Different Intestinal Microbial Profile in Over-Weight and Obese Subjects Consuming a Diet with Low Content of Fiber and Antioxidants
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Participants
2.2. Nutritional Assessment
2.3. Anthropometric Measures
2.4. Blood Biochemical Analyses
2.5. Fecal Collection and Microbiological Analyses
2.6. Short Chain Fatty Acids (SCFA) Analyses
2.7. Statistical Analysis
3. Results
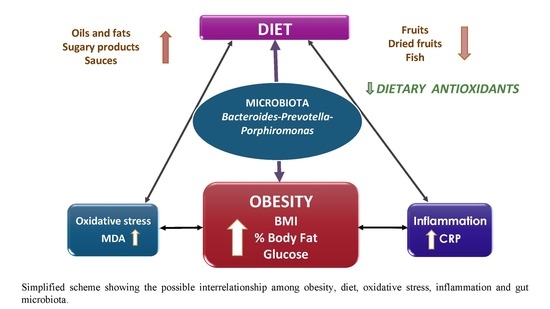
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Becer, E.; Cirakoglu, A. Association of the Ala16Val MnSOD gene polymorphism with plasma leptin levels and oxidative stress biomarkers in obese patients. Gene 2015, 568, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Taylor, A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006, 30, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Pego-Fernandes, P.M.; Bibas, B.J.; Deboni, M. Obesity: The greatest epidemic of the 21st century? Sao Paulo Med. J. 2011, 129, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Xu, J.; Leip, D.D.; Chen, C.H.; Westover, B.P.; Weatherford, J.; Buhler, J.D.; Gordon, J.I. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 2005, 307, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.L.; Devkota, S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Mol. Metab. 2016, 5, 737–742. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2015. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Montes-Borrego, S.M.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B.; et al. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J. Clin. Endocrinol. Metab. 2016, 101, 233–242. [Google Scholar] [CrossRef] [PubMed]
- De los Reyes-Gavilán, C.G.; Delzenne, N.M.; González, S.; Gueimonde, M.; Salazar, N. Development of functional foods to fight against obesity: Opportunities for probiotics and prebiotics. Agro FOOD Ind. Hi Tech. 2014, 25, 35–39. [Google Scholar]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Havinga, R.; Bleeker, A.; Rao, S.; Gerding, A.; van Eunen, K.; Groen, A.K.; Reijngoud, D.; Bakker, B.M. The short-chain fatty acid uptake fluxes by mice on a guar gum supplemented diet associate with amelioration of major biomarkers of the metabolic syndrome. PLoS ONE 2014, 9, e107392. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? Int. J. Obes. 2016, 40, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Rubio, M.A.; Barbany, M.; Moreno, B. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. 2007, 128, 184–196. [Google Scholar] [CrossRef]
- Cuervo, A.; Valdés, L.; Salazar, N.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Pilot study of diet and microbiota: Interactive associations of fibers and polyphenols with human intestinal bacteria. J. Agric. Food Chem. 2014, 62, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Hevia, A.; López, P.; Suárez, A.; Sánchez, B.; Margolles, A.; González, S. Association of polyphenols from oranges and apples with specific intestinal microorganisms in systemic lupus erythematosus patients. Nutrients 2015, 7, 1301–1317. [Google Scholar] [CrossRef] [PubMed]
- Centro de Enseñanza Superior de Nutrición Humana y Dietética (CESNID). Tablas de Composición de Alimentos por Medidas Caseras de Consumo Habitual en España; McGraw-Hill, Plublicaciones y Ediciones de la Universidad de Barcelona: Barcelona, Spain, 2008. [Google Scholar]
- United States Department of Agriculture (USDA). Agriculture Research Service, 2016 USDA National Nutrient Database for Standard References. Available online: http://www.ars.usda.gov/services/docs.htm?docid=8964 (accessed on 15 March 2017).
- Marlett, J.A.; Cheung, T.F. Database and quick methods of assessing typical dietary fiber intakes using data for 228 commonly consumed foods. J. Am. Diet. Assoc. 1997, 97, 1139–1151. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; Chaffaut, L.D.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database Oxf. 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Database for the Oxigen Radical Absorbance Capacity (ORAC) of Selected Foods. U.S. Dep. Agric, 2017. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 14 March 2017).
