (OMVs)
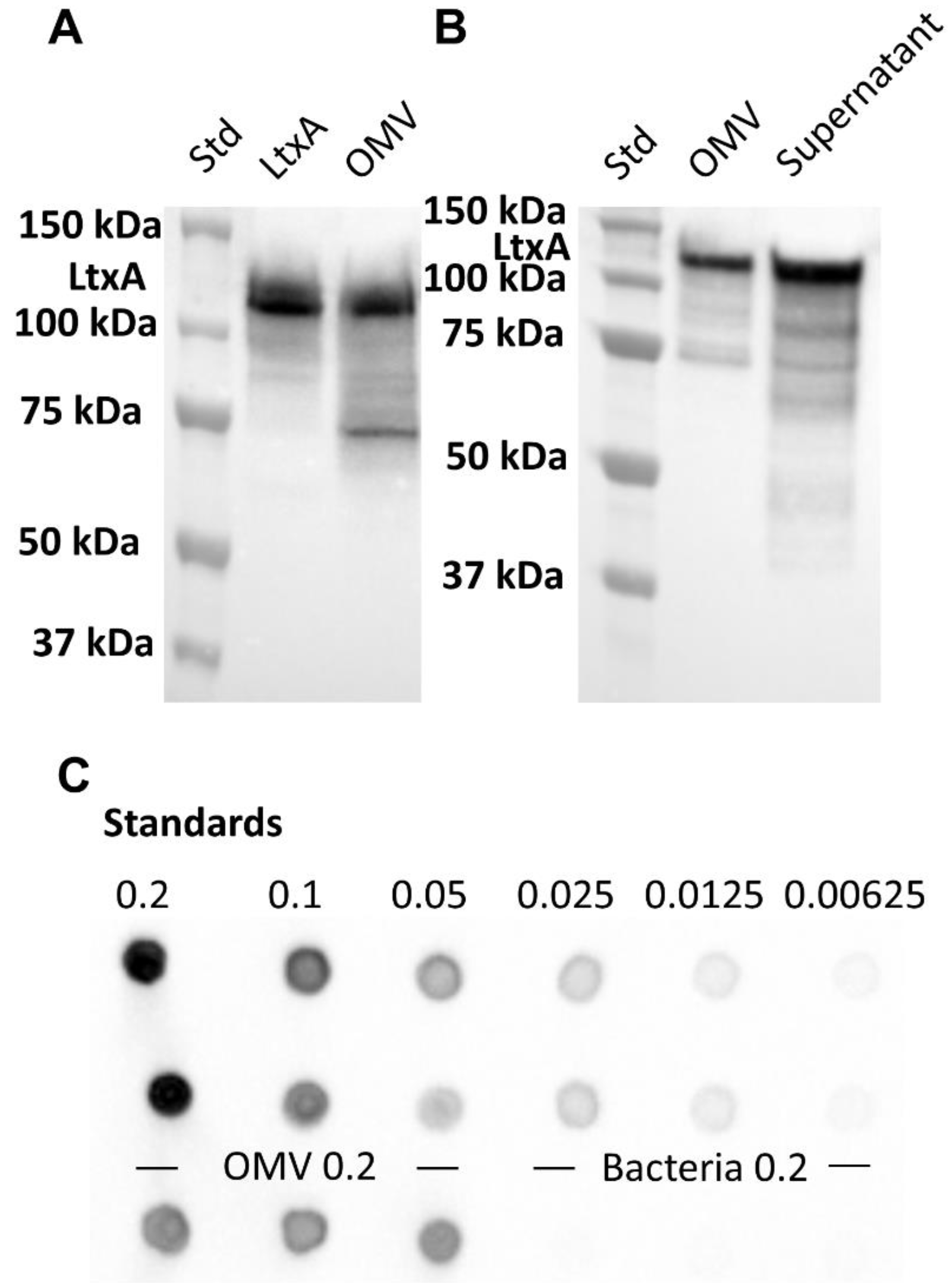
2.2. Association of LtxA with JP2 OMVs
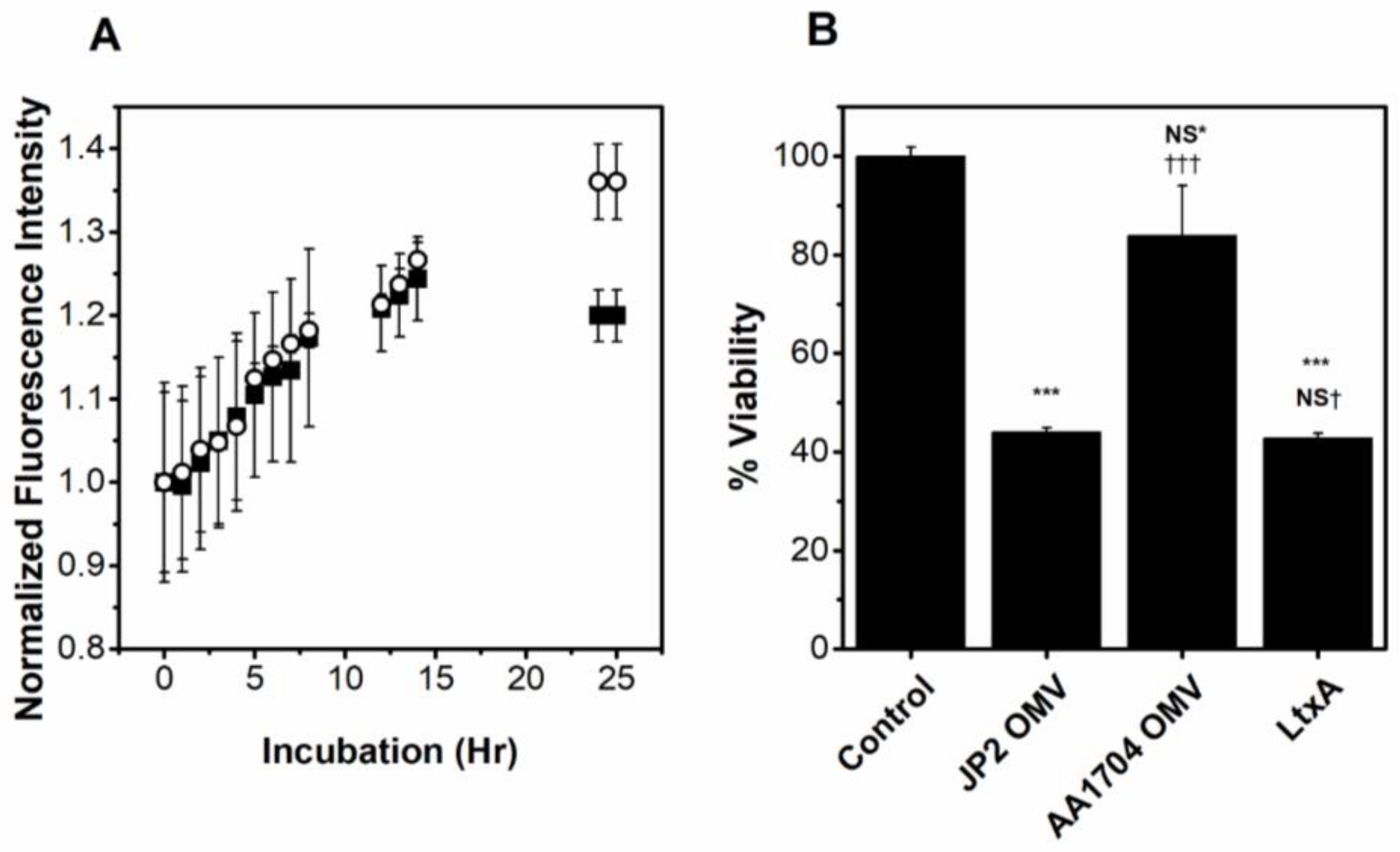
2.3. OMVs Associate with Host Cells
2.4. OMV-Associated LtxA Is Active
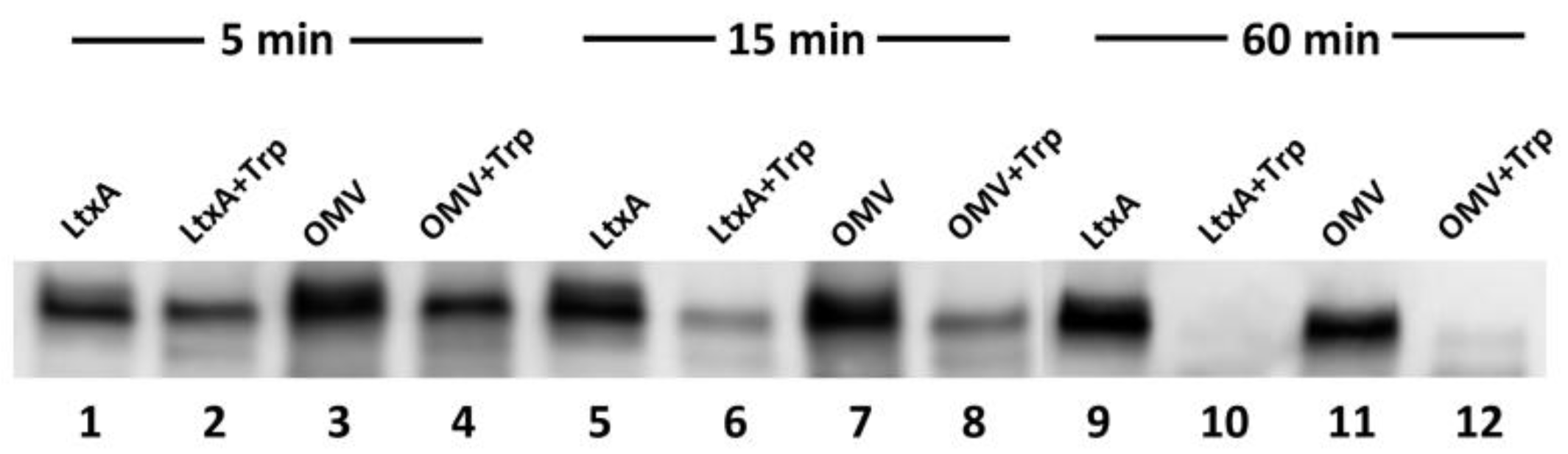
2.5. Inhibitors of LtxA Are Unable to Inhibit OMV-Associated LtxA
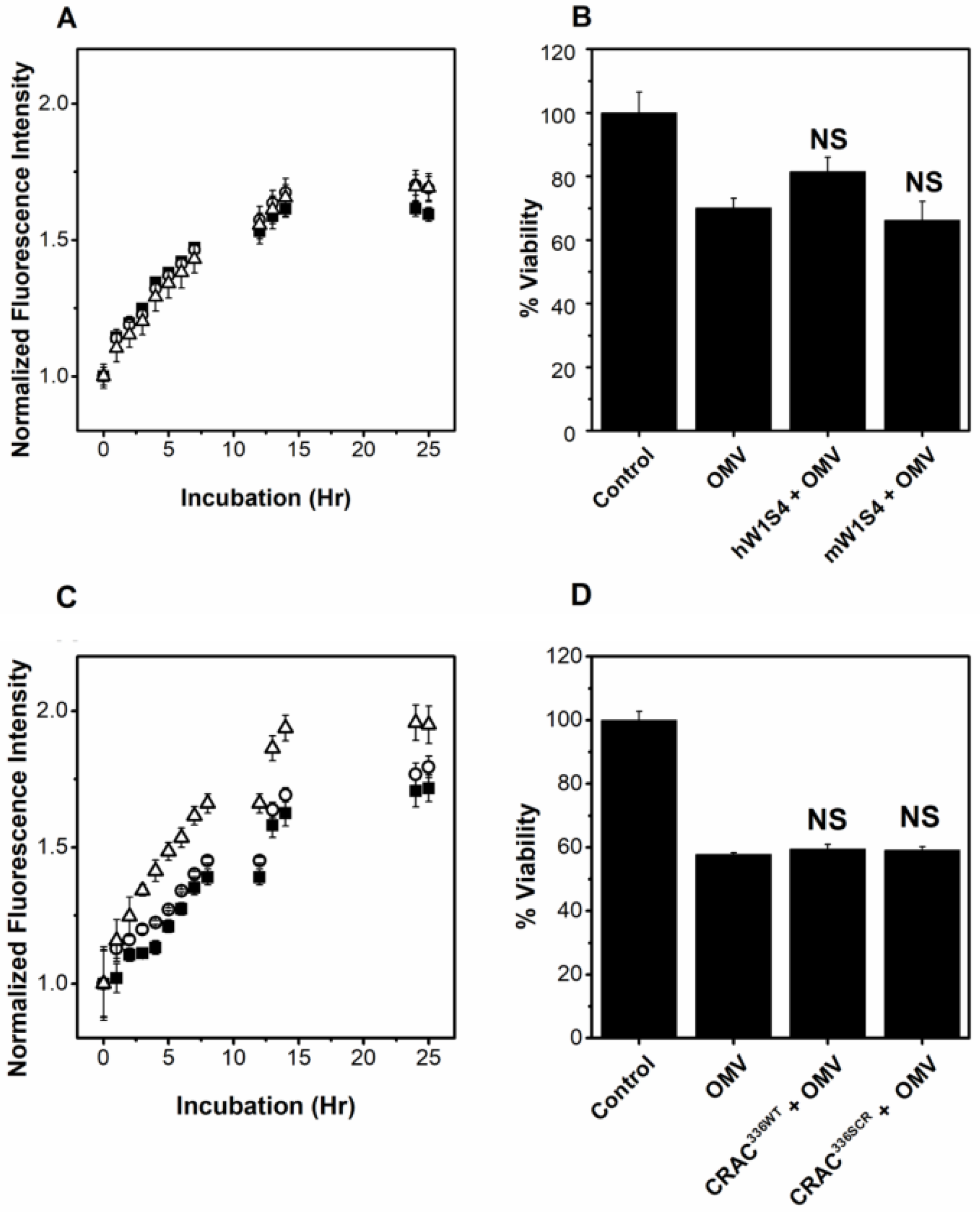
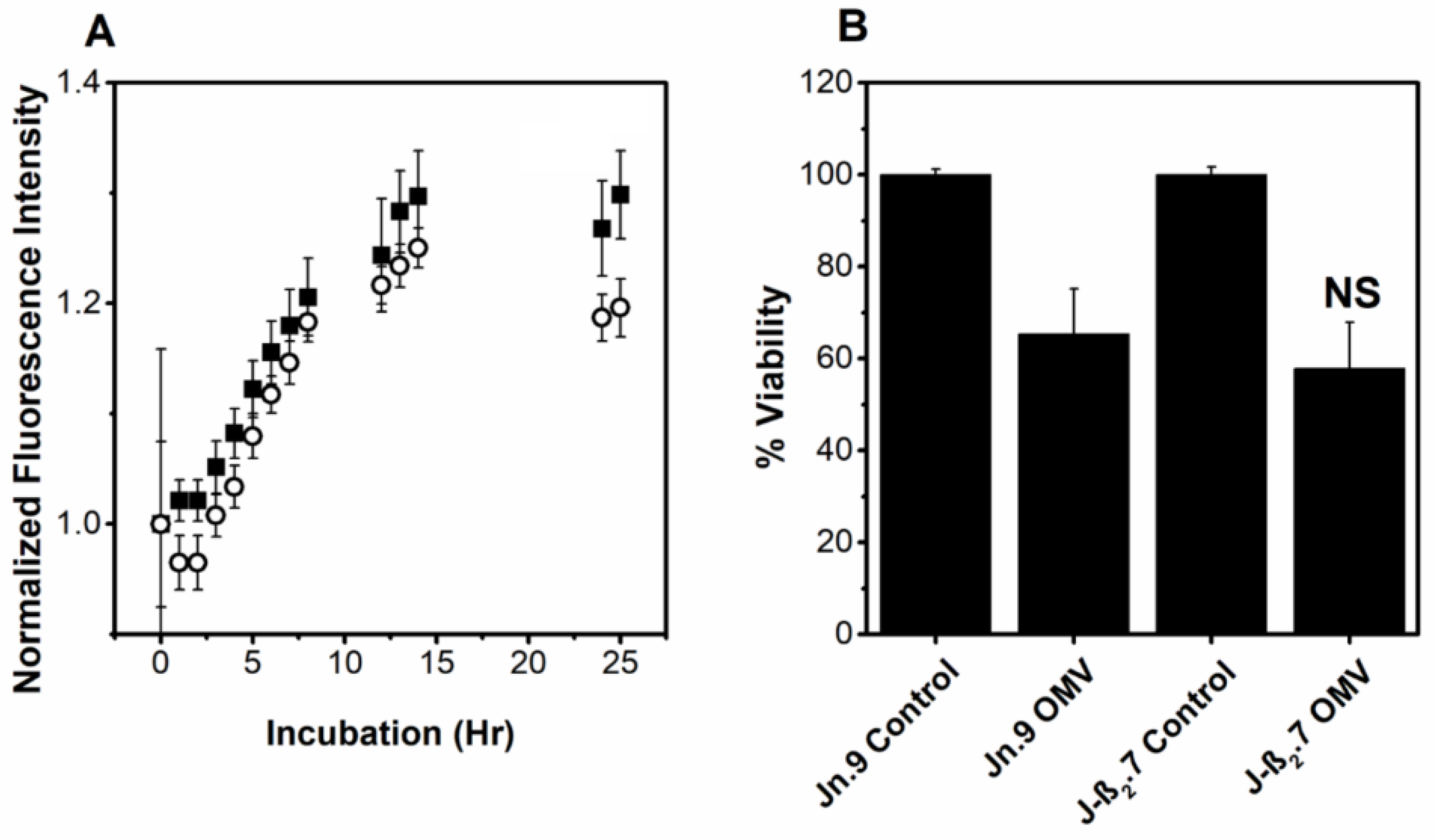
2.6. OMV-Associated LtxA Is Trafficked to Host Cells in an LFA-1- and Cholesterol-Independent Mechanism
2.7. OMV-Associated LtxA Is Active against Cells That Lack LFA-1
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain Cultivation
4.2. Cell Culture
4.3. Purification of OMVs
4.4. LtxA Purification
4.5. Peptide Synthesis
4.6. Dynamic Light Scattering (DLS)
4.7. Scanning Electron Microscopy (SEM)
4.8. Immunoblotting
4.9. Trypsin Digestion
4.10. Confocal Imaging
4.11. OMV Association Assay
4.12. Cytotoxicity Assays
4.13. Inhibition of Cholesterol Binding
4.14. Inhibition of LFA-1 Binding
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: Longitudinal cohort study of initially healthy adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.N. The case for periodontosis as a clinical entity. J. Periodontol. 1971, 42, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Lehner, T.; Wilton, J.M.; Ivanyi, L.; Manson, J.D. Immunological aspects of juvenile periodontitis (periodontosis). J. Period. Res. 1974, 9, 261–272. [Google Scholar] [CrossRef]
- Zambon, J.J. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 1985, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Deas, D.E.; Mealey, B.L. Response of chronic and aggressive periodontitis to treatment. Periodontology 2000 2010, 53, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Gmur, R.; Gobbi, C.; Lang, N.P. Actinobacillus actinomycetemcomitans in adult periodontitis. II. Characterization of isolated strains and effect of mechanical periodontal treatment. J. Periodontol. 1994, 65, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Christersson, L.A.; Slots, J.; Rosling, B.G.; Genco, R.J. Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. J. Clin. Periodontol. 1985, 12, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Saxen, L.; Asikainen, S. Metronidazole in the treatment of localized juvenile periodontitis. J. Clin. Periodontol. 