Abstract
The gut microbiota plays critical roles in development of obese-related metabolic diseases such as nonalcoholic fatty liver disease (NAFLD), type 2 diabetes(T2D), and insulin resistance(IR), highlighting the potential of gut microbiota-targeted therapies in these diseases. There are various ways that gut microbiota can be manipulated, including through use of probiotics, prebiotics, synbiotics, antibiotics, and some active components from herbal medicines. In this review, we review the main roles of gut microbiota in mediating the development of NAFLD, and the advances in gut microbiota-targeted therapies for NAFLD in both the experimental and clinical studies, as well as the conclusions on the prospect of gut microbiota-targeted therapies in the future.
1. Introduction
The mammalian gastrointestinal tract is the main site for commensal bacteria. There are over 1014 microorganisms inside human body [1], which play important roles in maintaining human health [2]. The abundance and composition of gut microbiota are highly variable in the context of different conditions contributing to the development of various diseases [3,4]. In recent years, a huge number of studies have revealed the critical roles of gut microbiota in the development of metabolic diseases including type 1 and 2 diabetes [5,6], obesity [7,8,9,10], cardiovascular disease [11,12,13], and chronic liver diseases [14].
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of chronic liver diseases including simple steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [9]. NAFLD is the most common chronic liver disease due to the prevalence of obesity worldwide [15]. In addition to the well-established “two-hit” theory [16], the alteration of gut microbiota also promotes the development of NAFLD by mediating processes of inflammation, insulin resistance, bile acids, and choline metabolism [17,18]. As a result, the elucidation on the roles of gut microbiota in NAFLD highlights the significance of gut microbiota-targeted therapies for NAFLD [19,20]. There are various ways to manipulate gut microbiota, for example through the use of probiotics, prebiotics, synbiotics, antibiotics, and some active components from herbal medicines.
In this review, we retrieved the publications on the topics of gut microbiota and NAFLD mainly published within the past 10 years through Pubmed. Based on all of the publications available, we first reviewed the main roles of gut microbiota in mediating NAFLD formation. Then, we discussed the status of gut microbiota-targeted therapies in NAFLD with both the experimental and clinical evidence, and made conclusions on the therapeutic potential of manipulating gut microbiota in the future.
2. Roles of the Gut Microbiota in NAFLD Development
Obesity is the common ground of most metabolic diseases. The gut microbiota plays critical roles in the development of obesity and obese-related metabolic diseases [21] by producing microbial metabolites like short-chain fatty acids (SCFAs) that regulate host energy harvest [22,23], or by modulating signaling pathways of host energy metabolism [24]. Study has revealed that the gut microbiota promotes the intestinal absorption of monosaccharides, accelerating de novo hepatic lipogenesis and suppressing fasting-induced adipocyte factor, resulting in the accumulation of triglycerides in adipocytes [25]. More evidence of gut microbiota affecting host energy metabolism has been acquired in numerous studies [25,26,27].
Insulin resistance is a basic pathophysiological process of metabolic diseases [28,29]. In NAFLD, insulin resistance accelerates the fat accumulation and inflammation in hepatocytes [30]. The enhanced inflammation and insulin resistance forms a “vicious cycle” deteriorating the development of NAFLD. The gut epithelium is a natural barrier for preventing the translocation of detrimental bacteria and harmful elements into circulation. NASH patients are typically characterized with small intestine bacterial overgrowth (SIBO) that may impair the intestinal tight junction and subsequently increase intestinal permeability. SIBO also induces hepatic expression of toll like receptor 4 (TLR4) and release of interleukin (IL)-8 that stimulates inflammatory reaction. The term “metabolic endotoxemia” was coined because of increased lipopolysaccharide (LPS) levels in the circulation in metabolic diseases [31], in which LPS combines with LPS binding protein (LBP) and then binds to themonocyte differentiation antigen(CD14)-TLR-4complex triggering an inflammatory reaction and insulin resistance [32,33,34]. Therefore, gut dysbiosis is causative for enhanced secretion of LPS and its mediated inflammation in NAFLD development.
Choline not only is an indispensable component of cell membrane phospholipids, but also plays important role in lipid metabolism. Choline facilitates the lipid transport in hepatocytes and prevents the abnormal accumulation of lipids in the liver, while choline deficiency usually leads to hepatic steatosis [35,36]. The gut microbiota is also involved in choline metabolism by converting it into toxic dimethylamine and trimethylamine, which are transported to liver and converted into trimethylamine oxide (TMAO) that causes liver inflammation and damage [37]. The increased production of TMAO is also the culprit for cardiovascular disease [37,38,39]. On the other hand, the content of dietary choline influences the composition and abundance of gut microbiota that are associated with the development of NAFLD [40]. The close relationship between gut microbiota and choline metabolism provides an important rationale for gut microbiota-targeted therapy for NAFLD.
Bile acids are synthesized from cholesterol with a wide range of physiological functions. Bile acids can not only facilitate digestion and absorption of fat-soluble food, but also preserve the intestinal barrier and prevent bacterial translocation [41,42]. Moreover, bile acids could function as signaling molecules that modulate the balance of bile acids metabolism by activating the farnesoid X receptor (FXR) and G protein-coupled bile acid receptor(TGR5) [43,44,45,46]. Studies reveal that antibiotics could attenuate the high-fat diet-induced NAFLD development by altering the composition of bile acids and inhibiting the FXR signaling pathway, whereas mice with intestine-specific FXR disruption have reduced triglyceride accumulation in the liver compared with control mice [47]. Bile acids usually have strong anti-microbial properties and gut microbiota can influence the homeostasis of bile acids pool by deconjugating and metabolizing the primary bile acids into secondary bile acids in the intestinal tract, which are involved in modulating lipids and energy metabolism pathways during NAFLD formation [44]. The crosstalk between gut microbiota and bile acids provides fundamental evidence for gut microbiota-targeted therapy of NAFLD. A schematic view on the roles of gut microbiota on NAFLD formation is summarized in Figure 1.
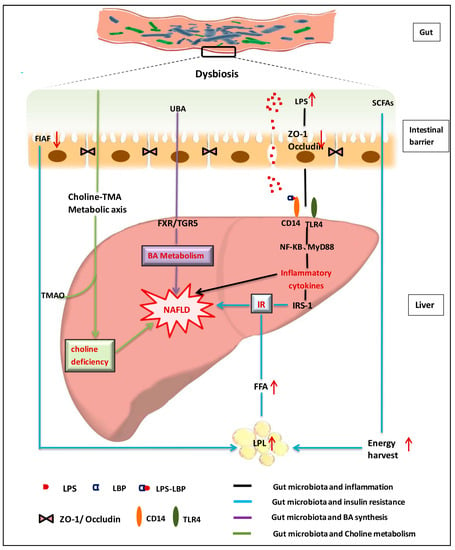
Figure 1.
