Anti-Atherogenic Activity of Polyphenol-Rich Extract from Bee Pollen
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Drugs
2.2. Preparation of Ethanol Extract of Bee Pollen (EEP)
2.3. Animals and Treatments
2.4. Collecting Biological Material for Tests
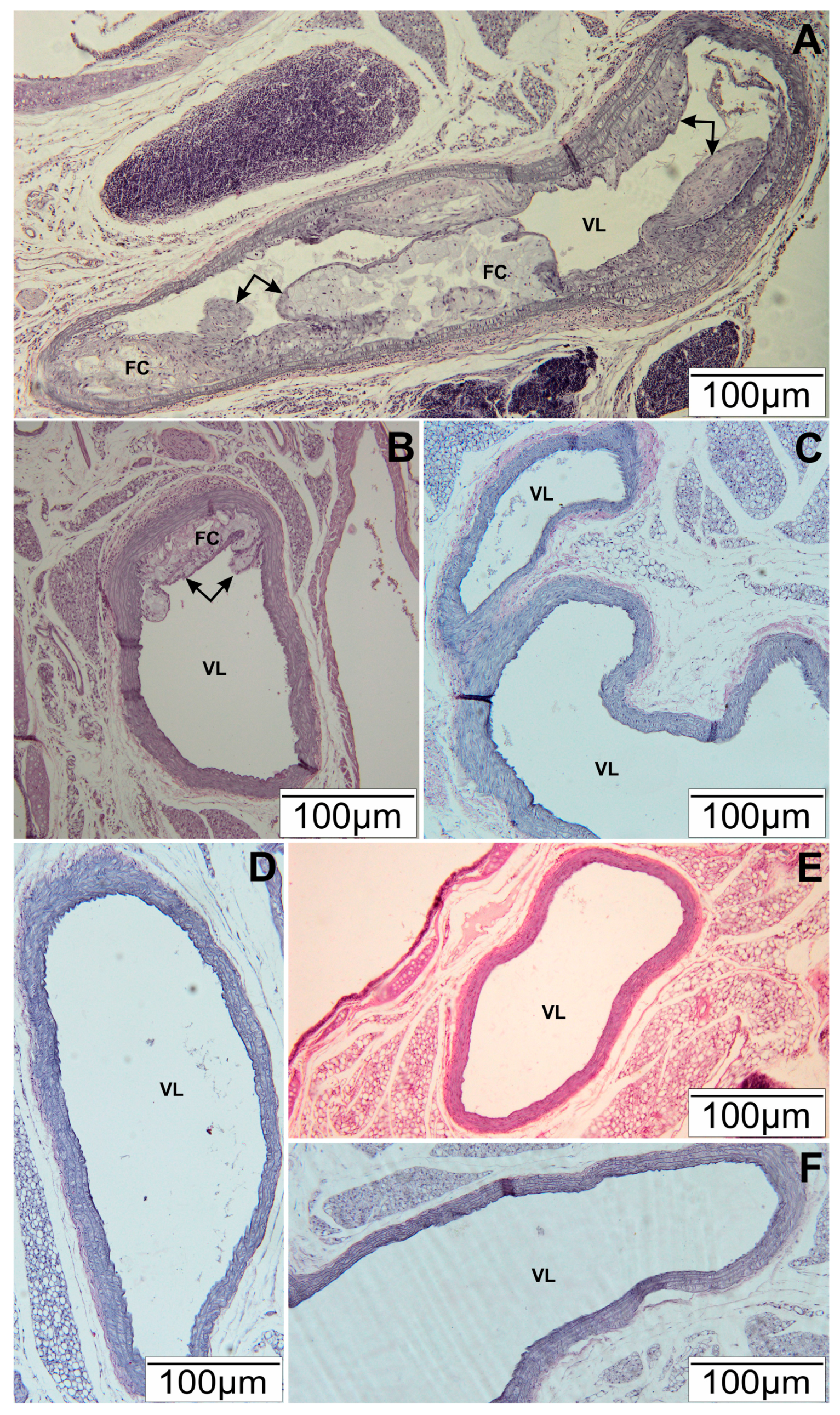
2.5. Histopathological Tests
2.6. Biochemical Tests
2.6.1. Determination of Total Cholesterol (TC)
2.6.2. Determination of Oxidized Low Density Lipoprotein (Ox-LDL) Concentration
2.6.3. Determination of Asymmetric Dimethylarginine (ADMA) Concentration
2.6.4. Determination of Angiotensin-Converting Enzyme (ACE) Concentration
2.6.5. Determination of Angiotensin II (ANG II) Concentration
2.7. Statistical Analysis
3. Results and Discussion
3.1. Histopathological Tests of Arteries
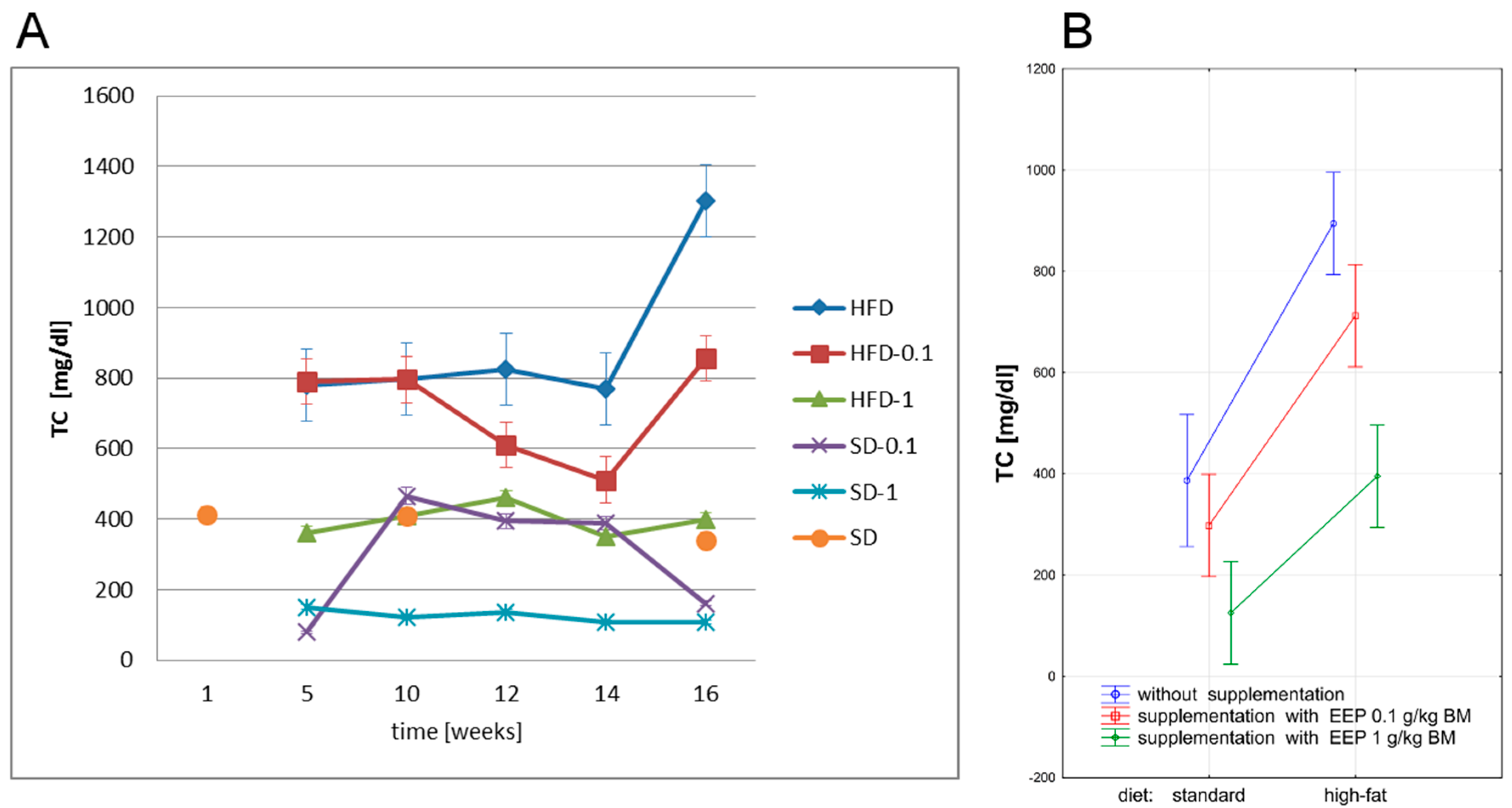
3.2. Effect of EEP on Total Cholesterol (TC)
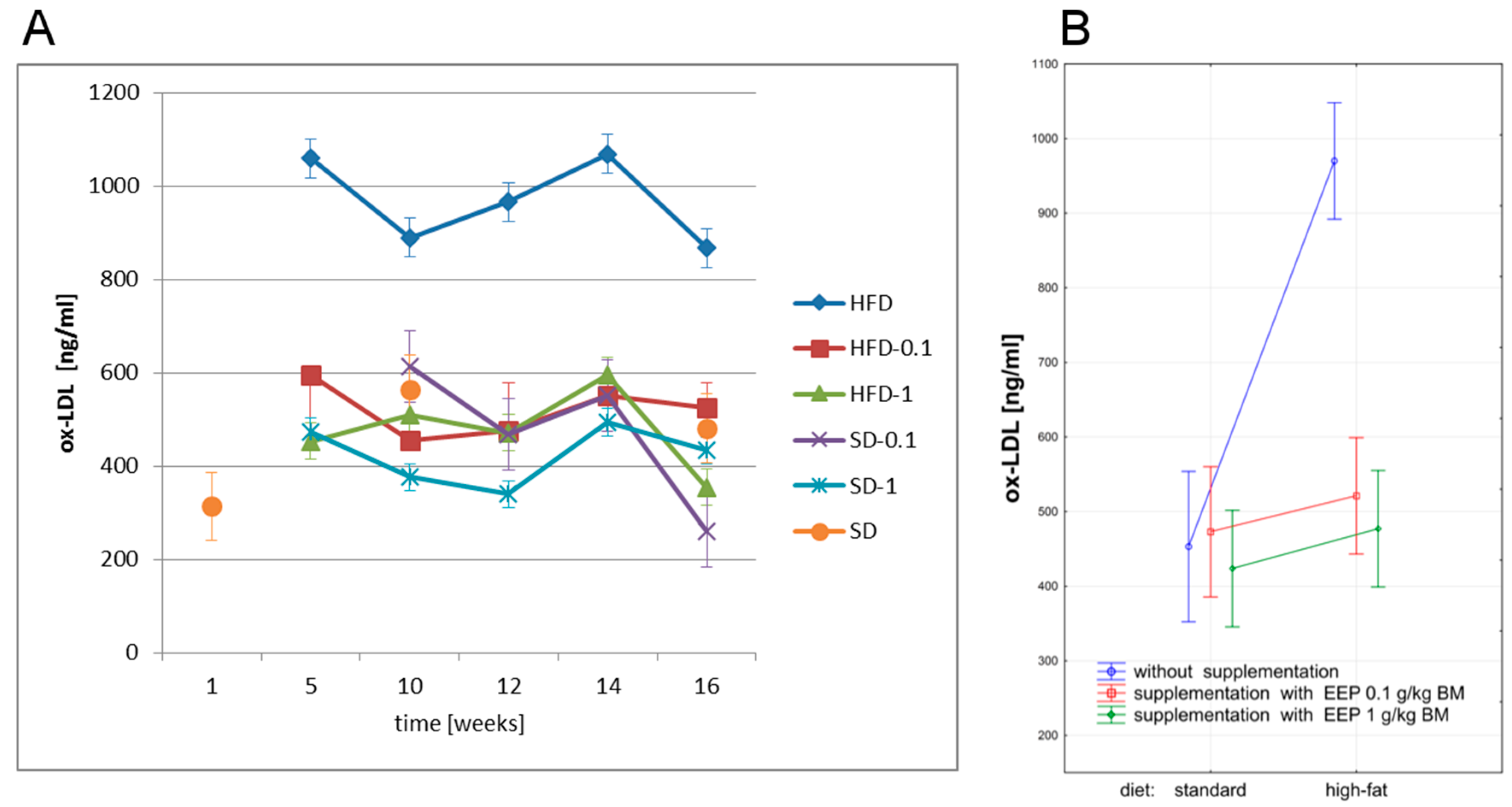
3.3. Effect of EEP on Oxidized Low Density Lipoprotein (Ox-LDL)
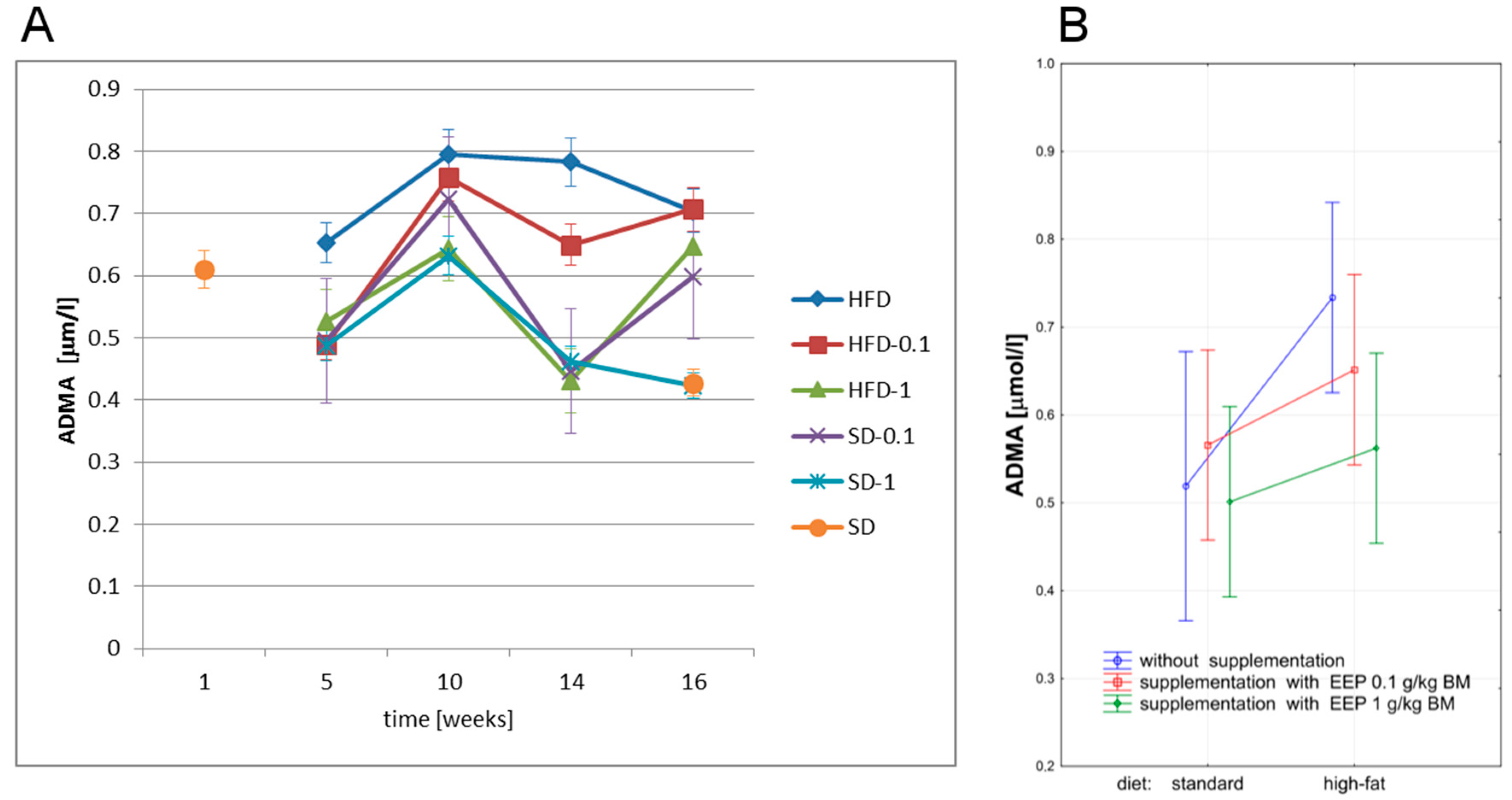
3.4. Effect of EEP on Asymmetric Dimethylarginine (ADMA)
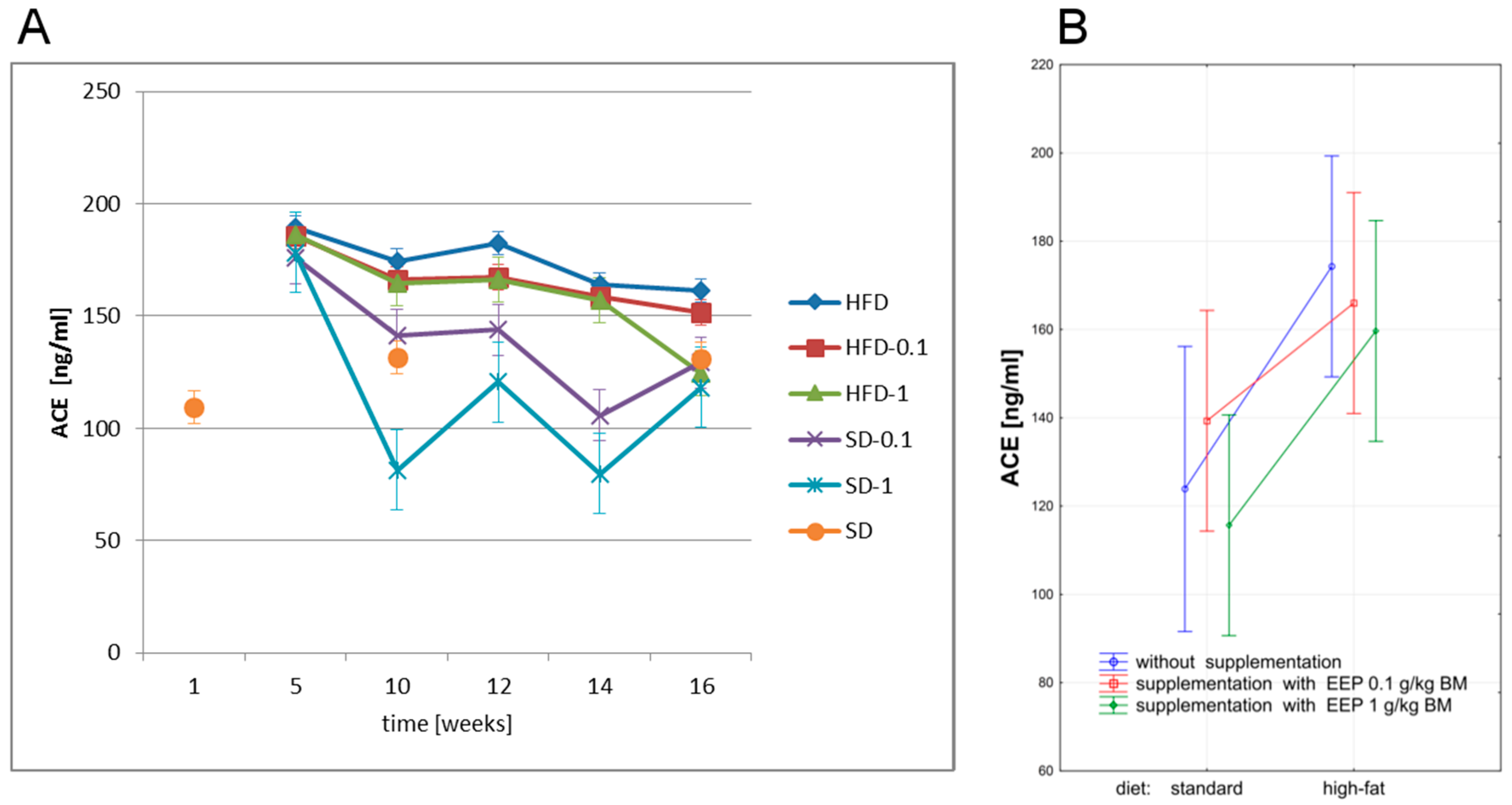
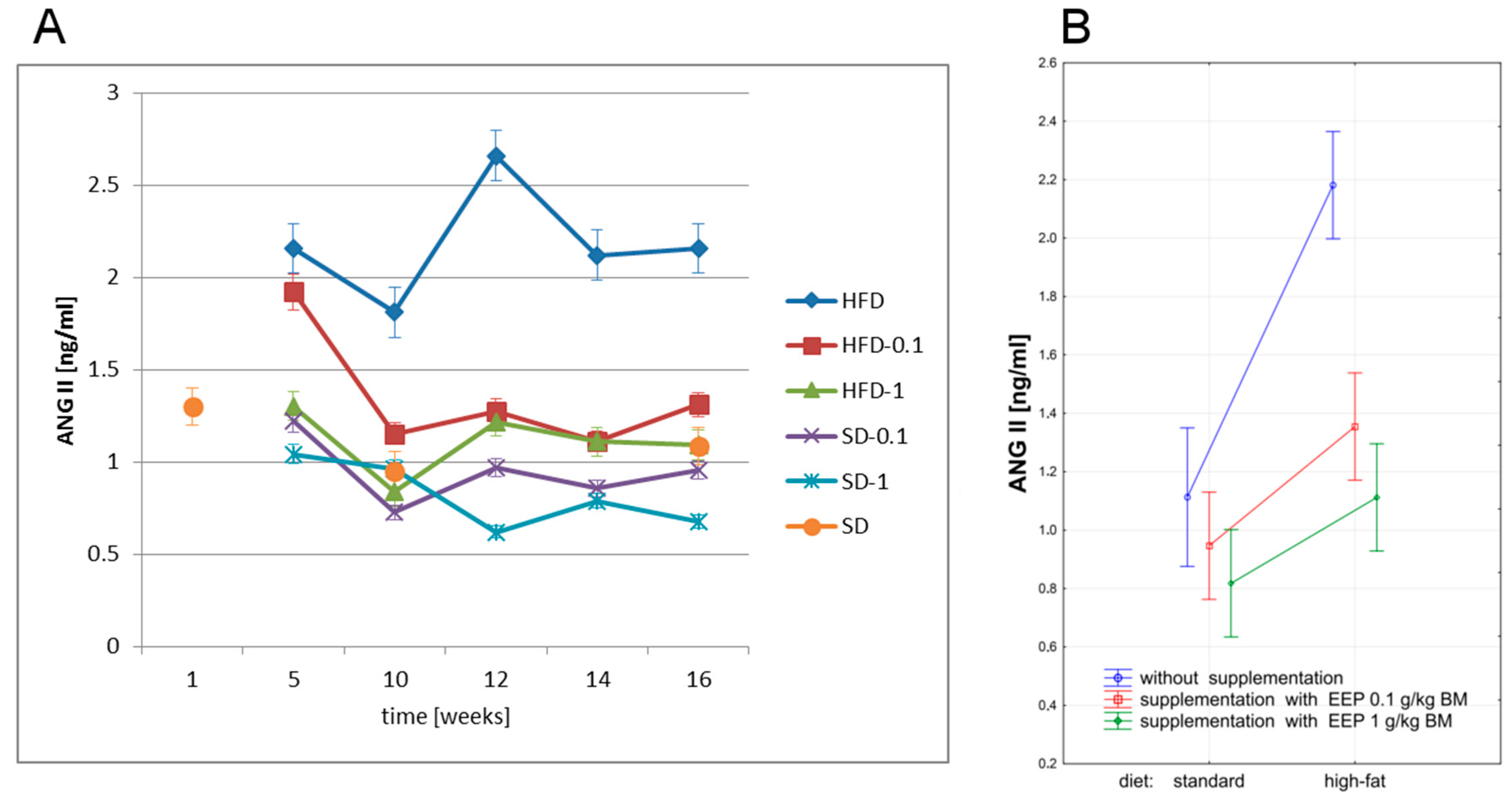
3.5. Effect of EEP on Angiotensin-Converting Enzyme (ACE) and Angiotensin II (ANG II)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campos, M.G.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of bee-pollen? J. ApiProd. ApiMed. Sci. 2010, 2, 131–144. [Google Scholar] [CrossRef]