1993, 20, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.B. The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000 1996, 10, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Fives-Taylor, P.M.; Meyer, D.H.; Mintz, K.P.; Brissette, C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology 2000 1999, 20, 136–167. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Crosby, J.A.; Kachlany, S.C. TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene 2007, 388, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kachlany, S.C.; Fine, D.H.; Figurski, D.H. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect. Immun. 2000, 68, 6094–6100. [Google Scholar] [CrossRef] [PubMed]
- Kieba, I.R.; Fong, K.P.; Tang, H.Y.; Hoffman, K.E.; Speicher, D.W.; Klickstein, L.B.; Lally, E.T. Aggregatibacter actinomycetemcomitans leukotoxin requires β-sheets 1 and 2 of the human CD11a β-propeller for cytotoxicity. Cell. Microbiol. 2007, 9, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Lally, E.T.; Hill, R.B.; Kieba, I.R.; Korostoff, J. The interaction between RTX toxins and target cells. Trend Microbiol. 1999, 7, 356–361. [Google Scholar] [CrossRef]
- Lally, E.T.; Kieba, I.R.; Sato, A.; Green, C.L.; Rosenbloom, J.; Korostoff, J.; Wang, J.F.; Shenker, B.J.; Ortlepp, S.; Robinson, M.K.; et al. RTX toxins recognize a β2 integrin on the surface of human target cells. J. Biol. Chem. 1997, 272, 30463–30469. [Google Scholar] [CrossRef] [PubMed]
- DiFranco, K.M.; Johnson-Farley, N.; Bertino, J.R.; Elson, D.; Vega, B.A.; Belinka, B.A.; Kachlany, S.C. Lfa-1-targeting leukotoxin (LtxA; Leukothera®) causes lymphoma tumor regression in a humanized mouse model and requires caspase-8 and Fas to kill malignant lymphocytes. Leuk. Res. 2015, 39, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.P.; Pacheco, C.M.F.; Otis, L.L.; Baranwal, S.; Kieba, I.R.; Harrison, G.; Hersh, E.V.; Boesze-Battaglia, K.; Lally, E.T. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell. Microbiol. 2006, 8, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Balashova, N.V.; Epand, R.M.; Epand, R.F.; Bragin, A.; Kachlany, S.C.; Walters, M.J.; Du, Y.; Boesze-Battaglia, K.; Lally, E.T. Aggregatibacter actinomycetemcomitans leukotoxin utilizes a cholesterol recognition/amino acid consensus site for membrane association. J. Biol. Chem. 2013, 288, 23607–23621. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Boesze-Battaglia, K.; Du, Y.; Stefano, F.P.; Kieba, I.R.; Epand, R.F.; Kakalis, L.; Yeagle, P.L.; Epand, R.M.; Lally, E.T. Aggregatibacter actinomycetemcomitans leukotoxin cytotoxicity occurs through bilayer destabilization. Cell. Microbiol. 2012, 14, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.J.; Brown, A.C.; Edrington, T.C.; Baranwal, S.; Du, Y.; Lally, E.T.; Boesze-Battaglia, K. Membrane association and destabilization by Aggregatibacter actinomycetemcomitans leukotoxin requires changes in secondary structures. Mol. Oral Microbiol. 2013, 28, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Krummenacher, C.; Brown, A.C.; Edrington, T.; Shenker, B.J.; Boesze-Battaglia, K. Mechanisms by which pathogens hijack and utilize membrane domains to mediate cytotoxicity. In The Structure of Biological Membranes, 3rd ed.; Yeagle, P., Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Balashova, N.; Dhingra, A.; Boesze-Battaglia, K.; Lally, E.T. Aggregatibacter actinomycetemcomitans leukotoxin induces cytosol acidification in LFA-1 expressing immune cells. Mol. Oral Microbiol. 2015, 106–114. [Google Scholar]
- DiFranco, K.M.; Gupta, A.; Galusha, L.E.; Perez, J.; Nguyen, T.-V.; Fineza, C.D.; Kachlany, S.C. Leukotoxin (Leukothera) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J. Biol. Chem. 2012, 287, 17618–17627. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, C.; Silvan, J.M.; Berglund, S.; Mizunoe, Y.; Uhlin, B.E.; Wai, S.N. Release of the Type I secreted α-haemolysin via outer membrane vesicles from Escherichia coli. Mol. Microbiol. 2006, 59, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Hozbor, D.; Rodriguez, M.E.; Fernandez, J.; Lagares, A.; Guiso, N.; Yantorno, O. Release of outer membrane vesicles from Bordetella pertussis. Curr. Microbiol. 1999, 38, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kowashi, Y.; Demuth, D.R. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 2002, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Wei, R.; Kachlany, S.C.; Kazi, M.; Balashova, N.V. Cytotoxic effects of Kingella kingae outer membrane vesicles on human cells. Microb. Pathog. 