Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Enzymatic Assessment for Biological Evaluation
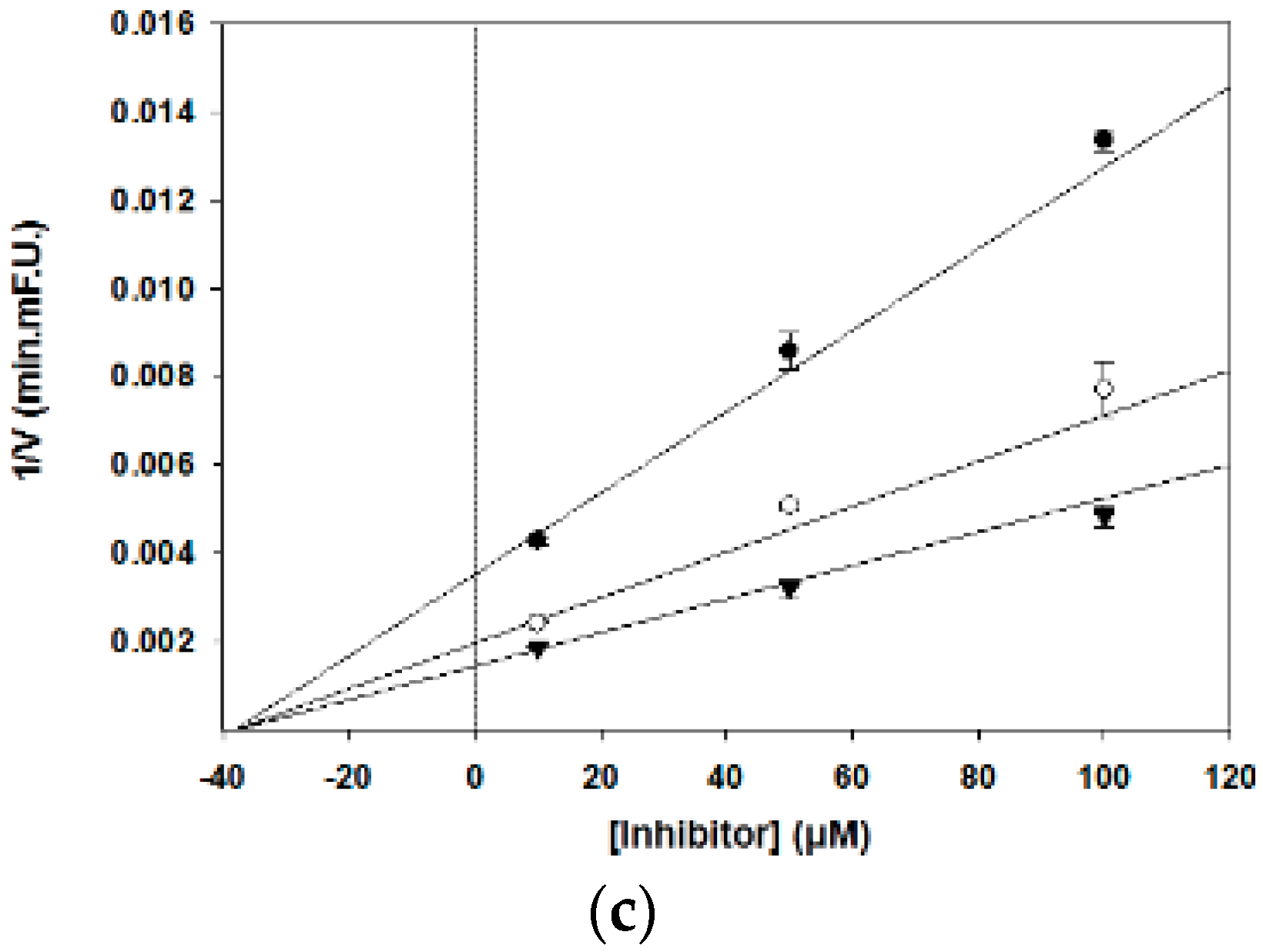
2.3. Assessment of the Inhibition Kinetics on BACE1
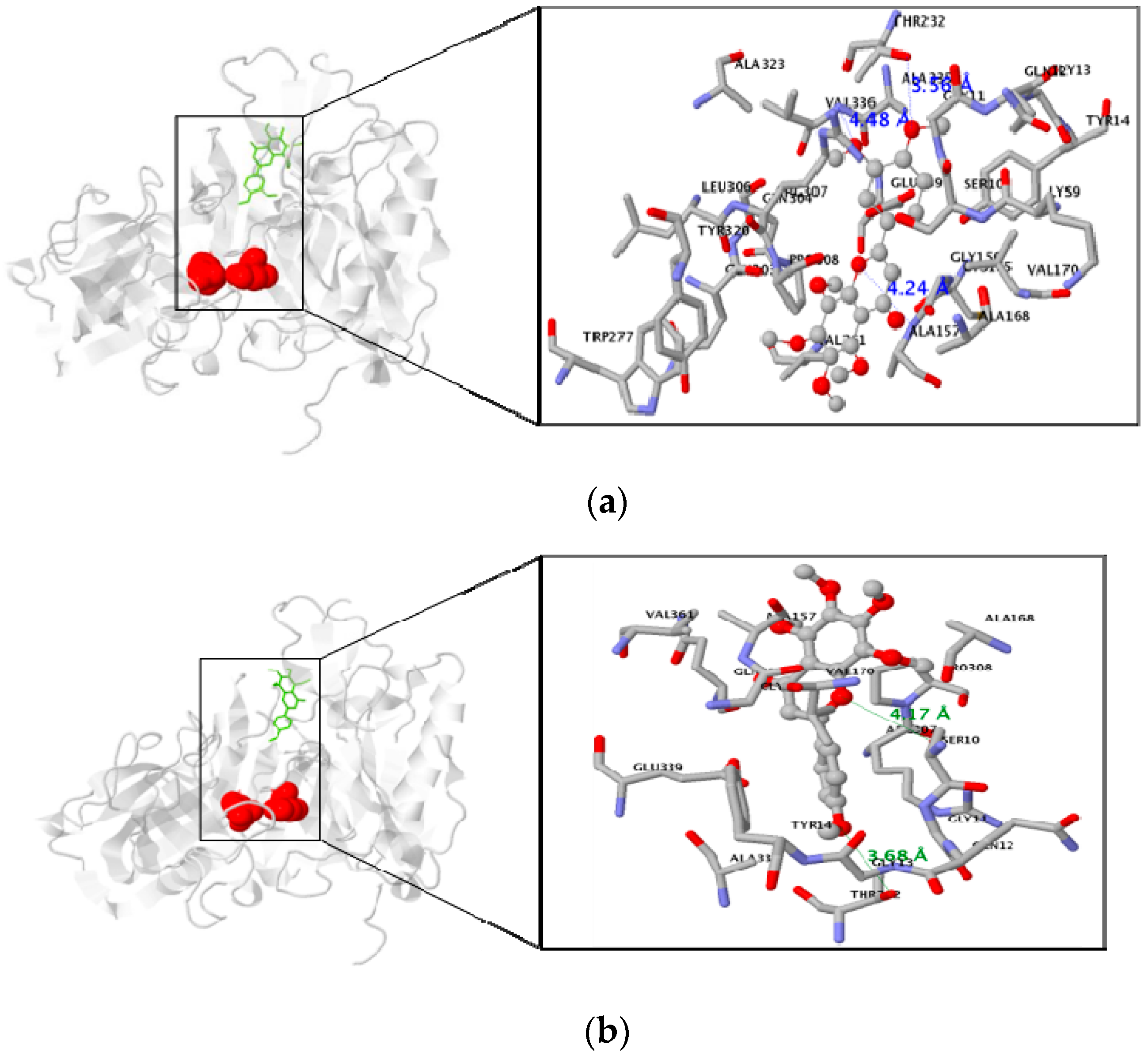
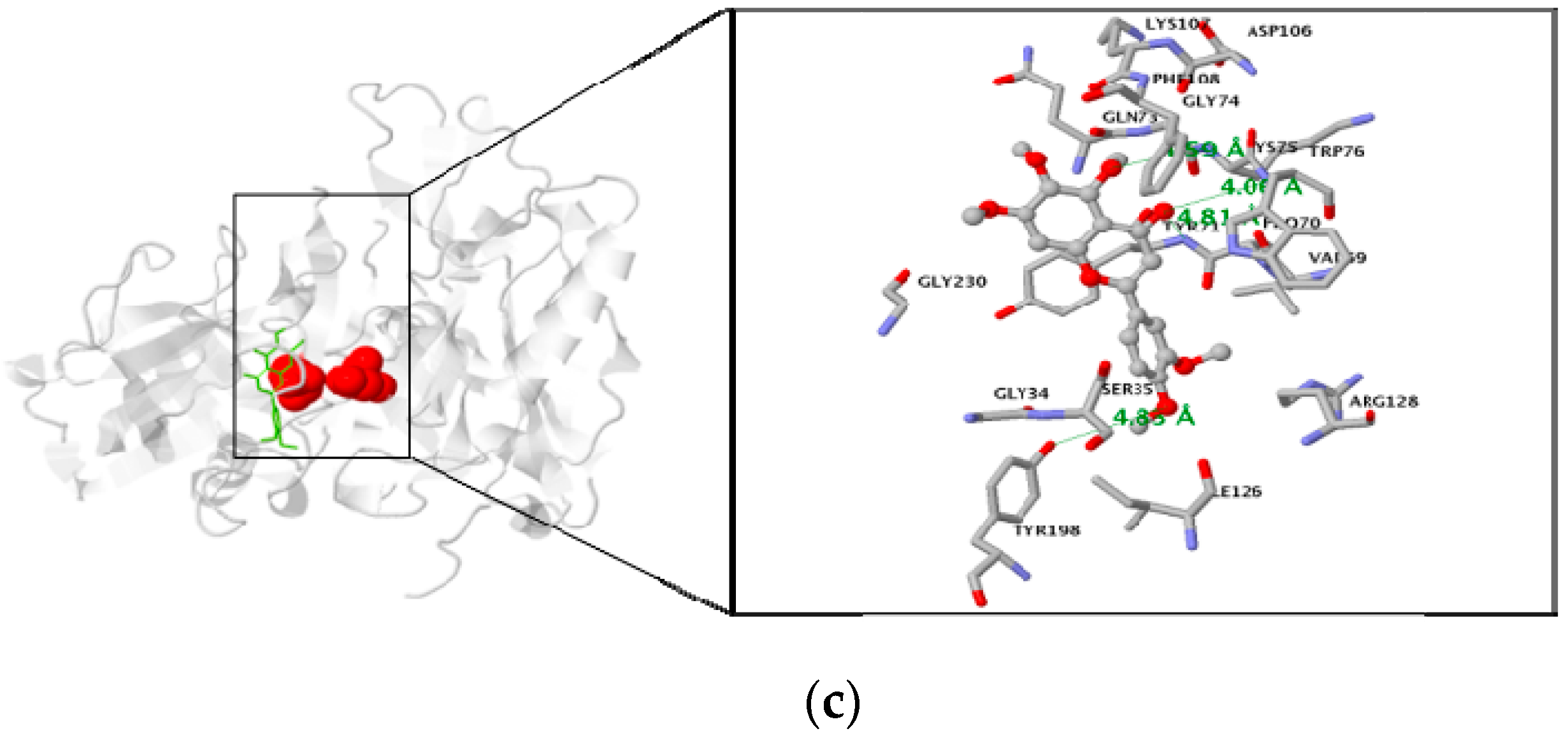
2.4. In Silico Docking Studies
2.5. Statistical Analysis
3. Results
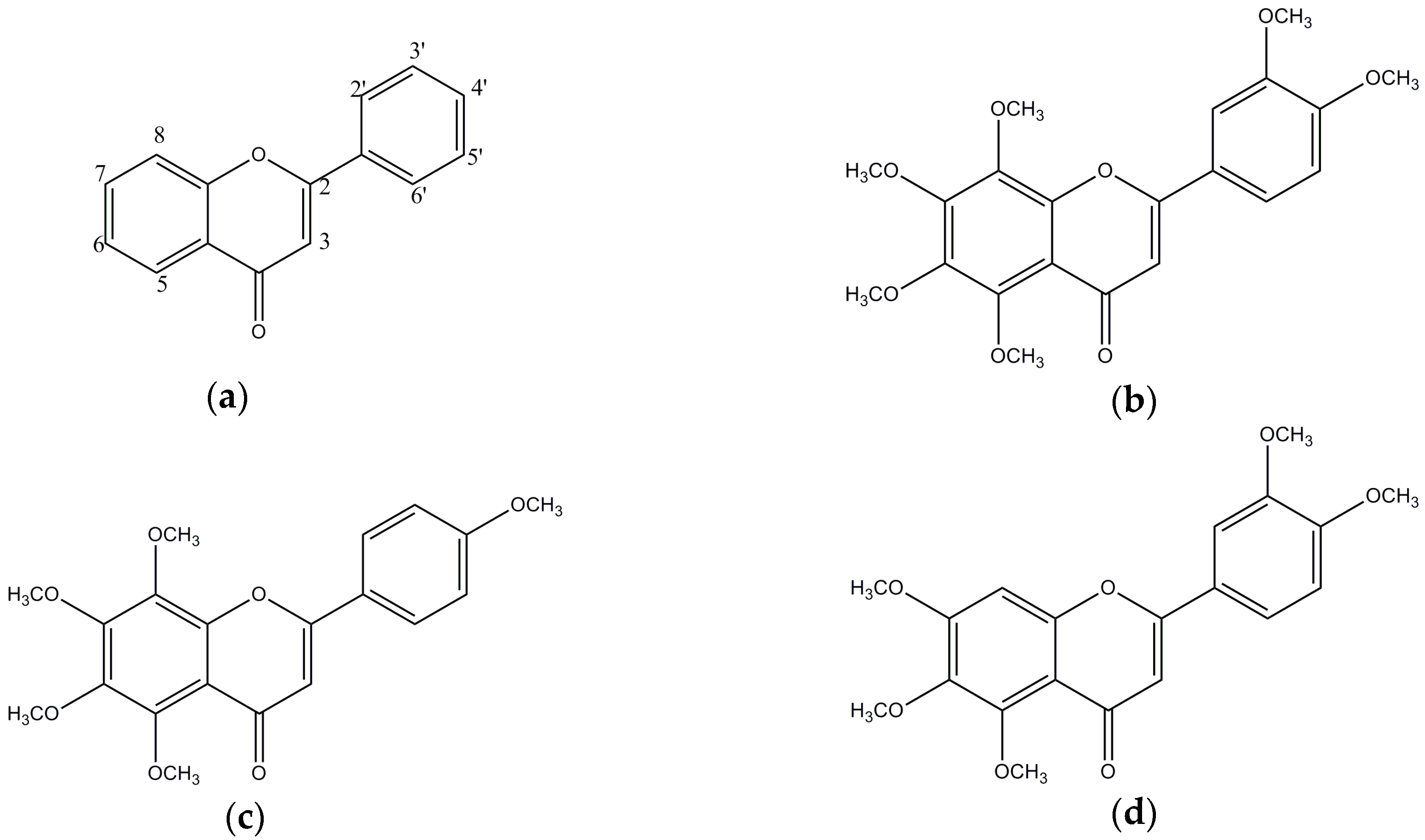
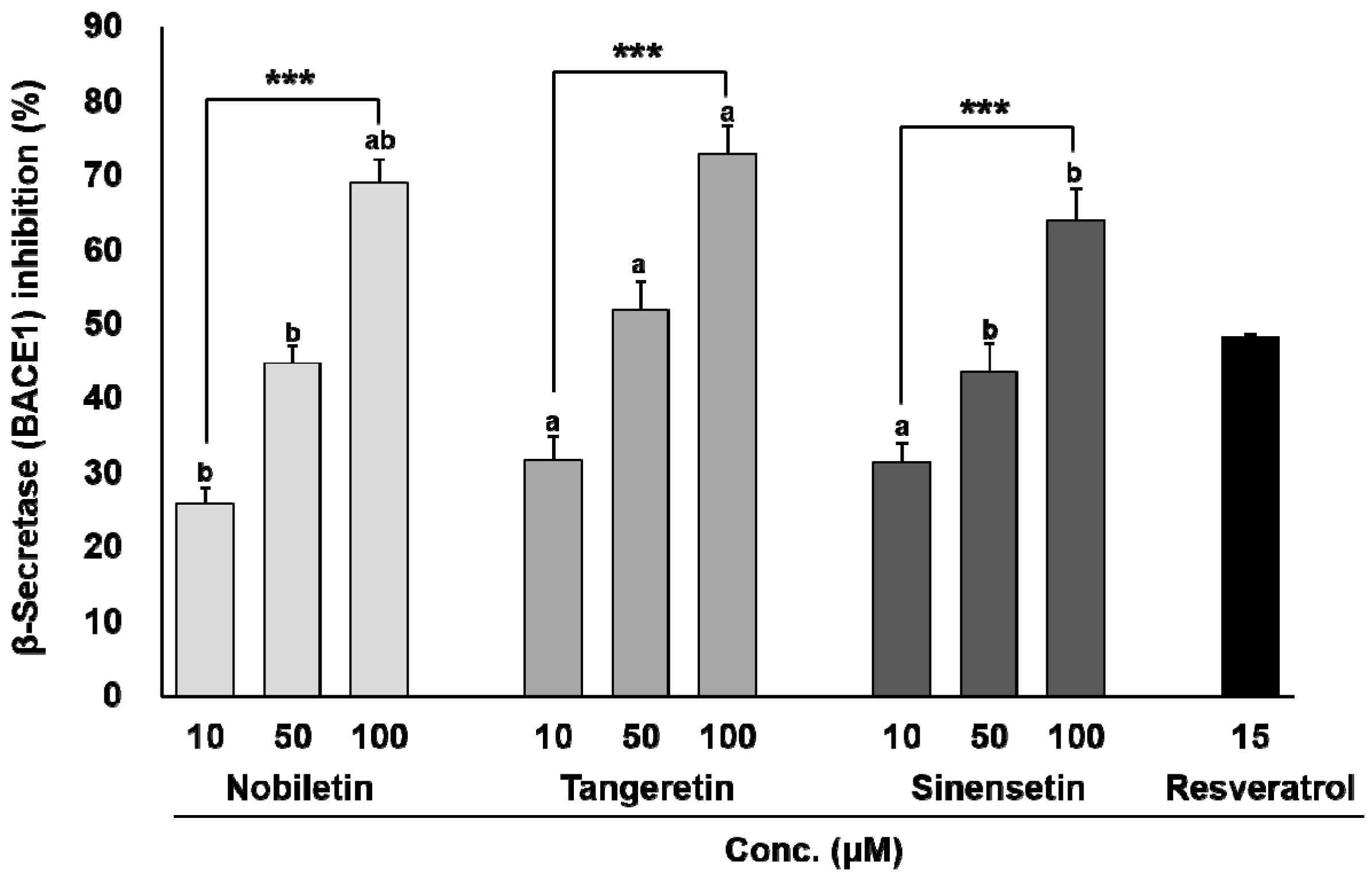
3.1. In Vitro BACE1 Inhibitory Activity of Biochanin A
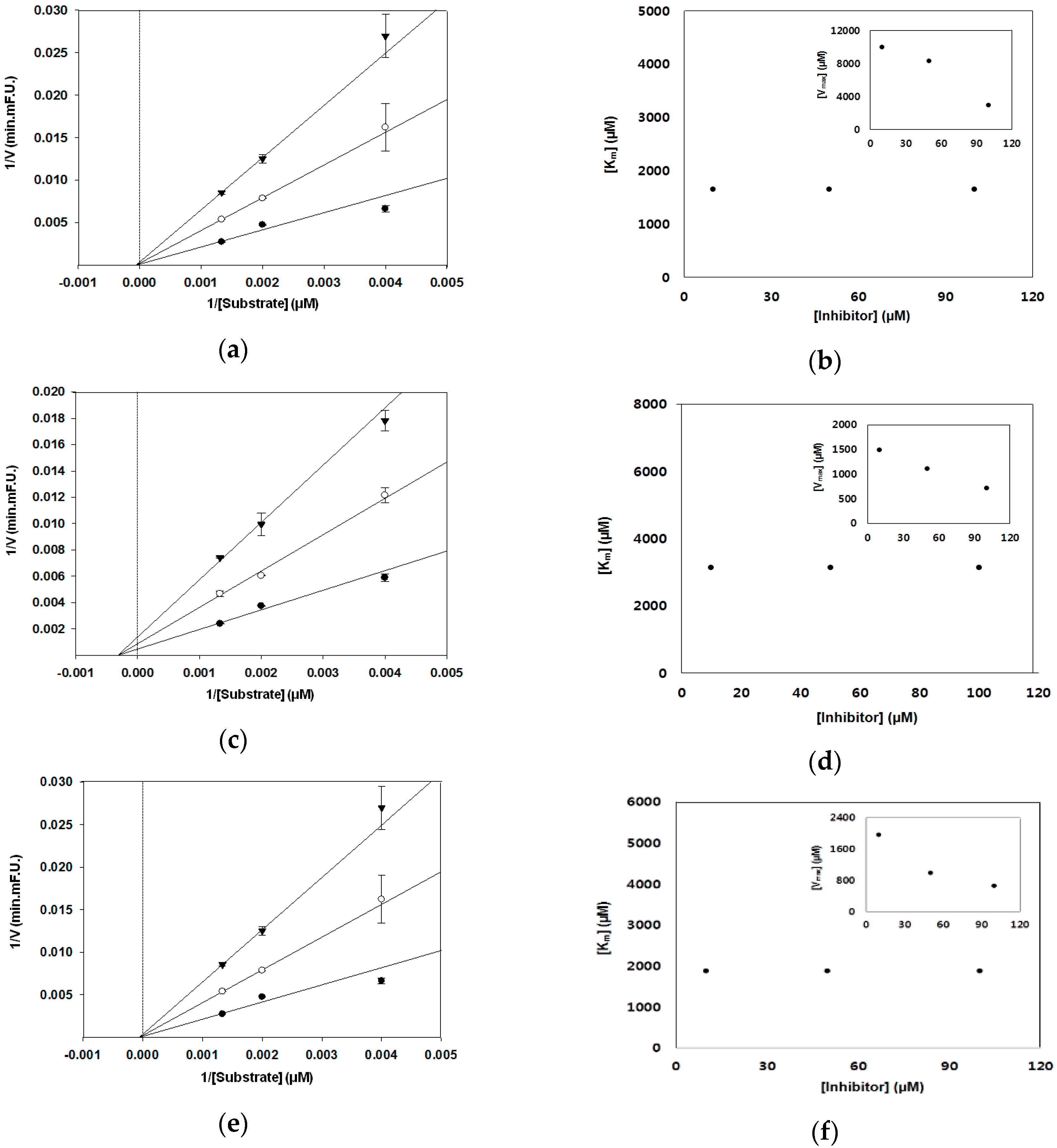
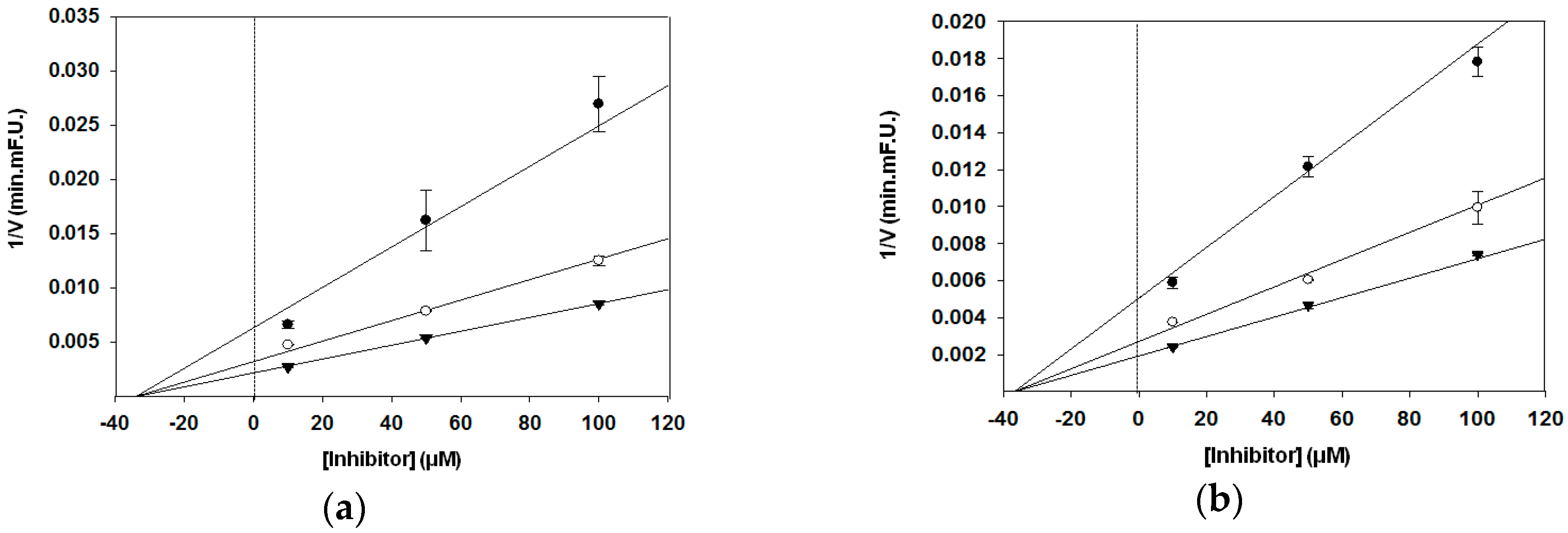
3.2. BACE1 Kinetic Assay
3.3. Molecular Docking Study of the Inhibitory Activity of PMFs against BACE1
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J. Alzheimer’s Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Osswald, H.L. BACE1 (beta-secretase) inhibitors for the treatment of Alzheimer’s disease. Chem. Soc. Rev. 2014, 7, 6765–6813. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Kuhn, P.H.; Haass, C.; Kennedy, M.E.; Rajendran, L.; Wong, P.C.; Lichtenthaler, S.F. Function, therapeutic potential and cell biology of BACE proteases: Current status and future prospects. J. Neurochem. 2014, 130, 4–28. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Annaert, W. Novel research horizons for presenilins and gamma-secretases in cell biology and disease. Annu. Rev. Cell Dev. Biol. 2010, 26, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, D.; Bradley, J.D.; Rominger, C.M.; Rominger, D.H.; Yang, F.; Meredith, J.E., Jr.; Wang, Q.; Roach, A.H.; Thompson, L.A.; Spitz, S.M.; et al. Presenilin-1 and -2 are molecular targets for γ-secretase inhibitors. J. Biol. Chem. 2000, 275, 34086–34091. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Bronson, R.T.; Chen, D.F.; Xia, W.; Selkoe, D.J.; Tonegawa, S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 1997, 89, 629–639. [Google Scholar] [CrossRef]
- Wong, P.C.; Zheng, H.; Chen, H.; Becher, M.W.; Sirinathsinghji, D.J.; Trumbauer, M.E.; Chen, H.Y.; Price, D.L.; Van der Ploeg, L.H.; Sisodia, S.S. Presenilin 1 is required for Notch1 DII1 expression in the paraxial mesoderm. Nature 1997, 387, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; De Strooper, B. The presenilins in Alzheimer’s disease-proteolysis holds the key. Science 1999, 286, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Yan, R. Physiological Functions of the β-Site Amyloid Precursor Protein Cleaving Enzyme 1 and 2. Front. Mol. Neurosci. 2017, 10, 97–106. [Google Scholar] [CrossRef] [PubMed]
- McConlogue, L.; Buttini, M.; Anderson, J.P.; Brigham, E.F.; Chen, K.S.; Freedman, S.B.; Games, D.; Johnson-Wood, K.; Lee, M.; Zeller, M.; et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J. Biol. Chem. 2007, 282, 26326–26334. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Devi, L.; Ohno, M. Partial reduction of BACE1 improves synaptic plasticity, recent and remote memories in Alzheimer’s disease transgenic mice. J. Nurochem. 2010, 113, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Barão, S.; Moechars, D.; Lichtenthaler, S.F.; De Strooper, B. BACE1 Physiological Functions May Limit Its Use as Therapeutic Target for Alzheimer’s Disease. Trends Neurosci. 2016, 39, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Physiol. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Itoh, T.; Ohguchi, K.; Iinuma, M.; Nozawa, Y. Interactive Effects of Polymethoxyflavones from Citrus on Cell Growth Inhibition in Human Neuroblastoma SH-SY5Y cells. Bioorg. Med. Chem. Lett. 2008, 16, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Ho, S.C. Polymethoxyflavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chem. 2010, 119, 868–873. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure-Activity Relationship of Flavonoids on Their Anti- Escherichia Coli Activity and Ihibition of DNA Gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Yoshida, M.; Sun, W.; Nakajima, A.; Lai, Y.; Osaka, N.; Matsuzaki, K.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; et al. Potent activity of nobiletin-rich citrus reticulata peel extract to facilitate cAMP/PKA/ERK/CREB signaling associated with learning and memory in cultured hippocampal neurons: Identification of the substances responsible for the pharmacological action. J. Neural Transm. 2013, 120, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Park, J.H.; Lee, J.; Jeong, W.S.; Ho, C.T.; Jun, M. The Identification of Biochanin A as a Potent and Selective β-Site App-Cleaving Enzyme 1 (Bace1) Inhibitor. Nutrients 2016, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Marvin 5.11.4, 2012, ChemAxon. Available online: http://www.chemaxon.com (accessed on 16 October 2016).
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun. 2005, 337, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739744. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain. Res. 2015, 289, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.P.; Yang, B.Y.; Zhao, H.; Xu, B.Q.; Jiao, W.J.; Wang, Q.H.; Wang, Z.B.; Kuang, H.X. Tangeretin exerts anti-neuroinflammatory effects via NF-κB modulation in lipopolysaccharide-stimulated microglial cells. Int. Immunopharmacol. 2014, 19, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of Citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Kuwahara, S.; Takahashi, Y.; Ito, C.; Furukawa, H.; Ju-Ichi, M.; Koshimizu, K.; Ohigashi, H. In vitro absorption and metabolism of nobiletin, a chemopreventive polymethoxyflavonoid in citrus fruits. Biosci. Biotechnol. Biochem. 