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Gerard-Monnier, D.; Erdelmeier, I.; Regnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudiere, J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solis, G.; Hernández-Barranco, A.; Margolles, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Gueimonde, M.; Hernández-Barranco, A.M.; Ruas-Madiedo, P.; de los Reyes-Gavilán, C.G. Exopolysaccharides produced by intestinal Bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl. Environ. Microbiol. 2008, 74, 4737–4745. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Shihabudeen, M.S.; David, H.P.; Thirumurugan, E.; Thirumurugan, K. Association between hyperleptinemia and oxidative stress in obese diabetic subjects. J. Diabetes Metab. Disord. 2015, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Cho, Y.H.; Lee, S.Y.; Jeong, D.W.; Cho, A.R.; Jeon, J.S.; Park, E.J.; Kim, Y.J.; Lee, J.G.; Yi, Y.H.; et al. Urinary malondialdehyde is associated with visceral abdominal obesity in middle-aged men. Mediat. Inflamm. 2015, 2015, 524291. [Google Scholar] [CrossRef] [PubMed]
- Gundala, R.; Chava, V.K.; Ramalingam, K. Association of leptin in periodontitis and acute myocardial infarction. J. Periodontol. 2014, 85, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, J.; Kochan, Z. Leptin as a mediator between obesity and cardiac dysfunction. Postepy Hig. Med. Dosw. 2012, 66, 267–274. [Google Scholar] [CrossRef]
- Paepegaey, A.C.; Genser, L.; Bouillot, J.C.; Oppert, J.M.; Clément, K.; Poitou, C. High levels of CRP in morbid obesity: The central role of adipose tissue and lessons for clinical practice before and after bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J.; Kasim-Karakas, S.; Dubuc, G.R.; Mueller, W.; Phinney, S.D. Gender differences in plasma leptin concentrations. Nat. Med. 1996, 2, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.F.; Riad-Gabriel, M.G.; Khan, A.; Sharma, A.; Michael, R.; Jinagouda, S.D.; Boyadjian, R.; Steil, G.M. Diurnal and ultradian rhythmicity of plasma leptin: Effects of gender and adiposity. J. Clin. Endocrinol. Metab. 