Flavonolignans Inhibit IL1-β-Induced Cross-Talk between Blood Platelets and Leukocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Blood Samples
2.3. Samples Preparation
2.4. Flow Cytometry Analysis of Platelet-Leukocyte Aggregates
2.5. Cytokine Level Analysis
2.6. Isolation of RNA and Reverse Transcription
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
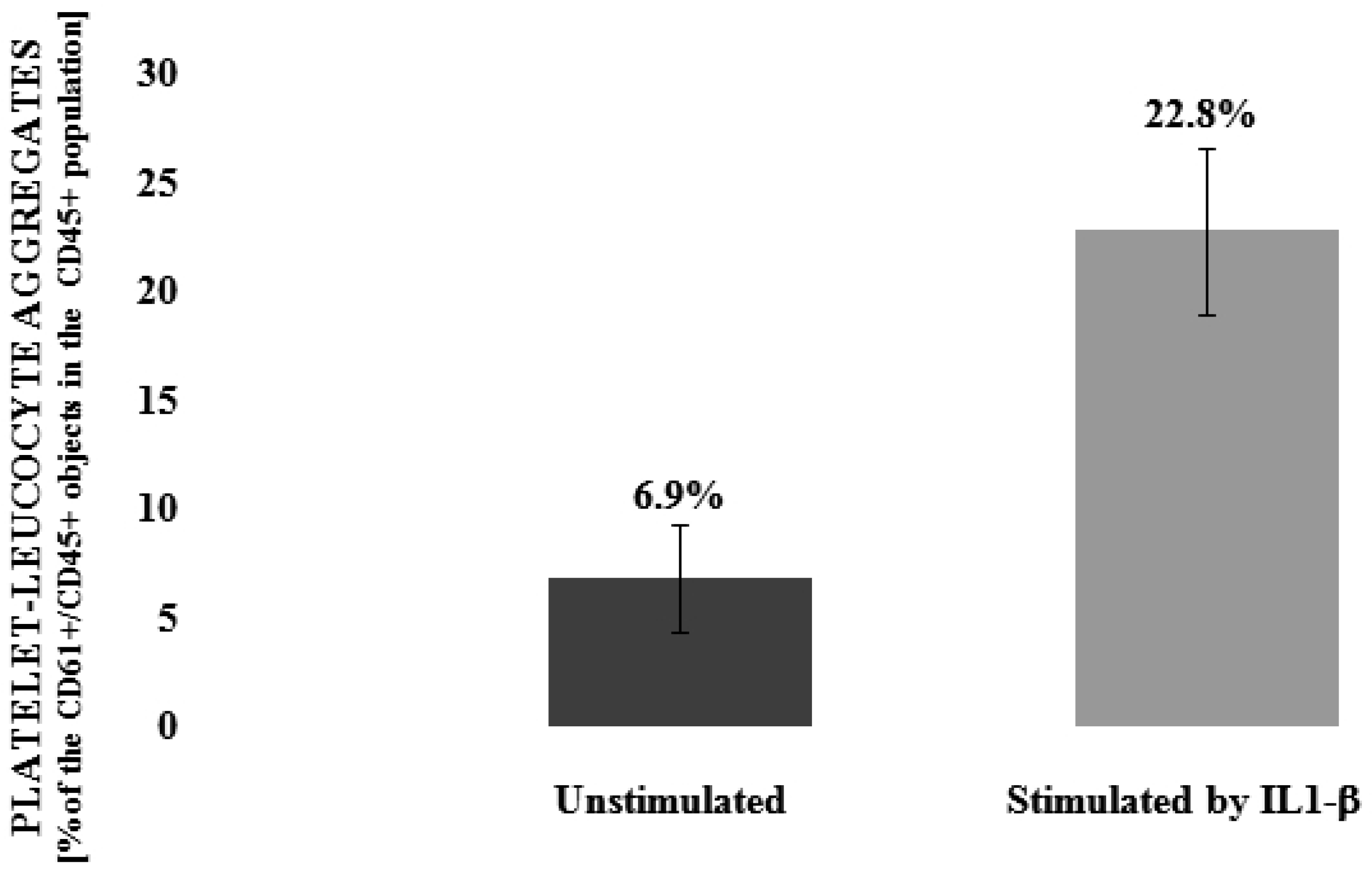
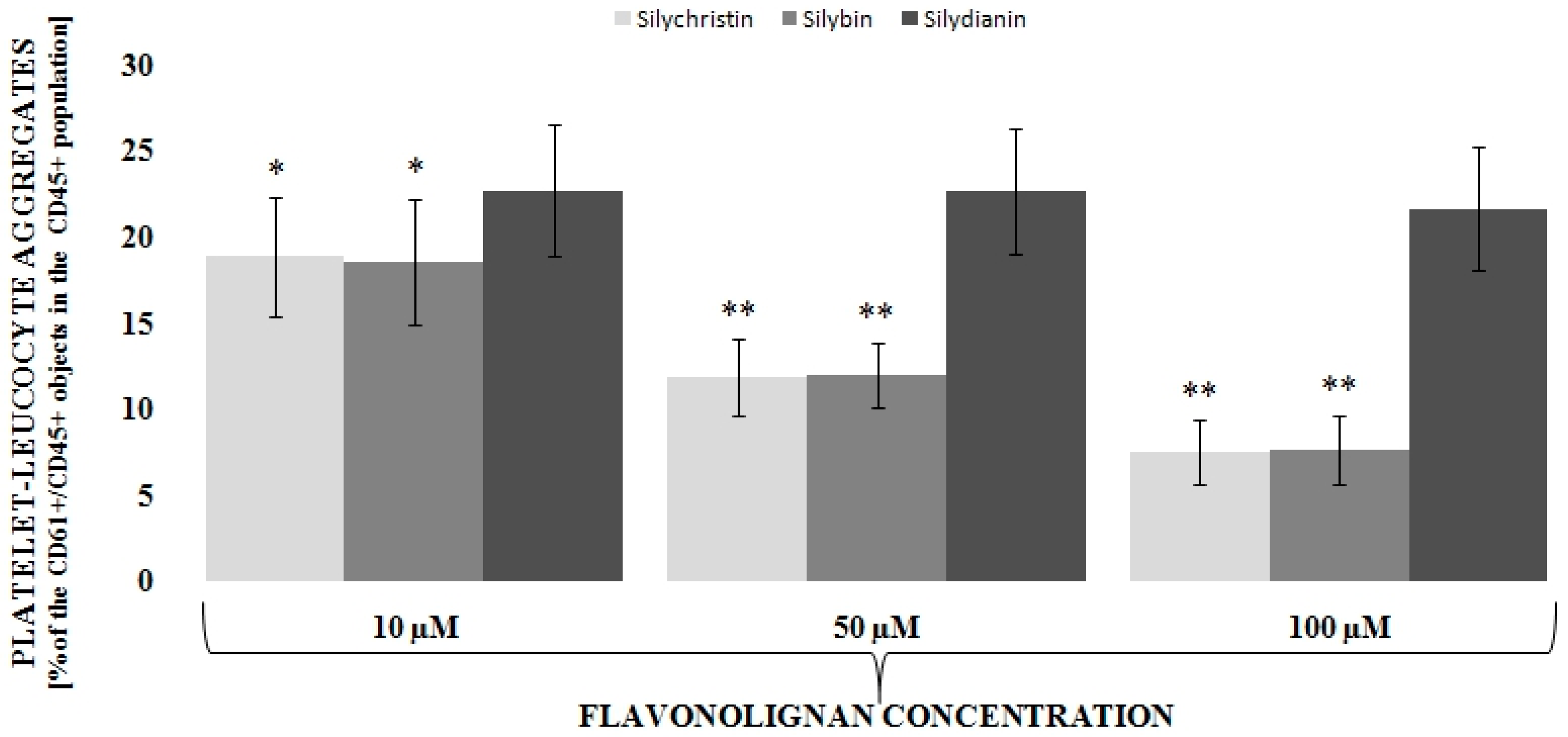
3.1. Flavonolignans Effect on IL-1β-Induced Formation of Blood Platelet-Leukocyte Aggregates
3.2. Flavonolignans Effect on IL-1β-Induced Cytokine Production (IL-2, TGF-β, TNF, INF-α and INF-γ)
3.3. Silychristin and Silybin Effect on mRNA Expression for INF-γ and TNF Genes
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1beta (IL-1beta) processing pathway. Sci. Signal. 2010, 3, cm2. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine levels in the serum of healthy subjects. Mediat. Inflamm. 2013, 2013, 434010. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.M.; Lin, E.; Mick, E.; Koupenova, M.; Weinberg, E.O.; Kramer, C.D.; Genco, C.A.; Tanriverdi, K.; Larson, M.G.; Benjamin, E.J.; et al. Interleukin 1 receptor 1 and interleukin 1beta regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Blocking IL-1 in systemic inflammation. J. Exp. Med. 2005, 201, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Rainone, F. Milk thistle. Am. Fam. Phys. 2005, 72, 1285–1288. [Google Scholar]
- Althagafy, H.S.; Meza-Avina, M.E.; Oberlies, N.H.; Croatt, M.P. Mechanistic study of the biomimetic synthesis of flavonolignan diastereoisomers in milk thistle. J. Org. Chem. 2013, 78, 7594–7600. [Google Scholar] [CrossRef] [PubMed]
- Kurkin, V.A. Phenylpropanoids from medicinal plants: Distribution, classification, structural analysis, and biological activity. Chem. Nat. Comp. 2003, 39, 123–153. [Google Scholar] [CrossRef]
- Nyiredy, S.; Samu, Z.; Szucs, Z.; Gulacsi, K.; Kurtan, T.; Antus, S. New insight into the biosynthesis of flavanolignans in the white-flowered variant of Silybum marianum. J. Chromatogr. Sci. 2008, 46, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Walterova, D. Silybin and silymarin--new effects and applications. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2005, 149, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin—New and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem. 2003, 1, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Hackett, E.S.; Twedt, D.C.; Gustafson, D.L. Milk thistle and its derivative compounds: A review of opportunities for treatment of liver disease. J. Vet. Intern. Med. 2013, 27, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Narayan, M.; Barrett, J.S. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B 2007, 845, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Flavonolignans-compounds not only for liver treatment. Pol. Merkur. Lek. 2017, 42, 34–37. [Google Scholar]
- Bijak, M.; Szelenberger, R.; Saluk, J.; Nowak, P. Flavonolignans inhibit ADP induced blood platelets activation and aggregation in whole blood. Int. J. Biol. Macromol. 2017, 95, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Saluk-Bijak, J. Flavonolignans inhibit the arachidonic acid pathway in blood platelets. BMC Complement. Altern. Med. 2017, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Dziedzic, A.; Saluk-Bijak, J. Flavonolignans reduce the response of blood platelet to collagen. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lach, D.; Cichon, N.; Dziedzic, A.; Bijak, M.; Saluk, J. Inflammatory processes in the pathogenesis of acute coronary syndromes. Pol. Merkur. Lek. 2017, 42, 183–186. [Google Scholar]
- Wyss, C.A.; Neidhart, M.; Altwegg, L.; Spanaus, K.S.; Yonekawa, K.; Wischnewsky, M.B.; Corti, R.; Kucher, N.; Roffi, M.; Eberli, F.R.; et al. Cellular actors, Toll-like receptors, and local cytokine profile in acute coronary syndromes. Eur. Heart J. 2010, 31, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Zeller, J.A.; Lenz, A.; Eschenfelder, C.C.; Zunker, P.; Deuschl, G. Platelet-leukocyte interaction and platelet activation in acute stroke with and without preceding infection. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hu, H.; Lindqvist, M.; Wikstrom-Jonsson, E.; Goodall, A.H.; Hjemdahl, P. Platelet-leukocyte cross talk in whole blood. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Totani, L.; Evangelista, V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Kral, J.B.; Schrottmaier, W.C.; Salzmann, M.; Assinger, A. Platelet Interaction with Innate Immune Cells. Transfus. Med. Hemother. 2016, 43, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Furman, M.I.; Barnard, M.R.; Krueger, L.A.; Fox, M.L.; Shilale, E.A.; Lessard, D.M.; Marchese, P.; Frelinger, A.L.; Goldberg, R.J.; Michelson, A.D. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 1002–1006. [Google Scholar] [CrossRef]