Schematic view on roles of gut microbiota in nonalcoholic fatty liver disease [2,48,49,50,51,52,53,54,55,56,57,58,59,60]. NAFLD: nonalcoholic fatty liver disease; LPS: lipopolysaccharides; LBP: LPS binding protein; SCFAs: short chain fatty acids; BA:Bile acid; TNF-α: tumor-necrosis factor alpha; TLR: Toll like receptor; CD14: monocyte differentiation antigen; UBA: unconjugated bile acid; ZO-1\Occludin: two tight junction proteins; FIAF: Fasting-induced adipocyte factor; NF-kB: Nuclear factor-κB; MyD88: myeloid differentiation factor 88; FXR: farnesoid X receptor; TGR5: Takeda G protein-coupled receptor 5; TMA: trimethylamine; TMAO: trimethylamine oxide; IR: Insulin resistance; IRS: Insulin receptor substrate; FFA: free fatty acid; LPL: lipoprteinlipase.
3. Gut Microbiota-Targeted Therapies in NAFLD
NAFLD is common with the current prevalence of obesity, however, clinical therapeutic options are still very scarce with respect to safety, effectiveness, and patient compliance [61]. As a result, the intricate relationship between gut microbiota and NAFLD opens up a new window for seeking effective and safe therapies on NAFLD by restoring gut homeostasis of NAFLD patients in various ways.
3.1. Gut Microbiota-Targeted Therapy with Probiotics
Probiotics are a collection of bacteria with a wide range of beneficial effects on host metabolism [2,62]. Bacteria of Lactobacillus, Bifidobacterium and Satreptococcus are most frequently used probiotics that can inhibit expansion of gram-negative pathogenic bacteria [63]. Okubo et al. investigated the effects of Lactobacillus caseistrain Shirota (LcS) on methionine-choline-deficient (MCD) diet-induced NASH mice [64]. They found that the MCD diet resulted in significant reduction in lactic acid bacteria (Bifidobacterium and Lactobacillusin) in feces, but values were increased by LcS supplementation. Moreover, the LcS supplement dramatically improved the symptoms of NASH induced by MCD such as hepatic histology and serum parameters triglycerides (TG), total cholesterol (TC), as well as the altered expression of hepatic genes and proteins (the mRNA levels of actin alpha/alpha-SMA(α-SMA) and tissue inhibitor of metalloproteinase 1(TIMP-1)). Meanwhile, metabolic beneficial effects of LcS supplement were observed in high-fat diet (HFD)-induced and genetic db/db obese mice, in which LcS supplementation significantly improved insulin resistance and lowered plasma levels of LBP [65]. Study revealed that LcS treatment protected against the fructose-induced NAFLD by suppressing the activation of the TLR4 signaling cascade in the liver [66]. Accordingly, the beneficial effect of LcS in metabolic diseases is due to the improvement of metabolic endotoxemia.
Lactobacillus is a genus of gram-positive bacteria which can convert sugars into lactic acid. Bacteria from Lactobacillus genus have been trialed as probiotics in studies [67,68,69]. Sohn et al. investigated the effects of Lactobacillus paracasei on NASH patients [70] and found that L. paracasei administration lowered inflammatory cytokines in NASH patients. However, probiotics with a single species of Lactobacillus bacteria did not show benefit in patients with irritable bowel syndrome or Crohn’s disease [71,72]. Meanwhile, the beneficial effects of Lactobacillus plantarum probiotics were investigated in NAFLD models such as L. plantarum MA2, L. plantarum A7 and L. plantarum NCU116. Results showed that either L. plantarum A7 or L. plantarum MA2 was effective in lowering serum lipids [73,74], while L. plantarum NCU116 improved liver function and decreased hepatic fat accumulation as well [75]. A similar effect was observed with L. rhamnosus supplementation in an NAFLD model. Probiotics of L. rhamnosus GG (LGG) protected mice from NAFLD by increasing the abundance of beneficial bacteria, improving gut barrier function and attenuating hepatic inflammation [76], as well as the cholesterol-lowering effect through inhibition of the FXR and FGF15(fibroblast growth factor) signaling pathway [77]. In addition, several other species of Lactobacilli bacteria have shown potential in NAFLD prevention, including L. johnsonii BS15 [78], L. reuteri GMNL-263 [79], L. gasseri BNR17 [80].
Bifidobacterium (Bif) belongs to the Bifidobacteria bacteria genera in the mammalian gastrointestinal tract, and is a frequently used probiotic [81,82,83]. Supplementation of Bif significantly improved visceral fat accumulation and insulin sensitivity in HFD-fed rats [84]. Administration of Bifidobacterium pseudocatenulatum CECT 7765 could reduce serum cholesterol and triglycerides, and improved glucose tolerance in obese mice [85]. It is proposed that probiotic of Bif is superior to Lactobacillus acidophilus in reducing hepatic fat accumulation [86]. Compared to probiotics with a single strain of bacteria, VSL#3 is a mixed probiotic with eight types of bacteria (Bifidobacteria (B. breve, B. longum, B. infantis), Streptococcus thermophilus, L. plantarum, L. acidophilus, L. paracasei and L. delbrueckii subsp. bulgaricus) which has shown great potential in treatment of various diseases [87,88,89,90,91]. Experimental evidence has indicated that VSL#3 could attenuate inflammation via modulation of the nuclear factor-kB (NF-kB) pathway [92], reduce hepatic fat accumulation and ALT levels [93], improve insulin sensitivity in NAFLD models [94], and prevent against liver fibrosis in NASH patients [95]. The probiotic with combined bacteria (LGG, Lactobacillus plantarum WCFS1 and anthraquinone from Cassia obtusifolia L.) was effective in reducing blood lipid levels and improving insulin resistance in NAFLD rats [96]. Meanwhile, supplementation of combined probiotic (Bifidobacterium infantis, Lactobacillus acidopilus, and Bacillus cereus) could improve gut dysbiosis and liver function via suppression of the LPS/TLR4 signaling pathway [97]. Kim et al. found that consumption of kefir (a probiotic beverage containing over 50 species of lactic acid bacteria and yeast) prevented obesity and NAFLD formation by restoring the gut microbiota and enhancing fatty acid oxidation in HFD-fed mice [98]. Further evidence of beneficial effects on NAFLD prevention has been acquired in many studies by administering probiotics with mixed bacteria [99,100,101]. In addition to the direct impacts on the composition of gut microbiota, the beneficial effects of probiotics on NAFLD are also associated with their metabolic activities [53]. It has been reported that probiotics of clostridium butyricum MIYAIRI 588—a butyrate-producing bacteria, decreased accumulation of lipid droplets in HFD-induced NAFLD models, improved insulin resistance [102], and reduced hepatic lipids and serum endotoxin levels in choline-deficient/l-amino acid-defined diet-induced NAFLD models [103], which may be associated with the stimulation of expression of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) andserine/threonine kinas (AKT) proteins, and lipogenesis- or lipolysis-related proteins.