2001, 65, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Matsuo, M.; Ohta, C.; Haraguchi, K.; Matsuoka, M.; Kato, Y.; Ishii, T.; Yano, M.; Ohta, H. Comparative study on nobiletin metabolism with liver microsomes from rats, Guinea pigs and hamsters and rat cytochrome p450. Biol. Pharm. Bull. 2007, 12, 2317–2323. [Google Scholar] [CrossRef]
- Al Rahim, M.; Nakajima, A.; Saigusa, D.; Tetsu, N.; Maruyama, Y.; Shibuya, M.; Yamakoshi, H.; Tomioka, Y.; Iwabuchi, Y.; Ohizumi, Y.; et al. 40-Demethylnobiletin, a bioactive metabolite of nobiletin enhancing PKA/ERK/CREB signaling, rescues learning impairment associated with NMDA receptor antagonism via stimulation of the ERK cascade. Biochemistry 2009, 48, 7713–7721. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sang, S.; Pan, M.H.; Lai, C.S.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg. Med. Chem. Lett. 2007, 17, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.M.; Rasmussen, S.E.; Brosen, K.; Friedberg, T.H. In vitro metabolism of genistein and tangeretin by human and murine cytochrome P450s. Pharmacol. Toxicol. 2003, 93, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.J.; Sheen, J.F.; Lu, W.C.; Hwang, L.S.; Ho, C.T.; Lin, C.I. Identification of sinensetin metabolites in rat urine by an isotope-labeling method and ultrahigh-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2012, 61, 5016–5021. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Ohizumi, Y.; Yamada, K. Anti-dementia activity of nobiletin, a citrus flavonoid: A review of animal studies. Clin. Psycopharmacol. Neurosci. 2014, 12, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, D.; Shibuya, M.; Jinno, D.; Yamakoshi, H.; Iwabuchi, Y.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; Ohizumi, Y.; Tomioka, Y.; et al. High-performance liquid chromatography with photodiode array detection for determination of nobiletin content in the brain and serum of mice administrated the natural compound. Anal. Bioanal. Chem. 2011, 400, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, J.; Zhong, Z.; Song, M.; Wu, X. Tissue distribution of nobiletin and its metabolites in mice after oral administration of nobiletin. FASEB J. 2013, 27 (Suppl. 1), 125.3. [Google Scholar]
- Onoue, S.; Uchida, A.; Takahashi, H.; Seto, Y.; Kawabata, Y.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Yamada, S. Development of high-energy amorphous solid dispersion of nanosized nobiletin, a citrus polymethoxylated flavone, with improved oral bioavailability. J. Pharm. Sci. 2011, 100, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood–brain barrier: In Vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, T.; Ushigome, F.; Koyabu, N.; Morimoto, S.; Shoyama, Y.; Naito, M.; Tsuruo, T. Inhibition of P-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Lett. 2000, 160, 21–28. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; De Castro, W.V.; Manthey, J.A.; Derendorf, H.; Butterweck, V. Polymethoxylated flavones and other phenolic derivates from citrus in their inhibitory effects on P-glycoprotein-mediated transport of talinolol in Caco-2 cells. J. Agric. Food Chem. 2007, 55, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Chiou, Y.S.; Jiang, Y.; Pan, M.H.; Lin, Z.; Huang, Q. Safety evaluation of tangeretin and the effect of using emulsion-based delivery system: Oral acute and 28-day sub-acute toxicity study using mice. Food Res. Int. 2015, 74, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kamiya, T.; Furukawa, K.; Azumi, M.; Ishizuka, S.; Takayama, S.; Nagase, S.; Arai, H.; Yamakuni, T.; Yaegashi, N. Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer’s disease: A case series. Geriatr. Gerontol. Int. 2013, 13, 236–238. [Google Scholar] [CrossRef] [PubMed]
| Sample (μM) | TACE | Trypsin | Chymotrypsin | Elastase |
|---|---|---|---|---|
| Nobiletin | ||||
| 50 | 5.49 ± 0.34 | 1.00 ± 0.07 | 1.39 ± 0.17 | 5.51 ± 1.17 |
| 100 | 11.61 ± 3.07 | 0.92 ± 0.06 | 1.77 ± 0.06 | 4.49 ± 1.00 |
| Tangeretin | ||||
| 50 | 8.00 ± 1.00 | 8.10 ± 0.99 | 5.68 ± 0.86 | 8.64 ± 0.65 |