1998, 83, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Piccoli de Melo, L.G.; Vargas Nunes, S.O.; Anderson, G.; Vargas, H.O.; Barbosa, D.S.; Galecki, P.; Carvalho, A.F.; Maes, M. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog Neuro-Psychopharmacol. Biol. Psychiatry 2017, 17. [Google Scholar] [CrossRef]
- Solomon, C.G.; Manson, J.E. Obesity and mortality: A review of the epidemiologic data. Am. J. Clin. Nutr. 1997, 66, 1044–1050. [Google Scholar]
- World Health Organisation. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation. WHO Technical Report Series 894, World Health Organization Technical Report Series; World Health Organisation: Geneva, Switzerland, 2000; Volume 894. [Google Scholar]
- He, Q.R.; Yu, T.; Fau-Li, P.; Li, P. Association of oxidative stress and serum adiponectine in patients with metabolic syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban 2009, 40, 623–627. [Google Scholar] [PubMed]
- Maes, M.; Ruckoanich, P.; Fau-Chang, Y.S.; Chang, Y.S.; Fau-Mahanonda, N.; Mahanonda, N.; Fau-Berk, M.; Berk, M. Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO&NS) pathways explain the co-association of depression and cardiovascular disorder (CVD), and the increased risk for CVD and due mortality in depressed patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 769–783. [Google Scholar]
- Sankhla, M.; Sharma, T.K.; Fau-Mathur, K.; Mathur, K.; Fau-Rathor, J.S.; Rathor, J.S.; Fau-Butolia, V.; Butolia, V.; Fau-Gadhok, A.K.; Gadhok, A.K.; et al. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin. Lab. 2012, 58, 385–392. [Google Scholar] [PubMed]
- Illán-Gómez, F.; Gonzálvez-Ortega, M.; Aragón-Alonso, A.; Orea-Soler, I.; Alcatraz-Tafalla, M.S.; Pérez-Paredes, M.; Lozano-Almeda, M.L. Obesity, endothelial function and inflammation: The effects of weight loss after bariatric surgery. Nutr. Hosp. 2016, 33, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Proctor, P.H.; Reynolds, E.S. Free radicals and disease in man. Physiol. Chem. Phys. Med. NMR 1984, 16, 175–195. [Google Scholar] [PubMed]