2011, 51, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Donato, G.M.; Goldsmith, C.S.; Paddock, C.D.; Eby, J.C.; Gray, M.C.; Hewlett, E.L. Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles. FEBS Lett. 2012, 586, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Kim, B.U.; Kim, S.Y.; Kim, C.M.; Na, H.S.; Koh, J.T.; Choy, H.E.; Rhee, J.H.; Lee, S.E. Outer membrane vesicles of Vibrio vulnificus deliver cytolysin-hemolysin VvhA into epithelial cells to induce cytotoxicity. Biochem. Bioph. Res. Commun. 2010, 399, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host–pathogen interaction. Gene. Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annual Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Mayrand, D.; Grenier, D. Biological activities of outer membrane vesicles. Can. J. Microbiol. 1989, 35, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Takeuchi, H.; Furata, N. Outer membrane vesicles function as offensive weapons in host-parasite interactions. Microbes Infect. 2010, 12, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Mamata, G.; Chan, M.D.; Won, C.C.; Hwa, L.J.; Chul, B.Y.; Jungmin, K.; Chul, L.Y.; Yong, S.S.; Taek, C.D.; Il, K.S. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 2011, 6, e27958. [Google Scholar]
- Schaar, V.; de Vries, S.P.W.; Vidakovics, M.L.A.P.; Boostsma, H.J.; Larsson, L.; Hermans, P.W.M.; Bjartell, A.; Morgelin, M.; Riesbeck, K. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell. Microbiol. 2011, 13, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Schaar, V.; Nordstrom, T.; Morgelin, M.; Riesbeck, K. Moraxella catarrhalis outer membrane vesicles carry {beta}-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob. Agents Chemother. 2011, 55, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Watson, E.; Sandu, P.; Gundogdu, O.; Mills, D.C.; Inglis, N.F.; Manson, E.; Imrie, L.; Bajaj-Elliott, M.; Wren, B.W.; et al. Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect. Immun. 2012, 80, 4089–4098. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Chaudhuri, K. Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a Nod1 protein-dependent manner and induce dendritic cell-mediated Th2/Th17 cell responses. J. Biol. Chem. 2013, 288, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, K.E.; Kuehn, M.J. Protein selection and export via outer membrane vesicles. Biochimica Biophysica Acta—Mol.Cell Res. 2014, 1843, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Ünal, C.M.; Schaar, V.; Riesbeck, K. Bacterial outer membrane vesicles in disease and preventive medicine. Semin. Immunopathol. 