Currently, although the beneficial effects of probiotics were mainly acquired in experimental studies, some consistent results have also been observed in clinical practice. Alisi et al. compared the therapeutic effects of VSL#3 in a randomized double-blind controlled study in obese children with biopsy-proven NAFLD [104]. They found that 4-month supplement of VSL#3 significantly improved liver function and increased glucagon-like peptide (GLP-1)/active glucagon-like peptide (aGLP1) levels suggesting the effects of VSL#3 might be GLP-1-dependent. Consistent effects were also observed on obese children with NAFLD by administering probiotics such as Lactobacillus rhamnosus strain GG [105] and mixed bacteria of Lactobacillus bulgaricus and Streptococcus thermophilus [106]. Sepideh et al. investigated the effects of a multistrain probiotic supplementation in NAFLD patients in a RCT study, and the results showed dramatic improvement in insulin sensitivity and inflammation [107]. Moreover, synergistic effects were also observed by combining probiotics with chemical drugs such as metformin in NASH and statins in NAFLD therapy [108,109], which highlights the great potential of clinical application of probiotics either alone or combined with other drugs. Nevertheless, the clinical efficacy of probiotics still needs further validation in well-designed studies with a larger scale of participants. Solga et al. observed that 4 months of probiotic supplements not only did not reduce hepatic steatosis, but increased fat accumulation in liver of four patients [110]. In 2010, Andreasen et al. conducted a randomized-double-blinded research on effects of L. acidophilus NCFM on insulin sensitivity and the systemic inflammation [111]. They found that insulin sensitivity was improved in the probiotic group, but not in the placebo group, and there were no differences in systemic inflammation in either group. Meanwhile, another study indicated that an 8-week probiotic supplement did not improve total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, TG, TG/ LDL and LDL/HDL ratios in diabetic patients [112]. Additionally, supplementation with Lactobacillus acidophilus did not improve the levels of plasma lipids in volunteers with elevated cholesterols in a double-blind placebo-controlled study [113]. A detailed summary of gut microbiota-targeted therapies on NAFLD with probiotics is provided in Table 1.

Table 1.
Gut microbiota-targeted therapies of NAFLD with probiotics.
3.2. Gut Microbiota-Targeted Therapy with Prebiotic
Prebiotics are indigestible food ingredients with beneficial effects, as they selectively stimulate the growth and/or activity of “good” and suppress the “bad” bacteria resident in the colon [123]. They can be defined as a fermented ingredient that allows changes both in the composition and/or activity in the gastrointestinal microflora conferring benefits upon host well-being and health [124,125]. Evidence suggested that prebiotic supplements prevented NAFLD development in both experimental and clinical studies [126,127].
In 2009, Cani et al. found that prebiotics of oligofructose (a mixture of fermentable dietary fibers) decreased plasma LPS and cytokine levels, and hepatic expression of inflammatory and oxidative stress markers in obese mice. An improvement in intestinal permeability and production of GLP-2 was also shown [128]. In an MCD diet-induced steatohepatitis mice model, a dietary fructooligosaccharide (FOS) supplement attenuated the extent of steatohepatitis by restoring the homeostasis of gut microbiota and intestinal epithelial barrier function [129]. Pachikian et al. reported that a FOS supplement reduced hepatic triglyceride accumulation in n-3 PUFA (polyunsaturated fatty acid)-depleted diet-induced NAFLD model by altering microbiota composition and increasing production of GLP-1 [130]. Meanwhile, the FOS supplement stimulated fatty acid oxidation by activating peroxisome proliferator-activated receptor-alpha (PPAR-α) and reduced cholesterol accumulation by inhibiting SREBP-2 (sterol-regulatory-element-binding protein isoform 2) in liver without affecting SREBP-1 expression and activity [130,131]. Lactulose is a prebiotic that promotes the growth of lactic acid bacteria and Bifidobacteria [132]. A study indicated that lactulose treatment decreased the hepatic inflammation and serum endotoxin levels in rats with steatohepatitis [133]. Chitin–glucan (CG) is another type of prebiotic from fungal source. Neyrinck et al. investigated the function of CG in HFD-induced obese mice and found CG treatment decreased body weight gain, improved glucose intolerance and hepatic triglyceride accumulation by restoring bacteria of clostridial cluster XIVa [134].
The combination of prebiotics with natural components will yield more benefits than prebiotics on their own. For example, combined therapy of isomalto-oligosaccharides (IMOs) with lycopene (an antioxidant) prevented body weight gain, enhanced adipose tissue fat mobilization, and improved insulin resistance and metabolic endotoxemia in HFD-induced NAFLD mice. The observed effects were associated with their modulation of microbial production of SCFAs [135].
In the clinic, prebiotics have also been tested for their benefits in various diseases [136,137,138,139,140]. Oligofructose (OFS), an inulin-type fructan, was added to diet of NASH patients in a pilot randomized double-blind study [127]. Their results showed that the OFS supplement decreased serum ALT and AST levels significantly. Prebiotics of mixed galacto-oligosaccharides and fructo-oligosaccharides (9:1) stimulated the abundance of Bifidobacteria bacteria in infants [141]. Similarly, administration of prebiotic inulin and oligofructose (50:50 in mixture) increased the abundance of Bifidobacterium and Faecalibacterium prausnitzii, which negatively correlated with serum LPS levels [142]. Prebiotics have shown great potential in prevention of obesity and NAFLD development by lowering the permeability of intestinal wall, attenuating metabolic endotoxemia, and reducing the accumulation of fat [143]. The gut microbiota-targeted therapies with prebiotics were summarized in Table 2.

Table 2.
Gut microbiota-targeted therapies of NAFLD with prebiotics.