- Feás, X.; Vázquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.M.; Rodrigues, S.; Pereira, A.P.; Feas, X. Portuguese bee pollen: Palynological study nutritional and microbiological evaluation. Int. J. Food Sci. Technol. 2012, 47, 429–435. [Google Scholar] [CrossRef]
- Marghitas, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobis, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Komosińska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef] [PubMed]
- Margaoan, R.; Mãrghitaş, L.; Dezmirean, D.; Mihai, C.M.; Bobis, O. Bee collected pollen—General aspects and chemical composition. Bull. UASVM Anim. Sci. Biotechnol. 2010, 67, 254–259. [Google Scholar]
- Maruyama, H.; Sakamoto, T.; Araki, Y.; Hara, H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement. Altern. Med. 2010, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Almaraz-Abarca, N.; Campos, M.G.; Ávila-Reyes, J.A.; Naranjo-Jiménez, N.; Carrol, H.J.; González-Valdez, L.S. Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J. Food Compos. Anal. 2007, 20, 119–124. [Google Scholar] [CrossRef]
- Carpes, S.T.; Mourão, G.B.; Alencar, S.M.; Masson, M.L. Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from Southern Brazil. Braz. J. Food Technol. 2009, 12, 220–229. [Google Scholar] [CrossRef]
- Campos, M.G.; Bogdanov, S.; Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardization of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feas, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Zurek, G.; Bässmann, C.; Almaraz-Abarca, N.; Quirantes, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds from pollen extracts using capillary electrophoresis-electrospray time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Moreira, L.; Feas, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Iriti, M. Introduction to polyphenols, plant chemicals for human health. Mini-Rev. Med. Chem. 2011, 11, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. Dietary polyphenols: Antioxidants or not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Mozdzierz, A.; Buszman, E. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A systematic review of the efficacy of bioactive compounds in cardiovascular disease: Phenolic compounds. Nutrients 2015, 7, 5177–5216. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Counterregulation rules in atherothrombosis. J. Am. Coll. Cardiol. 2012, 59, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Viola, J.; Soehnlein, O. Atherosclerosis—A matter of unresolved inflammation. Semin. Immunol. 2015, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Sobczak, A.; Stojko, R.; Buszman, E. Polyphenol content and antioxidant activity of bee pollen extracts from Poland. J. Apic. Res. 2015, 54, 482–490. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Pastore, J.J.; Giraud, F.; Sulpice, J.C.; Janmey, P.A. Flavonoid inhibition of platelet procoagulant activity and phosphoinositide synthesis. J. Thromb. Haemost. 