2011, 33, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Listgarten, M.A.; Hammond, B.F. Comparative ultrastructure of leukotoxic and non-leukotoxic strains of Actinobacillus actinomycetemcomitans. J. Period. Res. 1981, 16, 379–389. [Google Scholar] [CrossRef]
- Meyer, D.H.; Fives-Taylor, P.M. Evidence that extracellular components function in adherence of Actinobacillus actinomycetemcomitans to epithelial cells. Infect. Immun. 1993, 61, 4933–4936. [Google Scholar] [PubMed]
- Rompikuntal, P.K.; Thay, B.; Khan, M.K.; Alanko, J.; Penttinen, A.-M.; Asikainen, S.; Wai, S.N.; Oscarsson, J. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect. Immun. 2011, 80, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Thay, B.; Damm, A.; Kufer, T.A.; Wai, S.N.; Oscarsson, J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger Nod1- and Nod2-dependent NF-κB activation. Infect. Immun. 2014, 82, 4034–4046. [Google Scholar] [CrossRef] [PubMed]
- Kiley, P.; Holt, S.C. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect. Immun. 1980, 30, 862–873. [Google Scholar] [PubMed]
- Iino, Y.; Hopps, R.M. The bone-resorbing activities in tissue culture of lipopolysaccharides from the bacteria Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Capnocytophaga ochracea isolated from human mouths. Arch.Oral Biol. 1984, 29, 59–63. [Google Scholar] [CrossRef]
- Reddi, K.; Meghji, S.; Wilson, M.; Henderson, B. Comparison of the osteolytic activity of surface-associated proteins of bacteria implicated in periodontal disease. Oral Dis. 1995, 1, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kieselbach, T.; Zijnge, V.; Granström, E.; Oscarsson, J. Proteomics of Aggregatibacter actinomycetemcomitans outer membrane vesicles. PLoS ONE 2015, 10, e0138591. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Koufos, E.; Balashova, N.; Boesze-Battaglia, K.; Lally, E.T. Inhibition of LtxA toxicity by blocking cholesterol binding with peptides. Mol. Oral Microbiol. 2016, 31, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Koufos, E.; Chang, E.H.; Rasti, E.S.; Krueger, E.; Brown, A.C. Use of a cholesterol recognition amino acid consensus (CRAC) peptide to inhibit binding to cholesterol by a bacterial toxin. Biochemistry 2016, 55, 4787–4797. [Google Scholar] [CrossRef] [PubMed]