3.3. Gut Microbiota-Targeted Therapy with Synbiotic
Synbiotics are the combination of probiotics and prebiotics [144]. Synbiotics usually produce benefits by selectively stimulating the growth and/or activating the metabolism of health-promoting bacteria [145]. Administration of synbiotics containing Lactobacillus paracasei B21060 plus arabinogalactan and fructooligosaccharides attenuated hepatic inflammation and increased expression of nuclear PPARs and their targeted genes in HFD-induced NAFLD rats [146]. Synbiotics have shown various benefits in metabolic diseases, such as improvement of insulin resistance, glucose control, and inflammatory cytokine synthesis [147,148,149].
In the clinic, the therapeutic effect of a synbiotic containing seven probiotics and oligofructose was evaluated in patients with NAFLD in a double-blind RCT. The results showed that synbiotic therapy significantly decreased ALT levels [150]. Malaguarnera et al. observed that combination of synbiotic (B. longum and Fos) and lifestyle intervention in NASH patients resulted in a much greater improvement compared to lifestyle intervention alone, including reduction of serum TNFα, CRP (C-reactive protein), endotoxin, and AST levels, improvement in HOMA-IR( homeostasis model assessment of insulin resistance)and extent of NASH activity index [151]. Synbiotic therapy showed improvements in levels of fasting blood glucose, TG, and inflammatory cytokines in both obese and lean NAFLD patients [152,153]. Therefore, synbiotics are a promising gut microbiota-targeted intervention for NAFLD prevention or therapy. Nevertheless, more clinical validations are also needed. A summarized gut microbiota-targeted therapy on NAFLD with synbiotics was provided in Table 3.

Table 3.
Gut microbiota-targeted therapies of NAFLD with synbiotics.
3.4. Gut Microbiota-Targeted Therapies with Other Approaches
In addition to probiotics/prebiotics/synbiotics, gut microbiota-targeted interventions have also been investigated with other approaches. Butyrate is an SCFA and is an important gut microbial metabolite derived from fermentation of nondigestible polysaccharides. Butyrate has a critical role in affecting metabolic disease development in a variety of ways, including modulation on energy harvest, hepatic lipogenesis and gluconeogenesis, adipokine signaling in adipocytes, intestinal permeability, and appetite regulation in the brain [154,155]. Administration of sodium butyrate alleviated inflammation and fat accumulation in HFD-induced NAFLD mice by increasing the abundances of the beneficial bacteria Christensenellaceae, Blautia and Lactobacillus [156]. Therefore, appropriate approaches such as engineered bacteria could be developed to enhance the production of beneficial gut microbial metabolites (e.g., butyrate) or intervention with chemical drugs to promote the proliferation of “good” bacteria, and suppress the “bad” ones.
Antibiotics are frequently used in the clinic, although their disruption of gut microbial homeostasis is a double-edged sword [157]. On one hand, the short-term application of antibiotic can result in long-lasting impacts on host metabolism. On the other hand, administration of some kinds of antibiotics may attenuate diseases. For example, oral administration of cidomycin increased the small intestine transit rate and lowered serum ALT, AST, and TNF-α levels in NASH rats, suggesting the potential of cidomycin in alleviating the severity of NASH by intervening gut microbiota [158]. In the clinic, administration of rifaximin could decrease the circulating endotoxin and ALT levels in patients with NAFLD [159]. Although the improvement in NAFLD, especially in NASH, by short-term administration of antibiotic (e.g., rifaximin) can be observed, the long-term application of antibiotics is not encouraged because of probable side effects [160]. Nevertheless, the changes in gut microbiota resulting from antibiotics could provide important evidence for exploring alternative ways to modulate gut microbiota in disease therapy.
Compared to antibiotics, some ingredients from herbal medicines have shown more prospects for gut microbiota modulation with minor side effects [161,162]. Berberine is a typical herbal component with potent antibacterial activity, especially bacteria in intestinal tract, because berberine can hardly be absorbed in gut [163]. Currently, increasing evidence has confirmed the therapeutic effect of berberine on metabolic diseases including obesity, NAFLD, and type 2 diabetes via modulation on gut microbiota [164,165,166]. It has been revealed that berberine administration restored the relative abundance of Bifidobacteria and the ratio of Bacteroidetes/Firmicutes in HFD-induced NASH mice resulting in significant reduction in body weight, serum levels of lipids, glucose, insulin and inflammatory cytokines [167,168]. TSG (2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside) is an active component of the traditional Chinese medicine (TCM) Polygonum multiflorum Thunb, which has shown significant effects in NAFLD prevention by modulating gut microbiota, improving the intestinal mucosal barrier, and suppressing the expression of NF-κB [169]. Resveratrol is a natural polyphenol with anti-oxidative activity [170]. Recent studies showed resveratrol was also effective in preventing metabolic diseases such as obesity and NASH by regulating gut microbiota [171]. In addition to the individual component from herbal medicines, recent investigations revealed that the efficacy of some TCM formulas was associated with the modulation on gut microbiota. For example, Qushi Huayu Fang (a mixture of five herbs including Artemisia capillaries Thunb, Gardenia jasminoides Ellis, Fallopia japonica, Curcuma longa L., and Hypericum japonicum Thunb) is an ancient TCM formula which has been used for NAFLD treatment. Recent studies showed that administration of Qushi Huayu Decoction (QHD) significantly decreased body weight, alleviated hepatic steatosis, and reduced the content of TG and free fatty acids in liver in HFD-induced NAFLD rats. It showed that the QHD-treated group harbored significantly different gut microbiota from that of model rats, and the bacterial profiles of NAFLD rats could be modulated by the QHD [172,173]. Recently, the anti-obesity property of daesiho-tang (DSHT) was also investigated. It was found that DSHT treatment significantly reduced levels of serum TC and TG as well as hepatic fat accumulation that were associated with the regulation on abundance of gut microbiota [174]. Although the mechanisms underlying TCM therapy are extremely complicated and largely unknown, the gut microbiota was supposed to be an important target for many TCM formulas because many kinds of chemicals derived from TCM are unabsorbable. Those unabsorbed chemicals in TCM can influence gut microbiota directly or be metabolized into absorbable or active form by gut microbiota. A summary of gut microbiota-targeted therapies on NAFLD with other approaches were provided in Table 4.

Table 4.
Gut microbiota-targeted therapies of NAFLD—other approaches.