2003, 1, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.P.; Stevens, J.M.; Cicmil, M.; Sage, T.; Jordan, P.A.; Williams, C.M.; Lovegrove, J.A.; Gibbins, J.M. Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signaling pathway. J. Thromb. Haemost. 2003, 1, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Di Santo, S.; Buchetti, B.; Sanguigni, V.; Brunelli, A.; Violi, F. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: Effect on platelet recruitment. FASEB J. 2006, 20, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Marchesi, P.; Passamonti, S.; Pirillo, A.; Violi, F.; Catapano, A.L. Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis 2007, 191, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hayek, E.; Furhman, B.; Vaya, J.; Rosenblat, M.; Belikny, P.; Coleman, R.; Elis, A.; Aviram, M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler. Tromb. Vasc. Biol. 1997, 17, 2744–2752. [Google Scholar] [CrossRef]
- Daleprane, J.B.; Freitas, V.S.; Pacheco, A.; Rudnicki, M.; Faine, L.A.; Dorr, F.A.; Ikegaki, M.; Salazar, L.A.; Ong, T.P.; Abdalla, D.S. Anti-atherogenic and anti-angiogenic activities of polyphenols from propolis. J. Nutr. Biochem. 2012, 23, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.; Saavedra, N.; Cavalcant, M.F.; Salazar, L.A.; Abdalla, D.S. Identification of microRNAs involved in the modulation of pro-angiogenic factors in atherosclerosis by a polyphenol-rich extract from propolis. Arch. Biochem. Biophys. 