- Krueger, E.; Hayes, S.; Brown, A.C. Receptor-based peptides for inhibition of leukotoxin activity. ACS Infect. Dis. 2018, 4, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Lally, E.T.; Golub, E.E.; Kieba, I.R. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J. Biol. Chem. 1994, 269, 31289–31295. [Google Scholar] [PubMed]
- Ohta, H.; Kato, K.; Kokeguchi, S.; Hara, H.; Fukui, K.; Murayama, Y. Nuclease-sensitive binding of an Actinobacillus actinomycetemcomitans leukotoxin to the bacterial cell surface. Infect. Immun. 1991, 59, 4599–4605. [Google Scholar] [PubMed]
- Balashova, N.V.; Crosby, J.A.; Al Ghofaily, L.; Kachlany, S.C. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect. Immun. 2006, 74, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Demuth, D.; James, D.; Kowashi, Y.; Kato, S. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell. Microbiol. 2003, 5, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Dileepan, T.; Kachlany, S.C.; Balashova, N.V.; Patel, J.; Maheswaran, S.K. Human CD18 is the functional receptor for Aggregatibacter actinomycetemcomitans leukotoxin. Infect. Immun. 2007, 75, 4851–4856. [Google Scholar] [CrossRef] [PubMed]
- Bazil, V.; Stefanova, I.; Hilgert, I.; Kristofova, H.; Vanek, S.; Horejsi, V. Monoclonal antibodies against human leucocyte antigens IV. Antibodies against subunits of the LFA-1 (CD11a/CD18) leucocyte-adhesion glycoprotein. Folia Biologica. 1990, 36, 41–50. [Google Scholar] [PubMed]
- Balashova, N.V.; Shah, C.; Patel, J.K.; Megalla, S.; Kachlany, S.C. Aggregatibacter actinomycetemcomitans LtxC is required for leukotoxin activity and initial interaction between toxin and host cells. Gene 2009, 443, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Demel, R.A.; de Kruyff, B.; van Deenen, L.L.M. Studies on the biological properties of polyene antibiotics: Evidence for the direct interaction of filipin with cholesterol. J. Biol. Chem. 1972, 247, 1918–1929. [Google Scholar] [PubMed]