4. Conclusions and Perspectives
Currently, the gut microbiota has been recognized as a critical factor contributing to the development of NAFLD and the gut microbial-related mechanisms have also been well elucidated. As a result, the strategy of gut microbiota-targeted therapy on NAFLD is highly valued in the context of accumulating benefits of gut microbial modulation by using probiotics, prebiotics, synbiotics, antibiotics, and herbal medicines. Although many experimental reports were exciting, discrepant results were also observed in the clinic. Therefore, the clinical efficacy of gut microbiota-targeted therapies on NAFLD still need to be confirmed with large-scale and well-organized RCT studies. The main factors contributing to the variation of therapeutic outcomes in the clinic are differences in bacterial activity of probiotics or the diversified dysbiosis among NAFLD patients. In this sense, probiotics with mixed bacteria such as VSL#3 are more prospective than those with individual type of bacteria. Meanwhile, the gut microbiota-related efficacy of natural components from herbal medicines or the TCM formula itself highlighted the great potential of seeking novel medicines from TCM because some TCMs showed their effects by nourishing “good” bacteria and suppressing “bad” ones. Currently, 16S rDNA-based sequencing is still the major approach for most gut microbiota-involved studies because it is relatively affordable and applicable for most laboratories. Although 16S rDNA sequencing can provide a general description on the structural differences of the microbiome between samples, especially at the genus level, it is usually frustrating when information for specific bacteria species is necessary. Consequently, metagenomics will be more applicable for figuring out specific bacterial species that may contribute to the disease development or therapeutic efficacy, as well as the involved microbial functions.
In summary, gut microbiota-targeted therapies for diseases are still in their infancy. Nevertheless, we envision that more gut microbiota-targeted therapies will be tested in the context of accumulation of therapeutic evidence and advances in elucidation of gut microbial-related mechanisms in diseases, as well as the technological innovation of gut microbiome analysis.
Acknowledgments
Houkai Li was funded by National Natural Science Foundation of China (No. 81673662), The Program for Professor of Special Appointment (Eastern Scholar) and Shuguang Scholar (16SG36) at Shanghai Institutions of Higher Learning from Shanghai Municipal Education Commission.
Author Contributions
Junli Ma retrieved all the references and drafted the manuscript, Qihang Zhou helped in the references retrieving. Houkai Li designed the manuscript and made revision of the text.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Search strategy
- The main source of material was pubmed, and the search keywords used were as follows: “gut microbiota”, “gut flora”, “nonalcoholic fatty liver disease(NAFLD)”, “nonalcoholic steatohepatitis(NASH)”, “steatosis”,“probiotic”, “prebiotic”, “antibiotic”, “herbal medicince”;
- Selected papers have no language restrictions;
- Most of the papers selected were published during the past 10 years;
- References of some identified papers were further searched for related papers to cover this topic as completely as possible.
References
- Minemura, M.; Shimizu, Y. Gut microbiota and liver diseases. World J. Gastroenterol. 2015, 21, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, S.S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [PubMed]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, G.; Lopez-Ortiz, E.; Torres-Castro, I. Gut microbiota as a key player in triggering obesity, systemic inflammation and insulin resistance. Rev. Investig. Clin. 2014, 66, 450–459. [Google Scholar]
- Mehal, W.Z. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- DiBaise, J.K.; Zhang, H.; Crowell, M.D.; Krajmalnik-Brown, R.; Decker, G.A.; Rittmann, B.E. Gut microbiota and its possible relationship with obesity. Mayo Clin. Proc. 2008, 83, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Chong-Nguyen, C.; Duboc, H.; Sokol, H. The gut microbiota, a new cardiovascular risk factor? Presse Med. 2017, 46, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Tang, W.H.W. The Role and Impact of Gut Microbiota in Cardiovascular Disease. Rev. Esp. Cardiol. 2017, 70, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Wieland, A.; Frank, D.N.; Harnke, B.; Bambha, K. Systematic review: Microbial dysbiosis and nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2015, 42, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- He, X.; Ji, G.; Jia, W.; Li, H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanism and Application of Metabolomics. Int. J. Mol. Sci. 2016, 17, 300. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Bandsma, R. Targeting the Gut Microbiota for the Treatment of Non-Alcoholic Fatty Liver Disease. Curr. Drug Targets 2015, 16, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Farajian, S.; Mirlohi, M. Probiotics as a novel treatment for non-alcoholic Fatty liver disease; a systematic review on the current evidences. Hepat. Mon. 2013, 13, e7233. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Kaser, S.; Tilg, H. Non-alcoholic steatohepatitis: A microbiota-driven disease. Trends Endocrinol. Metab. 2013, 24, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Pacini, G.; Musso, G.; Gambino, R.; Mecca, F.; Depetris, N.; Cassader, M.; David, E.; Cavallo-Perin, P.; Rizzetto, M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology 2002, 35, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Caputi, A. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J. Gastroenterol. 2011, 17, 3785–3794. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C. Signalling links in the liver: Knitting SOCS with fat and inflammation. J. Hepatol. 2005, 43, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Reigstad, C.S.; Backhed, H.K.; Reinhardt, C.; Ketonen, M.; Lunden, G.O.; Cani, P.D.; Backhed, F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012, 61, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. Gut microflora as a target for energy and metabolic homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011, 140, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Zuniga, V.; Bartoli, R.; Planas, R.; Hofmann, A.F.; Vinado, B.; Hagey, L.R.; Hernandez, J.M.; Mane, J.; Alvarez, M.A.; Ausina, V.; et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 2003, 37, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Nishi, M.; Nakayama, H.; Kuwahara, T.; Ohnishi, Y.; Tashiro, S. Role of bile in intestinal barrier function and its inhibitory effect on bacterial translocation in obstructive jaundice in rats. J. Surg. Res. 2003, 115, 18–23. [Google Scholar] [CrossRef]