- Sarma, J.; Laan, C.A.; Alam, S.; Jha, A.; Fox, K.A.; Dransfield, I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation 2002, 105, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Naruko, T.; Ueda, M.; Haze, K.; van der Wal, A.C.; van der Loos, C.M.; Itoh, A.; Komatsu, R.; Ikura, Y.; Ogami, M.; Shimada, Y.; et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 2002, 106, 2894–2900. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Lichtman, A.H.; Hansson, G.K. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity 2013, 38, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Schulte, S.; Warrington, K.J.; Kopecky, S.L.; Frye, R.L.; Goronzy, J.J.; Weyand, C.M. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation 2002, 105, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Collagenases and cracks in the plaque. J. Clin. Investig. 2013, 123, 3201–3203. [Google Scholar] [CrossRef] [PubMed]
- Amento, E.P.; Ehsani, N.; Palmer, H.; Libby, P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. 1991, 11, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Jassim, N.A.; Numan, I.T.; Al-Khalifa, I.I.; Abdullah, T.A. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. A comparative study with piroxicam and meloxicam. Saudi Med. J. 2009, 30, 98–103. [Google Scholar] [PubMed]
- Manna, S.K.; Mukhopadhyay, A.; Van, N.T.; Aggarwal, B.B. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J. Immunol. 1999, 163, 6800–6809. [Google Scholar] [PubMed]
- Bannwart, C.F.; Nakaira-Takahagi, E.; Golim, M.A.; de Medeiros, L.T.; Romao, M.; Weel, I.C.; Peracoli, M.T. Downregulation of nuclear factor-kappa B (NF-kappaB) pathway by silibinin in human monocytes challenged with Paracoccidioides brasiliensis. Life Sci. 2010, 86, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Agarwal, R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008, 269, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Trappoliere, M.; Caligiuri, A.; Schmid, M.; Bertolani, C.; Failli, P.; Vizzutti, F.; Novo, E.; di Manzano, C.; Marra, F.; Loguercio, C.; et al. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J. Hepatol. 2009, 50, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yan, G.H. Silibinin attenuates mast cell-mediated anaphylaxis-like reactions. Biol. Pharm. Bull. 2009, 32, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Vasudev, S.S.; Ahmad, S. Oil based nanocarrier for improved oral delivery of silymarin: In vitro and in vivo studies. Int. J. Pharm. 2011, 413, 245–253. [Google Scholar] [CrossRef] [PubMed]


| Cytokine | Control (without IL1-β) | Control (with IL1-β) | IL1-β + Silychristin (µM) | IL1-β + Silybin (µM) | IL1-β + Silydianin (µM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 | |||
| INF-γ (pg/mL) | 255 ± 90 | 1701 ± 411 *** | 1278 ± 339 ** | 555 ± 175 *** | 262 ± 82 *** | 1339 ± 337 ** | 574 ± 149 *** | 283 ± 87 *** | 1677 ± 398 | 1665 ± 483 | 1542 ± 405 |
| TNF (pg/mL) | 355 ± 110 | 1632 ± 473 *** | 1216 ± 396 ** | 478 ± 160 *** | 338 ± 119 *** | 1286 ± 408 * | 466 ± 115 *** | 352 ± 115 *** | 1614 ± 453 | 1610 ± 423 | 1494 ± 360 |
| INF-α (pg/mL) | 13.3 ± 6.4 | 20.8 ± 8.0 ** | 16.8 ± 6.1 | 15.0 ± 4.9 * | 13.3 ± 4.0 *** | 16.3 ± 4.4 * | 14.8 ± 4.5 * | 12.6 ± 3.5 *** | 20.1 ± 5.1 | 18.8 ± 4.4 | 17.9 ± 3.9 |
| IL-2 (pg/mL) | 116 ± 28 | 189 ± 49 ** | 168 ± 45 | 129 ± 19 ** | 116 ± 24 *** | 164 ± 38 | 135 ± 21* | 112 ± 17 *** | 191 ± 46 | 175 ± 31 | 172 ± 30 |
| TGF-β (pM) | 78.3 ± 23.3 | 86.8 ± 27.9 | 84.2 ± 24.1 | 83.6 ± 28.1 | 83.6 ± 27.9 | 86.8 ± 31.8 | 82.8 ± 19.2 | 82.3 ± 27.7 | 84.1 ± 24.5 | 87.2 ± 23.5 | 85.3 ± 25.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bijak, M.; Dziedzic, A.; Synowiec, E.; Sliwinski, T.; Saluk-Bijak, J. Flavonolignans Inhibit IL1-β-Induced Cross-Talk between Blood Platelets and Leukocytes. Nutrients 2017, 9, 1022. https://doi.org/10.3390/nu9091022
Bijak M, Dziedzic A, Synowiec E, Sliwinski T, Saluk-Bijak J. Flavonolignans Inhibit IL1-β-Induced Cross-Talk between Blood Platelets and Leukocytes. Nutrients. 2017; 9(9):1022. https://doi.org/10.3390/nu9091022
Chicago/Turabian StyleBijak, Michal, Angela Dziedzic, Ewelina Synowiec, Tomasz Sliwinski, and Joanna Saluk-Bijak. 2017. "Flavonolignans Inhibit IL1-β-Induced Cross-Talk between Blood Platelets and Leukocytes" Nutrients 9, no. 9: 1022. https://doi.org/10.3390/nu9091022