- Murdolo, G.; Piroddi, M.; Luchetti, F.; Tortoioli, C.; Canonico, B.; Zerbinati, C.; Galli, F.; Iuliano, L. Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie 2013, 95, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Belza, A.; Toubro, S.; Stender, S.; Astrup, A. Effect of diet-induced energy deficit and body fat reduction on high-sensitive CRP and other inflammatory markers in obese subjects. Int. J. Obes. 2009, 33, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Aljada, A.; Mohanty, P.; Ghanim, H.; Abdo, T.; Tripathy, D.; Chaudhuri, A.; Dandona, P. Increase in intranuclear nuclear factor κB and decrease in inhibitor κB inmononuclear cells after a mixed meal: Evidence for a proinflammatory effect. Am. J. Clin. Nutr. 2004, 79, 682–690. [Google Scholar] [PubMed]
- Dalmas, E.; Rouault, C.; Abdennour, M.; Rovere, C.; Rizkalla, S.; Bar-Hen, A.; Nahon, J.L.; Bouillot, J.L.; Guerre-Millo, M.; Clément, K.; et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am. J. Clin. Nutr. 2011, 94, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.N.; Ragab, S.H.; Abd, E.A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar]
- Bervoets, L.; Van, H.K.; Kortleven, I.; Van, N.C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Sakar, Y.; Lepage, P.; Devime, F.; Langelier, B.; Dore, J.; Covasa, M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes 2014, 63, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Kassis, A.; Major, G.; Chou, C.J. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J. Obes. 2012, 2012, 879151. [Google Scholar] [PubMed]
- Hartstra, A.V.; Bouter, K.E.; Backhed, F.; Nieuwdorp, M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Seo, J.H.; Youn, H.S. Gut microbiota and clinical disease: Obesity and nonalcoholic fatty liver disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, G.; López-Ortíz, E.; Torres-Castro, I. Gut microbiota as a key player in triggering obesity, systemic inflammation and insulin resistance. Rev. Investig. Clin. 2014, 66, 450–459. [Google Scholar]