- Fuchs, C.; Claudel, T.; Trauner, M. Bile acid-mediated control of liver triglycerides. Semin. Liver Dis. 2013, 33, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Watanabe, M.; Auwerx, J. Endocrine functions of bile acids. EMBO J. 2006, 25, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile acids as regulatory molecules. J. Lipid Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Claudel, T.; Staels, B.; Kuipers, F. The Farnesoid X receptor: A molecular link between bile acid and lipid and glucose metabolism. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.Z.; Takahashi, S.; et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Bashiardes, S.; Shapiro, H.; Rozin, S.; Shibolet, O.; Elinav, E. Non-alcoholic fatty liver and the gut microbiota. Mol. Metab. 2016, 5, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shanab, A.; Quigley, E.M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T. Pathophysiological role of host microbiota in the development of obesity. Nutr. J. 2016, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Paolella, G.; Mandato, C.; Pierri, L.; Poeta, M.; Di Stasi, M.; Vajro, P. Gut-liver axis and probiotics: Their role in non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 15518–15531. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Miyoshi, M.; Yamashita, H. Gut microbiota and host metabolism in liver cirrhosis. World J. Gastroenterol. 2015, 21, 11597–11608. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Alligier, M.; Salazar, N.; Neyrinck, A.M.; Delzenne, N.M. Modulation of the gut microbiota by nutrients with prebiotic and probiotic properties. Adv. Nutr. 2014, 5, 624S–633S. [Google Scholar] [CrossRef] [PubMed]
- Finelli, C.; Tarantino, G. Non-alcoholic fatty liver disease, diet and gut microbiota. EXCLI J. 2014, 13, 461–490. [Google Scholar] [PubMed]
- Patel, R.; DuPont, H.L. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S108–S121. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, S.M.; Armiliato, G.N.; Couto, C.A.; Ferrari, T.C. The role of intestinal bacteria overgrowth in obesity-related nonalcoholic fatty liver disease. Nutrients 2014, 6, 5583–5599. [Google Scholar] [CrossRef] [PubMed]
- Fialho, A.; Thota, P.; McCullough, A.J.; Shen, B. Small Intestinal Bacterial Overgrowth Is Associated with Non-Alcoholic Fatty Liver Disease. J. Gastrointestin. Liver Dis. 2016, 25, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, M.; Tomasic, V.; Gomercic, M.; Smircic Duvnjak, L.; Barsic, N.; Lerotic, I. Therapy of nonalcoholic fatty liver disease: Current status. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 57–66. [Google Scholar] [PubMed]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46 (Suppl. 2), S58–S61; discussion S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Ohnishi, H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 7381–7791. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Sakoda, H.; Kushiyama, A.; Fujishiro, M.; Nakatsu, Y.; Fukushima, T.; Matsunaga, Y.; Kamata, H.; Asahara, T.; Yoshida, Y.; et al. Lactobacillus casei strain Shirota protects against nonalcoholic steatohepatitis development in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G911–G918. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Yoshida, Y.; Makino, K.; Kounoshi, Y.; Kunihiro, S.; Takahashi, R.; Matsuzaki, T.; Miyazaki, K.; Ishikawa, F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J. Appl. Microbiol. 2011, 110, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Wagnerberger, S.; Spruss, A.; Kanuri, G.; Stahl, C.; Schroder, M.; Vetter, W.; Bischoff, S.C.; Bergheim, I. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: A mouse model. J. Nutr. Biochem. 2013, 24, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Yamada, A.; Endo, T.; Nakano, M. Effects of a mixture of organisms, Lactobacillus acidophilus or Streptococcus faecalis on delta6-desaturase activity in the livers of rats fed a fat- and cholesterol-enriched diet. Nutrition 1999, 15, 373–378. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Gilliland, S.E. Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J. Am. Coll. Nutr. 1999, 18, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Jun, D.W.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Choi, H.S.; Yoon, B.C. Lactobacillus paracasei Induces M2-Dominant Kupffer Cell Polarization in a Mouse Model of Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561; quiz 1546, 1562. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, Y.A.; Richards, D.M.; Rahimi, E.F.; Krill, J.T.; Jelinek, K.A.; DuPont, A.W. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2014, 7, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, H.; Moshtaghian, J.; Mirlohi, M.; Shirzadi, M. Reduction in serum lipid parameters by incorporation of a native strain of Lactobacillus Plantarum A7 in Mice. Iran. J. Diabetes Lipid Disord. 2010, 9, 1–7. [Google Scholar]
- Wang, Y.; Xu, N.; Xi, A.; Ahmed, Z.; Zhang, B.; Bai, X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2009, 84, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nie, S.P.; Zhu, K.X.; Ding, Q.; Xiong, T.; Xie, M.Y. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014, 5, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Ritze, Y.; Bardos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, K.Y.; Ji, Y.; Park, S.; Holzapfel, W.; Hyun, C.K. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem. Biophys. Res. Commun. 2016, 473, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zeng, D.; Wang, H.; Ni, X.; Yi, D.; Pan, K.; Jing, B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 2014, 98, 6817–6829. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.C.; Lee, C.L.; Chai, C.Y.; Chen, W.T.; Lu, Y.C.; Wu, C.S. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr. Metab. (Lond.) 2013, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Yun, S.I.; Park, M.H.; Park, J.H.; Jeong, S.Y.; Park, H.O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Huang, C.; Cheng, M. Dietary blueberry and bifidobacteria attenuate nonalcoholic fatty liver disease in rats by affecting SIRT1-mediated signaling pathway. Oxid. Med. Cell. Longev. 2014, 2014, 469059. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.G.; Santacruz, A.; Trejo, F.M.; Sanz, Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity 2013, 21, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.Y.; Wan, Y.P.; Fang, Q.Y.; Lu, W.; Cai, W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nutr. 2012, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Gupta, S.S.; Chellani, H.; Maliye, C.; Kumari, V.; Arya, S.; Garg, B.S.; Gaur, S.D.; Gaind, R.; Deotale, V.; et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: A randomised controlled trial. BMJ Open 2015, 5, e006564. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Pare, P.; et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.K.; Rana, B.; Agrawal, S.; Garg, A.; Chopra, M.; Thumburu, K.K.; Khattri, A.; Malhotra, S.; Duseja, A.; Chawla, Y.K. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: A randomized, controlled trial. Gastroenterology 2014, 147, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.K.; Yang, C.; Song, G.H.; Wong, J.; Ho, K.Y. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: A randomized double-blinded placebo study. Dig. Dis. Sci. 2015, 60, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Cipriani, S.; Renga, B.; Bruno, A.; D’Amore, C.; Distrutti, E.; Fiorucci, S. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS ONE 2012, 7, e45425. [Google Scholar] [CrossRef]