- Weber, K.S.; York, M.R.; Springer, T.A.; Klickstein, L.B. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: The alphal and beta2 subunits are interdependent for cell surface expression. J. Immunol. 1997, 158, 273–279. [Google Scholar] [PubMed]
- Lally, E.T.; Golub, E.E.; Kieba, I.R.; Taichman, N.S.; Decker, S.; Berthold, P.; Gibson, C.W.; Demuth, D.R.; Rosenbloom, J. Structure and function of the B and D genes of the Actinobacillus actinomycetemcomitans leukotoxin complex. Microb. Pathog. 1991, 11, 111–121. [Google Scholar] [CrossRef]
- Tsai, C.C.; Shenker, B.J.; DiRienzo, J.M.; Malamud, D.; Taichman, N.S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect. Immun. 1984, 43, 700–705. [Google Scholar] [PubMed]
- Bielaszewska, M.; Rüter, C.; Kunsmann, L.; Greune, L.; Bauwens, A.; Zhang, W.; Kuczius, T.; Kim, K.S.; Mellmann, A.; Schmidt, M.A.; et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013, 9, e1003797. [Google Scholar] [CrossRef] [PubMed]
- Furuta, N.; Tsuda, K.; Omori, H.; Yoshimori, T.; Yoshimura, F.; Amano, A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect. Immun. 2009, 77, 4187–4196. [Google Scholar] [CrossRef] [PubMed]
- Kesty, N.C.; Mason, K.M.; Reedy, M.; Miller, S.E.; Kuehn, M.J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004, 23, 4538–4549. [Google Scholar] [CrossRef] [PubMed]
- Bomberger, J.M.; MacEachran, D.P.; Coutermarsh, B.A.; Ye, S.; O′Toole, G.A.; Stanton, B.A. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009, 5, e1000382. [Google Scholar] [CrossRef] [PubMed]
- Bumba, L.; Masin, J.; Fiser, R.; Sebo, P. Bordetella adenylate cyclase toxin mobilizes its β2 integrin receptor into lipid rafts to accomplish translocation across target cell membrane in two steps. PLoS Pathog. 2010, 6, e1000901. [Google Scholar] [CrossRef] [PubMed]
- Rasti, E.S.; Schappert, M.L.; Brown, A.C. Association of Vibrio cholerae 569B outer membrane vesicles with host cells occurs in a GM1-independent manner. Cell. Microbiol. 2018, 20, e12828. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Brown, A.; Walker, L.; Besack, D.; Zekavat, A.; Wrenn, S.; Krummenacher, C.; Shenker, B.J. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J. Biol. Chem. 2009, 284, 10650–10658. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Chao, K.; Patel, K.; Dreyfus, L. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect. Immun. 2001, 69, 3418–3422. [Google Scholar] [CrossRef] [PubMed]