2014, 557, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, E.; Wang, F.; Wang, T.; Qin, Z.; Niu, S.; Qiu, C. Quercetin increases macrophage cholesterol efflux to inhibit foam cell formation through activating PPARγ-ABCA1 pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 10854–10860. [Google Scholar] [PubMed]
- Steinberg, D.; Witztu, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Kamiloglu, S.; Capanoglu, E.; Van Camp, J. Cell systems to investigate the impact of polyphenols on cardiovascular health. Nutrients 2015, 7, 9229–9255. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Tani, M.; Kondo, K. Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur. J. Clin. Nutr. 2013, 67, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Natsume, M.; Baba, S. Suppressive effects of cacao polyphenols on the development of atherosclerosis in apolipoprotein E-deficient mice. Subcell. Biochem. 2014, 77, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Freund, M.A.; Flammer, A.J.; Sexton, J.; Lennon, R.; Romani, A.; Mulinacci, N.; Vinceri, F.F.; Lerman, L.O.; Lerman, A. Beneficial effects of polyphenol-rich olive oil in patients with early atherosclerosis. Eur. J. Nutr. 2013, 52, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Massaro, M.; Scoditti, E.; De Caterina, R. Chapter 69—Atherosclerosis and mediterranean diet polyphenols. In Polyphenols in Human Health and Disease, 1st ed.; Watson, R.R., Preedy, V., Zibadi, S., Eds.; Elsevier: Oxford, UK, 2014; pp. 895–903. ISBN 9780123984678. [Google Scholar]