- Esposito, E.; Iacono, A.; Bianco, G.; Autore, G.; Cuzzocrea, S.; Vajro, P.; Canani, R.B.; Calignano, A.; Raso, G.M.; Meli, R. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J. Nutr. 2009, 139, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; Desimone, C.; Song, X.Y.; Diehl, A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hua, J.; Li, Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J. Hepatol. 2008, 49, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Velayudham, A.; Dolganiuc, A.; Ellis, M.; Petrasek, J.; Kodys, K.; Mandrekar, P.; Szabo, G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 2009, 49, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Tang, Y.; Li, M.; Yang, P.; Liu, Z.; Yuan, J.; Zheng, P. Co-Administration of Cholesterol-Lowering Probiotics and Anthraquinone from Cassia obtusifolia L. Ameliorate Non-Alcoholic Fatty Liver. PLoS ONE 2015, 10, e0138078. [Google Scholar] [CrossRef]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.; Jeong, D.; Kang, I.B.; Chon, J.W.; Kim, H.S.; Song, K.Y.; Seo, K.H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Karahan, N.; Isler, M.; Koyu, A.; Karahan, A.G.; Basyigit Kilic, G.; Ciris, I.M.; Sutcu, R.; Onaran, I.; Cam, H.; Keskin, M. Effects of probiotics on methionine choline deficient diet-induced steatohepatitis in rats. Turk. J. Gastroenterol. 2012, 23, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.S.; Kim, H.N.; Park, H.J.; Lee, J.E.; Yeo, S.Y.; Yang, J.S.; Park, S.Y.; Yoon, H.S.; Cho, G.S.; Franz, C.M.; et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef. Microbes 2012, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Bodnar, P.; Beregova, T. Probiotics Supplemented with Omega-3 Fatty Acids are More Effective for Hepatic Steatosis Reduction in an Animal Model of Obesity. Probiotics Antimicrob. Proteins 2017, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Inoue, I.; Tanaka, M.; Matsuda, N.; Nakano, T.; Awata, T.; Katayama, S.; Alpers, D.H.; Komoda, T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig. Dis. Sci. 2013, 58, 3534–3544. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014, 39, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Mandato, C.; Licenziati, M.R.; Franzese, A.; Vitale, D.F.; Lenta, S.; Caropreso, M.; Vallone, G.; Meli, R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; De Luis, D.A.; Izaola, O.; Conde, R.; Gonzalez Sagrado, M.; Primo, D.; De La Fuente, B.; Gonzalez, J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1090–1095. [Google Scholar] [PubMed]
- Sepideh, A.; Karim, P.; Hossein, A.; Leila, R.; Hamdollah, M.; Mohammad, E.G.; Mojtaba, S.; Mohammad, S.; Ghader, G.; Seyed Moayed, A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2016, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Shavakhi, A.; Minakari, M.; Firouzian, H.; Assali, R.; Hekmatdoost, A.; Ferns, G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int. J. Prev. Med. 2013, 4, 531–537. [Google Scholar] [PubMed]
- Zvenigorodskaia, L.A.; Cherkashova, E.A.; Samsonova, N.G.; Nilova, T.V.; Sil’verstova, S. Advisability of using probiotics in the treatment of atherogenic dyslipidemia. Eksp. Klin. Gastroenterol. 2011, 2, 37–43. [Google Scholar]
- Solga, S.F.; Buckley, G.; Clark, J.M.; Horska, A.; Diehl, A.M. The effect of a probiotic on hepatic steatosis. J. Clin. Gastroenterol. 2008, 42, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Moller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Mahboobi, S.; Iraj, B.; Maghsoudi, Z.; Feizi, A.; Ghiasvand, R.; Askari, G.; Maayeshi, N. The effects of probiotic supplementation on markers of blood lipids, and blood pressure in patients with prediabetes: A randomized clinical trial. Int. J. Prev. Med. 2014, 5, 1239–1246. [Google Scholar] [PubMed]
- Lewis, S.J.; Burmeister, S. A double-blind placebo-controlled study of the effects of Lactobacillus acidophilus on plasma lipids. Eur. J. Clin. Nutr. 2005, 59, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Chen, X.; Chen, Y.; MenghebiligeBao, Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J. Dairy Sci. 2012, 95, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Gauffin Cano, P.; Santacruz, A.; Moya, A.; Sanz, Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE 2012, 7, e41079. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Federico, A.; Tuccillo, C.; Terracciano, F.; D’Auria, M.V.; De Simone, C.; Del Vecchio Blanco, C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J. Clin. Gastroenterol. 2005, 39, 540–543. [Google Scholar] [PubMed]
- Al-Muzafar, H.M.; Amin, K.A. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement. Altern. Med. 2017, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Famouri, F.; Shariat, Z.; Hashemipour, M.; Keikha, M.; Kelishadi, R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Won, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Li, K.C.; Chan, H.L. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [PubMed]
- Nabavi, S.; Rafraf, M.; Somi, M.H.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V.; Akbarian-Moghari, A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 2011, 94, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [PubMed]
- Roberfroid, M.B. Inulin-type fructans: Functional food ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [PubMed]
- Parnell, J.A.; Raman, M.; Rioux, K.P.; Reimer, R.A. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012, 32, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Daubioul, C.A.; Horsmans, Y.; Lambert, P.; Danse, E.; Delzenne, N.M. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: Results of a pilot study. Eur. J. Clin. Nutr. 2005, 59, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Tsunashima, H.; Omagari, K.; Hara, M.; Yasuda, I.; Miyakawa, H.; Kikuchi, K. Fructo-oligosaccharides and intestinal barrier function in a methionine-choline-deficient mouse model of nonalcoholic steatohepatitis. PLoS ONE 2017, 12, e0175406. [Google Scholar] [CrossRef] [PubMed]