- Sanz, Y.; Santacruz, A.; Gauffin, P. Gut microbiota in obesity and metabolic disorders. Proc. Nutr. Soc. 2010, 69, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed]
- Da, R.R.; Assaloni, R.; Ceriello, A. Postprandial hyperglycemia and diabetic complications. Recenti Prog. Med. 2005, 96, 436–444. [Google Scholar]
- Hennig, B.; Toborek, M.; McClain, C.J. High-energy diets, fatty acids and endothelial cell function: Implications for atherosclerosis. J. Am. Coll. Nutr. 2001, 20, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Proctor, S.D.; Mamo, J.C.; Botham, K.M.; Castro, C.M. Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int. J. Vasc. Med. 2012, 2012, 947417. [Google Scholar] [CrossRef] [PubMed]
- Nappo, F.; Esposito, K.; Cioffi, M.; Giugliano, G.; Molinari, A.M.; Paolisso, G.; Marfella, R.; Giugliano, D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002, 39, 1145–1150. [Google Scholar] [CrossRef]
- Ong, P.J.; Dean, T.S.; Hayward, C.S.; Della Monica, P.L.; Sanders, T.A.; Collins, P. Effect of fat and carbohydrate consumption on endothelial function. Lancet 1999, 354, 2134. [Google Scholar] [CrossRef]
- Roberts, C.K.; Barnard, R.J.; Sindhu, R.K.; Jurczak, M.; Ehdaie, A.; Vaziri, N.D. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 2006, 55, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.P.; Johnson, B.; Padilla, J.; Mather, K. Postprandial lipaemia, oxidative stress and endothelial function: A review. Int. J. Clin. Pract. 2010, 64, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Boque, N.; Campion, J.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; San, R.B.; Banuelos, O.; Martínez, J.A. Screening of polyphenolic plant extracts for anti-obesity properties in Wistar rats. J. Sci. Food Agric. 2013, 93, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.C.; Gao, W.; Wang, J.S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
| Microbial Target | Strain Used for Standard Curve | Primer Sequence 5′–3′ | Tm (°C) | Reference |
|---|---|---|---|---|
| Akkermansia | Akkermansia muciniphila CIP 107961 | F: CAGCACGTGAAGGTGGGGAC | 60 | [32] |
| R: CCTTGCGGTTGGCTTCAGAT | ||||