- Boger, R.H. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 2003, 59, 824–833. [Google Scholar] [CrossRef]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Landim, M.B.; Filho, A.C.; Chagas, A.C. Asymmetric dimethylarginine (ADMA) and endothelial dysfunction: Implications for atherogenesis. Clinics 2009, 64, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Boger, R.H. Asymmetric dimethylarginine (ADMA) and cardiovascular disease: Insight from prospective clinical trials. Vasc. Med. 2005, 10, 19–25. [Google Scholar] [CrossRef]
- Li Volti, G.; Salomone, S.; Sorrenti, V.; Mangiameli, A.; Urso, V.; Siarkos, I.; Galvano, F.; Salamone, F. Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc. Diabetol. 2011, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Luna, R.; Munoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.; Villar, J.; Stiefel, P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflamm. 2014, 2014, 689360. [Google Scholar] [CrossRef] [PubMed]
- Kokot, F.; Hyla-Klekot, L. Renin-angiotensin-aldosterone system (RAA) yesterday and today. Nefrol. Dial. Polska 2008, 12, 181–185. [Google Scholar]
- Velez, J.C.Q. The importance of intrarenal renin-angiotensin system. Nat. Rev. Nephrol. 2009, 5, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Darland, G.; Bland, J.S.; Tripp, M.L.; Konda, V.R. META060 attenuates TNF-α-activated inflammation, endothelial-monocyte interactions, and matrix metalloproteinase-9 expression, and inhibits NF-κB and AP-1 in THP-1 monocytes. Atherosclerosis 2012, 223, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Arutyunyan, T.V.; Korystova, A.F.; Kublik, L.N.; Levitman, M.K.H.; Shaposhnikova, V.V.; Korystov, Y.N. Effects of taxifolin on the activity of angiotensin-converting enzyme and reactive oxygen and nitrogen species in the aorta of aging rats and rats treated with the nitric oxide synthase inhibitor and dexamethasone. Age (Dordr) 2013, 35, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Yang, C.; Li, S.N. Effects of genistein on angiotensin-converting enzyme in rats. Life Sci. 2006, 79, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Ocete, M.A.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.A.; de Oliveira, P.R.; de Bem, G.F.; de Cavalho, L.C.; Ognibene, D.T.; da Silva, A.F.; Dos Santos Valença, S.; Pires, K.M.; da Cunha Sousa, P.J.; de Moura, R.S.; et al. Euterpe oleracea Mart.—Derived polyphenols prevent endothelial dysfunction and vascular structural changes in renovascular hypertensive rats: Role of oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.Z.; Xu, S.J.; Jiang, D.X.; Chen, S.X.; Wang, L.L.; Huang, S.; Lai, X.P. Effect of the flavonoid fraction of Lithocarpus polystachyus Rehd on spontaneously hypertensive and normotensive rats. J. Ethnopharmacol. 