- Lara-Tejero, M.; Galán, J.E. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 2001, 69, 4358–4365. [Google Scholar] [CrossRef] [PubMed]
- Nešić, D.; Hsu, Y.; Stebbins, C.E. Assembly and function of a bacterial genotoxin. Nature 2004, 429, 429. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Tsuzukibashi, O.; Takada, K. Anticytotoxic effect of green tea catechin on Aggregatibacter actinomycetemcomitans vesicles. Int. J. Oral-Med. Sci. 2012, 11, 101–105. [Google Scholar] [CrossRef][Green Version]
- Hallett, F.R.; Watton, J.; Krygsman, P. Vesicle sizing number distributions by dynamic light scattering. Biophys. J. 1991, 59, 357–362. [Google Scholar] [CrossRef]
- Alberts, B.; Hopkin, J.; Lewis, R.; Roberts, W. Essential Cell Biology, 3rd ed.; Taylor & Francis: New York, NY, USA, 2010. [Google Scholar]
- McDowell, E.M.; Trump, B.F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch. Pathol. Lab. Med. 1976, 100, 405–414. [Google Scholar] [PubMed]
- Nation, J.l. A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol. 1983, 58, 347. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nice, J.B.; Balashova, N.V.; Kachlany, S.C.; Koufos, E.; Krueger, E.; Lally, E.T.; Brown, A.C. Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Indepdendent Manner When Associated with Outer Membrane Vesicles. Toxins 2018, 10, 414. https://doi.org/10.3390/toxins10100414
Nice JB, Balashova NV, Kachlany SC, Koufos E, Krueger E, Lally ET, Brown AC. Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Indepdendent Manner When Associated with Outer Membrane Vesicles. Toxins. 2018; 10(10):414. https://doi.org/10.3390/toxins10100414
Chicago/Turabian StyleNice, Justin B., Nataliya V. Balashova, Scott C. Kachlany, Evan Koufos, Eric Krueger, Edward T. Lally, and Angela C. Brown. 2018. "Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Indepdendent Manner When Associated with Outer Membrane Vesicles" Toxins 10, no. 10: 414. https://doi.org/10.3390/toxins10100414
APA StyleNice, J. B., Balashova, N. V., Kachlany, S. C., Koufos, E., Krueger, E., Lally, E. T., & Brown, A. C. (2018). Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Indepdendent Manner When Associated with Outer Membrane Vesicles. Toxins, 10(10), 414. https://doi.org/10.3390/toxins10100414