| Bacteroides group Bacteroides-Prevotella-Porphiromonas | Bacteroides thetaiotaomicron DSMZ 2079 | F: GAGAGGAAGGTCCCCCAC | 60 | [32] |
| R: CGCKACTTGGCTGGTTCAG | ||||
| Bifidobacterium | Bifidobacterium longum NCIMB 8809 | F:GATTCTGGCTCAGGATGAACGC | 60 | [32] |
| R: CTGATAGGACGCGACCCCAT | ||||
| Faecalibacterium | Faecalibacterium prausnitzi DSMZ 17677 | F:GGAGGAAGAAGGTCTTCGG | 60 | [33] |
| R: AATTCCGCCTACCTCTGCACT | ||||
| Clostridia XIVa Blautia coccoides—Eubacterium rectale group | Blautia coccoides DSMZ 935 | F: CGGTACCTGACTAAGAAGC | 55 | [32] |
| R: AGTTTYATTCTTGCGAACG | ||||
| Lactobacillus group | Lactobacillus gasseri IPLA IF7/5 | F: AGCAGTAGGGAATCTTCCA | 60 | [32] |
| R: CATGGAGTTCCACTGTCCTC |
| Normal Weight BMI ≤ 25.0 n = 20 | Over Weight BMI 25.0–30.0 n = 35 | p | Obesity BMI ≥ 30.0 n = 13 | p | |
|---|---|---|---|---|---|
| Age (years) a | 56.4 ± 10.1 | 51.7 ± 11.7 | 0.152 | 47.8 ± 10.2 | 0.033 |
| Female (%) | 80.0 | 51.4 | 0.036 | 53.8 | 0.110 |
| BMI (kg/m2) a | 23.0 ± 1.5 | 27.5 ± 1.4 | <0.001 | 34.1 ± 2.7 | <0.001 |
| Energy intake (Kcal/day) a | 1958 ± 537 | 1790 ± 482 | 0.261 | 2040 ± 548 | 0.681 |
| Basal Metabolic rate (Kcal/day) a | 1280 ± 167 | 1416 ± 228 | <0.001 | 1548 ± 324 | <0.001 |
| Sedentary lifestyle (%) | 20.0 | 17.1 | 0.792 | 30.8 | 0.481 |
| Current smokers (%) | 27.8 | 26.5 | 0.729 | 25.0 | 0.978 |
| Alcohol consumers (%) | 70.0 | 54.3 | 0.252 | 61.5 | 0.614 |
| Body fat (%) a | 26.2 ± 7.5 | 35.6 ± 9.3 | <0.001 | 51.7 ± 10.8 | <0.001 |
| Blood parameters | |||||
| Serum glucose (mg/dL) a | 97.1 ± 14.2 | 96.0 ± 9.1 | 0.711 | 108 ± 11.1 | 0.020 |
| Triglycerides (mg/dL) a | 100 ± 47.8 | 117 ± 56.9 | 0.288 | 147 ± 91.0 | 0.070 |
| LDL/HDL ratio a | 2.5 ± 0.8 | 2.6 ± 0.8 | 0.869 | 2.3 ± 0.6 | 0.347 |
| Leptin (ng/mL) a | 6.1 ± 4.3 | 9.2 ± 5.2 | 0.021 | 14.7 ± 6.8 | <0.001 |
| MDA (μM) a | 2.1 ± 0.6 | 2.2 ± 0.9 | 0.700 | 3.2 ± 1.6 | 0.012 |
| CRP (mg/L) a | 0.9 ± 0.8 | 3.8 ± 8.4 | 0.150 | 5.4 ± 7.4 | 0.009 |
| Normal Weight BMI ≤ 25.0 n = 20 | Over Weight BMI 25.0–30.0 n = 31 | Obesity BMI ≥ 30.0 n = 13 | BMI | |||
|---|---|---|---|---|---|---|
| R2 | β | p | ||||