2012, 143, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, X.; Liang, Y.; Head, R.; Bennett, L. Inhibition of angiotensin converting enzyme (ACE) activity by polyphenols from tea (Camellia sinensis) and links to processing method. Food Funct. 2011, 2, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Inoue, R.; Suzuki, N.; Tanoue, Y.; Kai, N.; Nagashima, T. Antihypertensive activities of enzymatic hydrolysates from honeybee-collected pollen of Cistus ladaniferus. J. Food Agric. Environ. 2007, 5, 86–89. [Google Scholar]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzepecka-Stojko, A.; Stojko, J.; Jasik, K.; Buszman, E. Anti-Atherogenic Activity of Polyphenol-Rich Extract from Bee Pollen. Nutrients 2017, 9, 1369. https://doi.org/10.3390/nu9121369
Rzepecka-Stojko A, Stojko J, Jasik K, Buszman E. Anti-Atherogenic Activity of Polyphenol-Rich Extract from Bee Pollen. Nutrients. 2017; 9(12):1369. https://doi.org/10.3390/nu9121369
Chicago/Turabian StyleRzepecka-Stojko, Anna, Jerzy Stojko, Krzysztof Jasik, and Ewa Buszman. 2017. "Anti-Atherogenic Activity of Polyphenol-Rich Extract from Bee Pollen" Nutrients 9, no. 12: 1369. https://doi.org/10.3390/nu9121369
APA StyleRzepecka-Stojko, A., Stojko, J., Jasik, K., & Buszman, E. (2017). Anti-Atherogenic Activity of Polyphenol-Rich Extract from Bee Pollen. Nutrients, 9(12), 1369. https://doi.org/10.3390/nu9121369