| 100 | 11.28 ± 1.66 | 11.80 ± 1.04 | 6.16 ± 0.38 | 10.58 ± 1.09 |
| Sinensetin | ||||
| 50 | 7.88 ± 1.12 | 5.16 ± 0.69 | 9.08 ± 0.48 | 7.40 ± 2.09 |
| 100 | 7.19 ± 1.35 | 4.70 ± 0.58 | 8.78 ± 0.80 | 11.41 ± 1.38 |
| Ligand | Binding Energy (kcal/mol) | No. of Hydrogen Bonds | Hydrogen Bonds Interacting Residues |
|---|---|---|---|
| Nobiletin | −7.0 | 3 | Residue in 5 Å : ALA157, VAL336, THR232 |
| Tangeretin | −6.8 | 2 | Residue in 5 Å :SER10, THR232 |
| Sinensetin | −7.2 | 4 | Residue in 5 Å : TYR71, LYS75, TRP76, TYR198 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, K.; Yu, Y.; Lee, J.; Jeong, W.-S.; Ho, C.-T.; Jun, M. Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels. Nutrients 2017, 9, 973. https://doi.org/10.3390/nu9090973
Youn K, Yu Y, Lee J, Jeong W-S, Ho C-T, Jun M. Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels. Nutrients. 2017; 9(9):973. https://doi.org/10.3390/nu9090973
Chicago/Turabian StyleYoun, Kumju, Yoonjin Yu, Jinhyuk Lee, Woo-Sik Jeong, Chi-Tang Ho, and Mira Jun. 2017. "Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels" Nutrients 9, no. 9: 973. https://doi.org/10.3390/nu9090973
APA StyleYoun, K., Yu, Y., Lee, J., Jeong, W.-S., Ho, C.-T., & Jun, M. (2017). Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels. Nutrients, 9(9), 973. https://doi.org/10.3390/nu9090973