| Model 1. Fecal SCFA concentration (mM) | ||||||
| Acetate | 35.7 ± 14.6 | 38.0 ± 16.8 | 46.6 ± 17.0 | 0.081 | 0.282 | 0.025 |
| Propionate | 14.4 ± 6.5 | 13.7 ± 6.7 | 17.1 ± 8.2 | 0.022 | 0.136 | 0.288 |
| Butyrate | 11.6 ± 8.7 | 10.3 ± 6.4 | 12.3 ± 9.0 | 0.047 | 0.040 | 0.748 |
| Model 2. Microbial target(log nº cells/gram of faeces) | ||||||
| Akkermansia | 6.3 ± 2.2 | 5.6 ± 1.6 | 5.6 ± 2.1 | 0.026 | −0.143 | 0.264 |
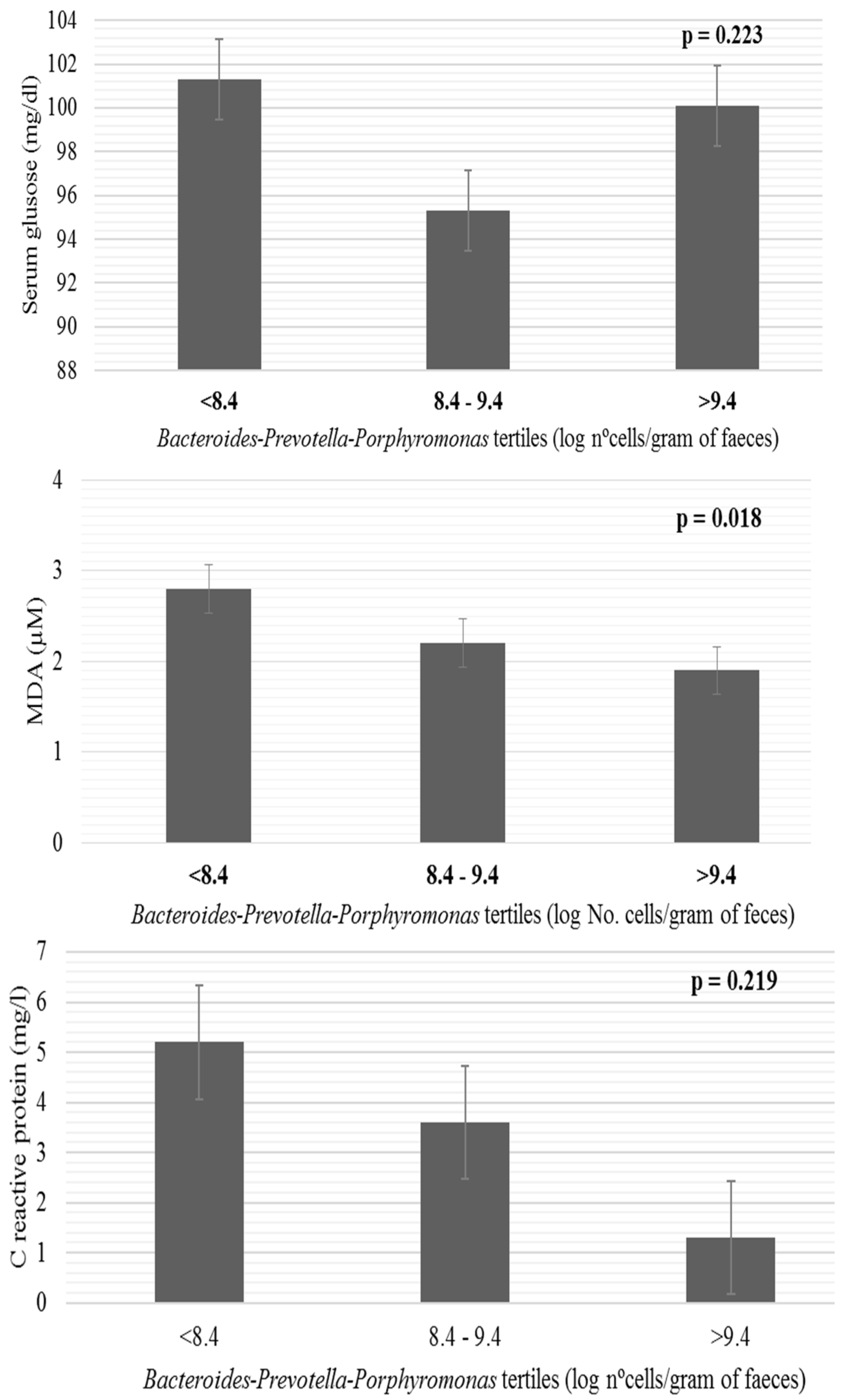
| Bacteroides-Prevotella-Porphyromonas | 8.8 ± 1.3 | 8.9 ± 1.1 | 8.2 ± 1.2 | 0.067 | −0.245 | 0.052 |
| Bifidobacterium | 7.7 ± 2.0 | 8.2 ± 0.8 | 8.2 ± 0.7 | 0.090 | 0.126 | 0.305 |
| Clostridia cluster XIVa group | 7.7 ± 1.7 | 8.2 ± 1.3 | 8.4 ± 1.1 | 0.048 | 0.150 | 0.236 |
| Lactobacillus group | 5.7 ± 1.3 | 6.0 ± 1.1 | 6.7 ± 0.9 * | 0.194 | 0.256 | 0.029 |
| Faecalibacterium prausnitzii | 7.3 ± 1.0 | 7.5 ± 1.0 | 7.7 ± 0.9 | 0.024 | 0.152 | 0.233 |
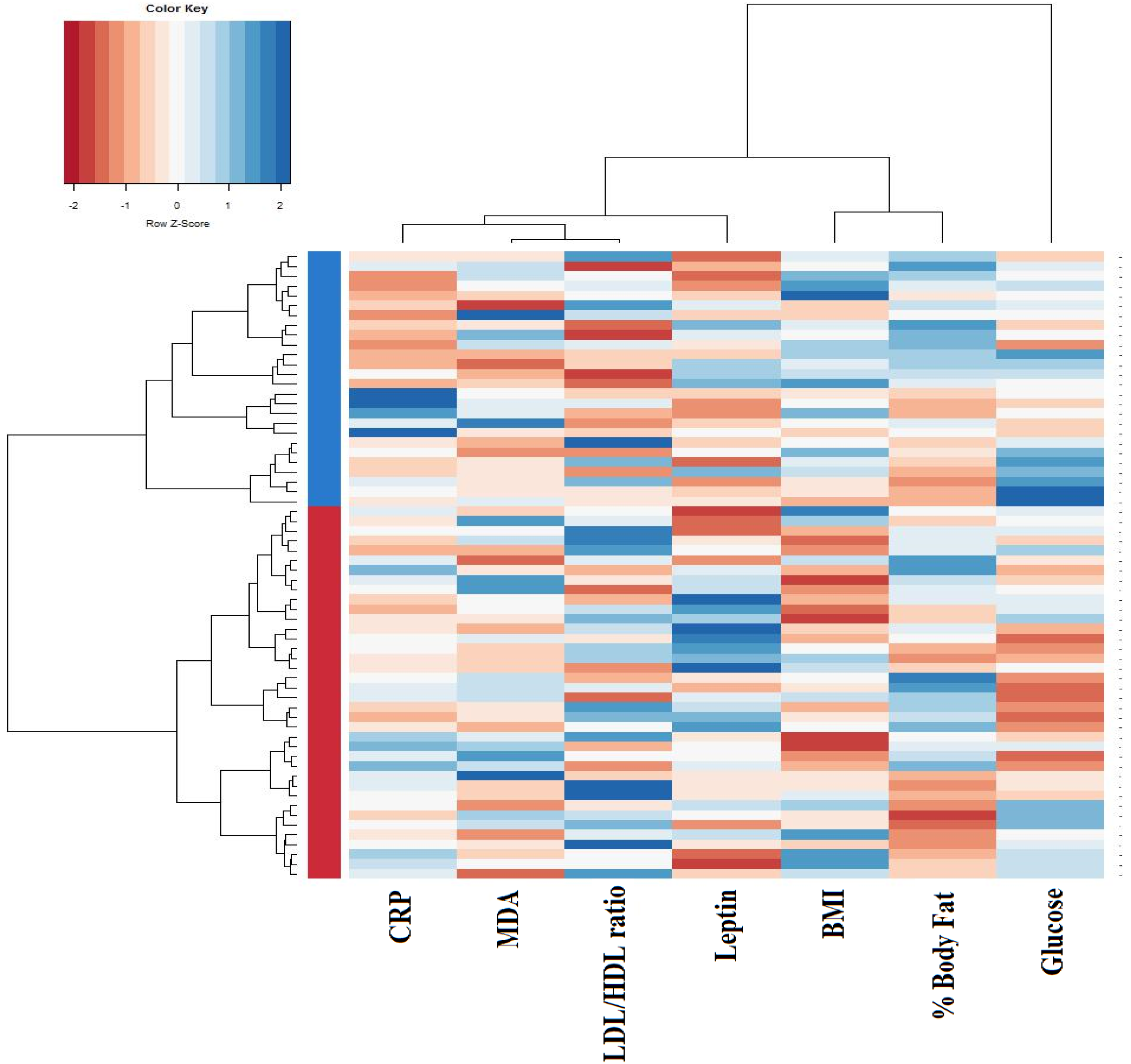
| Cluster I n = 38 | Cluster II n = 26 | p | |
|---|---|---|---|
| BMI (kg/m2) | 25.2 ± 2.7 | 30.3 ± 4.3 | <0.001 |
| Body fat (%) | 30.5 ± 8.0 | 42.3 ± 13.3 | <0.001 |
| Blood parameters | |||
| Serum glucose (mg/dL) | 92.7 ± 8.2 | 108.2 ± 10.9 | <0.001 |
| LDL/HDL ratio | 2.6 ± 0.8 | 2.5 ± 0.8 | 0.921 |
| Leptin (ng/mL) | 8.2 ± 5.1 | 10.8 ± 6.4 | 0.074 |
| MDA (μM) | 2.0 ± 0.6 | 2.8 ± 1.3 | 0.001 |
| CRP (mg/L) | 0.9 ± 0.9 | 6.7 ± 10.4 | 0.001 |
| Cluster I n = 37 | Cluster II n = 24 | |
|---|---|---|
| Model 1. Fecal SCFA concentration (mM) | ||
| Acetate | 35.8 ± 14.8 | 44.8 ± 17.7 * |
| Propionate | 14.0 ± 6.7 | 16.2 ± 7.3 |
| Butyrate | 11.1 ± 8.0 | 11.8 ± 7.7 |
| Model 2. Microbial target (log nº cells/gram of feces) | ||
| Akkermansia | 6.0 ± 1.8 | 5.6 ± 2.2 |
| Bacteroides-Prevotella-Porphyromonas | 9.0 ± 1.0 | 8.3 ± 1.3 * |
| Bifidobacterium | 8.1 ± 0.9 | 7.9 ± 1.8 |
| Clostridia cluster XIVa group | 7.9 ± 1.4 | 8.2 ± 1.4 |
| Lactobacillus group | 6.0 ± 1.1 | 6.1 ± 1.3 |
| Faecalibacterium prausnitzii | 7.4 ± 0.9 | 7.5 ± 0.9 |
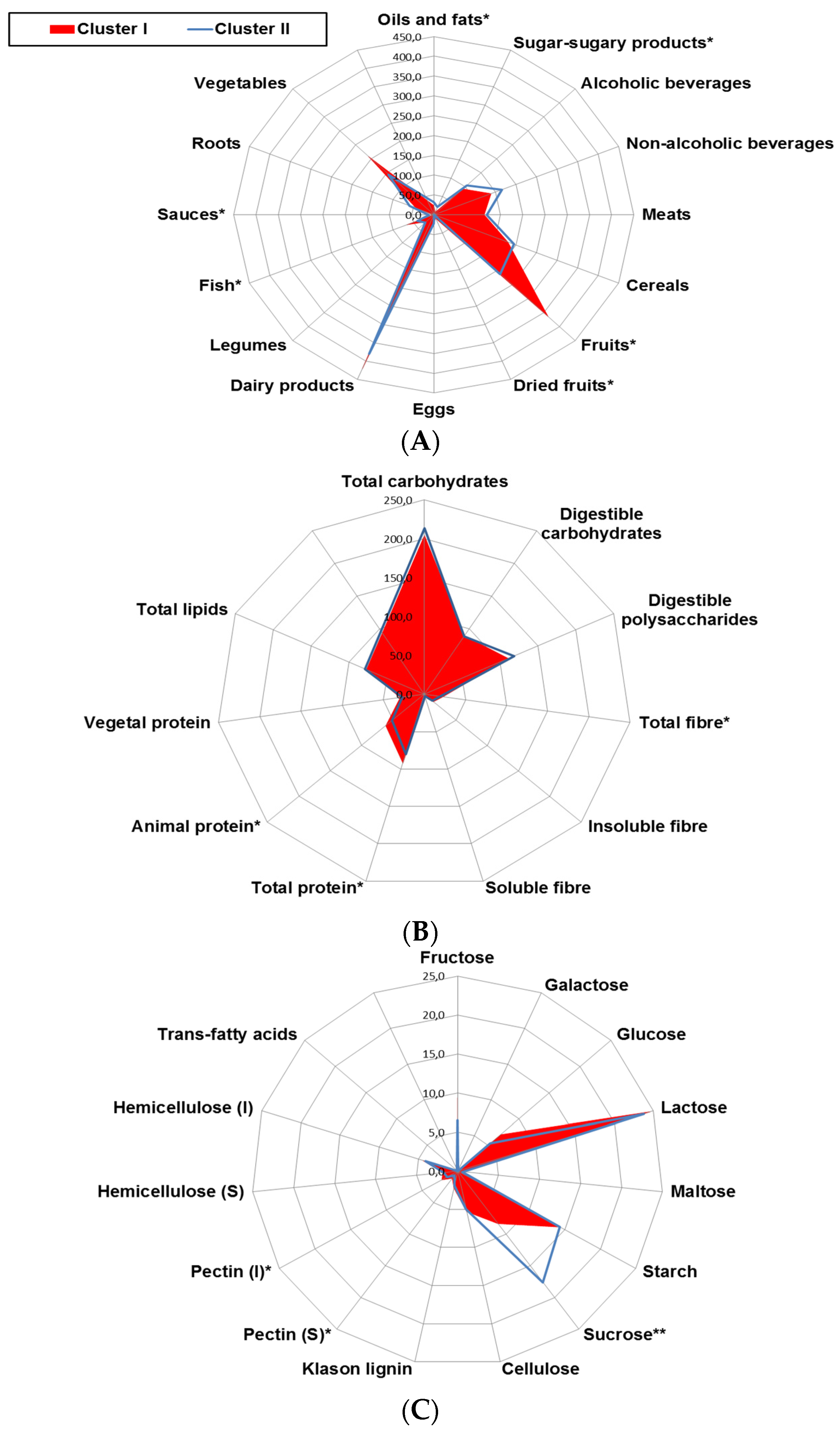
| Cluster I n = 38 | Cluster II n = 26 | p | |