- Pachikian, B.D.; Essaghir, A.; Demoulin, J.B.; Catry, E.; Neyrinck, A.M.; Dewulf, E.M.; Sohet, F.M.; Portois, L.; Clerbaux, L.A.; Carpentier, Y.A.; et al. Prebiotic approach alleviates hepatic steatosis: Implication of fatty acid oxidative and cholesterol synthesis pathways. Mol. Nutr. Food Res. 2013, 57, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Carvalho, D.; Freitas, P. Gut Microbiota: Association with NAFLD and Metabolic Disturbances. Biomed. Res. Int. 2015, 2015, 979515. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Salminen, E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand. J. Gastroenterol. Suppl. 1997, 222, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.G.; Xu, Z.J.; Wang, G.L. Effect of lactulose on establishment of a rat non-alcoholic steatohepatitis model. World J. Gastroenterol. 2005, 11, 5053–5056. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Khare, P.; Zhu, J.; Kondepudi, K.K.; Singh, J.; Baboota, R.K.; Boparai, R.K.; Khardori, R.; Chopra, K.; Bishnoi, M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. Int. J. Obes. (Lond.) 2016, 40, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Micka, A.; Siepelmeyer, A.; Holz, A.; Theis, S.; Schon, C. Effect of consumption of chicory inulin on bowel function in healthy subjects with constipation: A randomized, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2017, 68, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Delcour, J.A.; Courtin, C.M.; Kuypers, D.; Augustijns, P.; Verbeke, K.; Meijers, B. The Influence of Prebiotic Arabinoxylan Oligosaccharides on Microbiota Derived Uremic Retention Solutes in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0153893. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Parnell, J.A.; Eksteen, B.; Raman, M.; Bomhof, M.R.; Rioux, K.P.; Madsen, K.L.; Reimer, R.A. Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: A randomized controlled trial protocol. BMC Gastroenterol. 2015, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Davy, B.M.; Halliday, T.M.; Hulver, M.W.; Neilson, A.P.; Ponder, M.A.; Davy, K.P. The effect of prebiotic supplementation with inulin on cardiometabolic health: Rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials 2015, 45, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Savaiano, D.A.; Ritter, A.J.; Klaenhammer, T.R.; James, G.M.; Longcore, A.T.; Chandler, J.R.; Walker, W.A.; Foyt, H.L. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): A randomized, double-blind clinical trial. Nutr. J. 2013, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Faust, K.L.; Czerkies, L.A.; Litov, R.; Ziegler, E.E.; Lessin, H.; Hatch, T.; Sun, S.; Tappenden, K.A. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J. Parenter. Enteral Nutr. 2012, 36, 95S–105S. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The gut microbiome as therapeutic target. Pharmacol. Ther. 2011, 130, 202–212. [Google Scholar] [CrossRef] [PubMed]
- de Vrese, M.; Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 2008, 111, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Simeoli, R.; Iacono, A.; Santoro, A.; Amero, P.; Paciello, O.; Russo, R.; D’Agostino, G.; Di Costanzo, M.; Canani, R.B.; et al. Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J. Nutr. Biochem. 2014, 25, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Aminorroaya, A.; Feizi, A.; Jafari, P.; Amini, M. The effects of probiotic and synbiotic supplementation on metabolic syndrome indices in adults at risk of type 2 diabetes: Study protocol for a randomized controlled trial. Trials 2017, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: A pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 2017, 117, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, A.; Askari, G.; Esmailzade, A.; Feizi, A.; Mohammadi, V. The Effect of Symbiotic Supplementation on Liver Enzymes, C-reactive Protein and Ultrasound Findings in Patients with Non-alcoholic Fatty Liver Disease: A Clinical Trial. Int. J. Prev. Med. 2016, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Mass, K.; Parks, E.J.; Wagner, D.A.; McClure, E.A.; Graf, J.; Savoye, M.; Pierpont, B.; Cline, G.; Santoro, N. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J. Clin. Endocrinol. Metab. 2016, 101, 4367–4376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, S.; Chen, J.; Zhao, R.; Yang, X. Maternal sodium butyrate supplement elevates the lipolysis in adipose tissue and leads to lipid accumulation in offspring liver of weaning-age rats. Lipids Health Dis. 2016, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Lofmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C. Small intestinal bacteria overgrowth decreases small intestinal motility in the NASH rats. World J. Gastroenterol. 2008, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Gangarapu, V.; Ince, A.T.; Baysal, B.; Kayar, Y.; Kilic, U.; Gok, O.; Uysal, O.; Senturk, H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sripada, L. Side effects of antibiotics during bacterial infection: Mitochondria, the main target in host cell. Mitochondrion 2014, 16, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.J.; Xie, Z.S.; Yang, H.; Li, P.; Xu, X.J. Moutan Cortex and Paeoniae Radix Rubra reverse high-fat-diet-induced metabolic disorder and restore gut microbiota homeostasis. Chin. J. Nat. Med. 2017, 15, 210–219. [Google Scholar] [CrossRef]
- Xu, J.; Chen, H.B.; Li, S.L. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med. Res. Rev. 2017, 37, 1140–1185. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Ding, L.; Chen, Y.; Gong, B.; He, J.; Xu, G. Determination of berberine in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, J.Z.; Zhou, X.D.; Xu, X. Berberine regulates type 2 diabetes mellitus related with insulin resistance. Zhongguo Zhong Yao Za Zhi 2017, 42, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Liu, X.Z.; Pan, W.; Zou, D.J. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol. Med. Rep. 2017, 15, 2765–2787. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pan, Q.; Cai, W.; Shen, F.; Chen, G.Y.; Xu, L.M.; Fan, J.G. Modulation of Gut Microbiota by Berberine Improves Steatohepatitis in High-Fat Diet-Fed BALB/C Mice. Arch. Iran. Med. 2016, 19, 197–203. [Google Scholar]
- Xie, W.; Gu, D.; Li, J.; Cui, K.; Zhang, Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS ONE 2011, 6, e24520. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lu, J.; Wang, Y.; Gu, W.; Yu, J.; Zhao, R. Naturally Occurring Stilbenoid TSG Reverses Non-Alcoholic Fatty Liver Diseases via Gut-Liver Axis. PLoS ONE 2015, 10, e0140346. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Dey, C.S. Resveratrol regulates neuronal glucose uptake and insulin sensitivity via P21-activated kinase 2 (PAK2). Biochem. Biophys. Res. Commun. 2017, 485, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Peng, J.; Zhao, L.; Yu, Y.; Zhang, X.; Liu, P.; Feng, Q.; Hu, Y.; Pang, X. Structural changes of gut microbiota in a rat non-alcoholic fatty liver disease model treated with a Chinese herbal formula. Syst. Appl. Microbiol. 2013, 36, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, W.; Baker, S.S.; Li, H.; Chen, C.; Liu, Q.; Tang, S.; Guan, L.; Tsompana, M.; Kozielski, R.; et al. Multi-targeting therapeutic mechanisms of the Chinese herbal medicine QHD in the treatment of non-alcoholic fatty liver disease. Oncotarget 2017, 8, 27820–27838. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Yadav, M.K.; Bose, S.; Wang, J.H.; Lim, D.; Song, Y.K.; Ko, S.G.; Kim, H. Daesiho-Tang Is an Effective Herbal Formulation in Attenuation of Obesity in Mice through Alteration of Gene Expression and Modulation of Intestinal Microbiota. PLoS ONE 2016, 11, e0165483. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, Z.M.; Zhang, Y.; Liang, F.F.; He, X.X. Influence of gut microecology on the pathogenesis and treatment of nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi 2016, 24, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lian, F.; Zhao, L.; Zhao, Y.; Chen, X.; Zhang, X.; Guo, Y.; Zhang, C.; Zhou, Q.; Xue, Z.; et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015, 9, 552–562. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).