|---|---|---|---|
| ORAC, hydrophilic (μmol TE/day) | 10367 ± 6641 | 6089 ± 4179 | 0.004 |
| ORAC, lipophilic (μmol TE/day) | 244 ± 193 | 138 ± 103 | 0.011 |
| ORAC, Total (μmol TE/day) | 10609 ± 6790 | 6229 ± 4224 | 0.004 |
| Selenium (μg/day) | 123 ± 40.3 | 115 ± 43.6 | 0.250 |
| Total carotenoids (μg/day) | 2391 ± 1538 | 1660 ± 1001 | 0.034 |
| γ-Tocopherol (mg/day) | 2.6 ± 1.1 | 2.1 ± 2.2 | 0.249 |
| Vitamin C (mg/day) | 222 ± 196 | 131 ± 102 | 0.021 |
| Vitamin E (mg/day) | 10.1 ± 4.1 | 12.7 ± 8.2 | 0.063 |
| Total polyphenols (mg/day) | 2057 ± 1076 | 1553 ± 975 | 0.043 |
| Total flavonoids (mg/day) | 435 ± 291 | 303 ± 232 | 0.049 |
| Total phenolics (mg/day) | 198 ± 192 | 224 ± 236 | 0.626 |
| Flavanols (mg/day) | 222 ± 187 | 189 ± 194 | 0.505 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Navarro, T.; Salazar, N.; Gutiérrez-Díaz, I.; De los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. Different Intestinal Microbial Profile in Over-Weight and Obese Subjects Consuming a Diet with Low Content of Fiber and Antioxidants. Nutrients 2017, 9, 551. https://doi.org/10.3390/nu9060551
Fernández-Navarro T, Salazar N, Gutiérrez-Díaz I, De los Reyes-Gavilán CG, Gueimonde M, González S. Different Intestinal Microbial Profile in Over-Weight and Obese Subjects Consuming a Diet with Low Content of Fiber and Antioxidants. Nutrients. 2017; 9(6):551. https://doi.org/10.3390/nu9060551
Chicago/Turabian StyleFernández-Navarro, Tania, Nuria Salazar, Isabel Gutiérrez-Díaz, Clara G. De los Reyes-Gavilán, Miguel Gueimonde, and Sonia González. 2017. "Different Intestinal Microbial Profile in Over-Weight and Obese Subjects Consuming a Diet with Low Content of Fiber and Antioxidants" Nutrients 9, no. 6: 551. https://doi.org/10.3390/nu9060551
APA StyleFernández-Navarro, T., Salazar, N., Gutiérrez-Díaz, I., De los Reyes-Gavilán, C. G., Gueimonde, M., & González, S. (2017). Different Intestinal Microbial Profile in Over-Weight and Obese Subjects Consuming a Diet with Low Content of Fiber and Antioxidants. Nutrients, 9(6), 551. https://doi.org/10.3390/nu9060551