Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions
Abstract
:1. Introduction
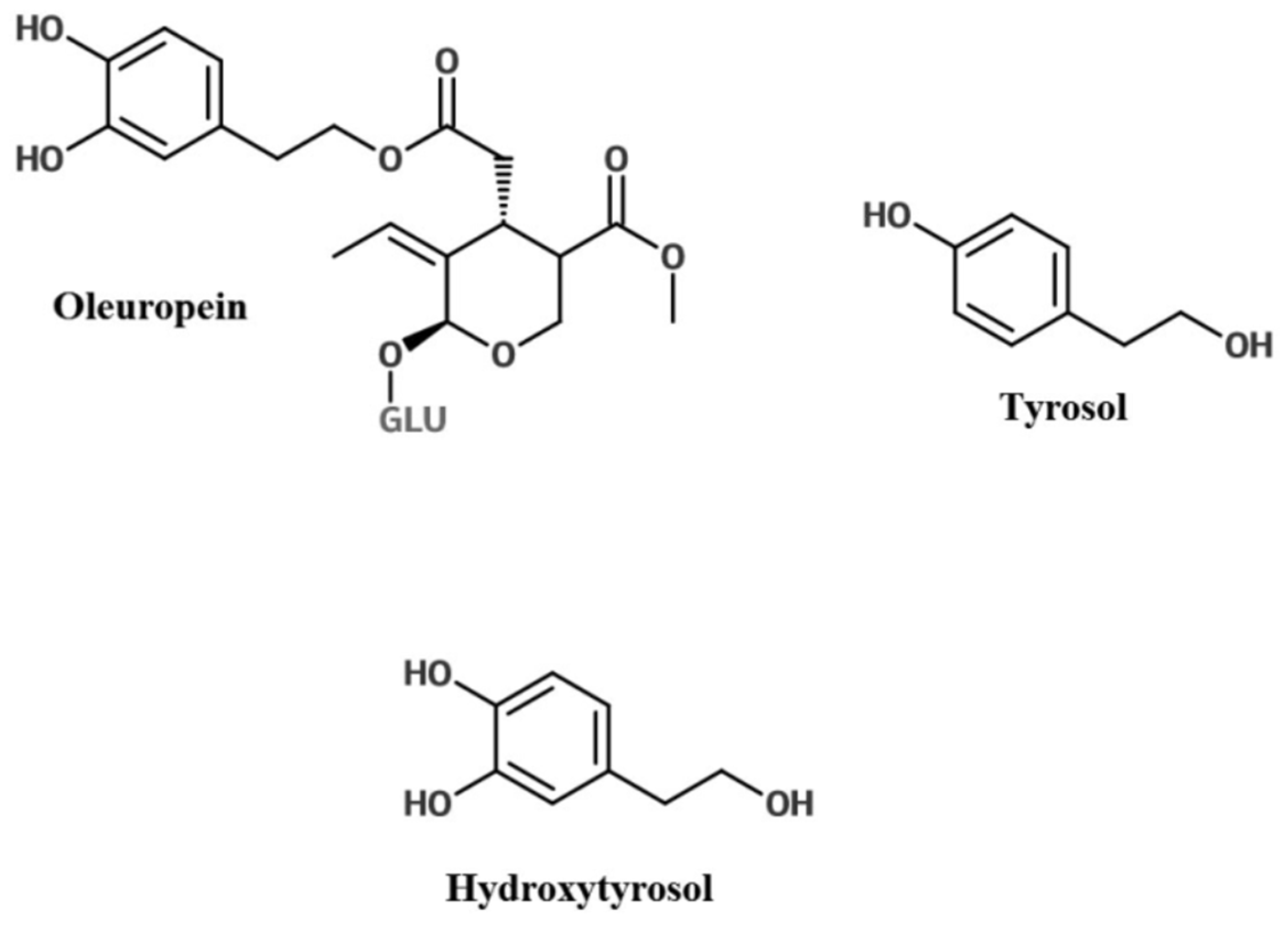
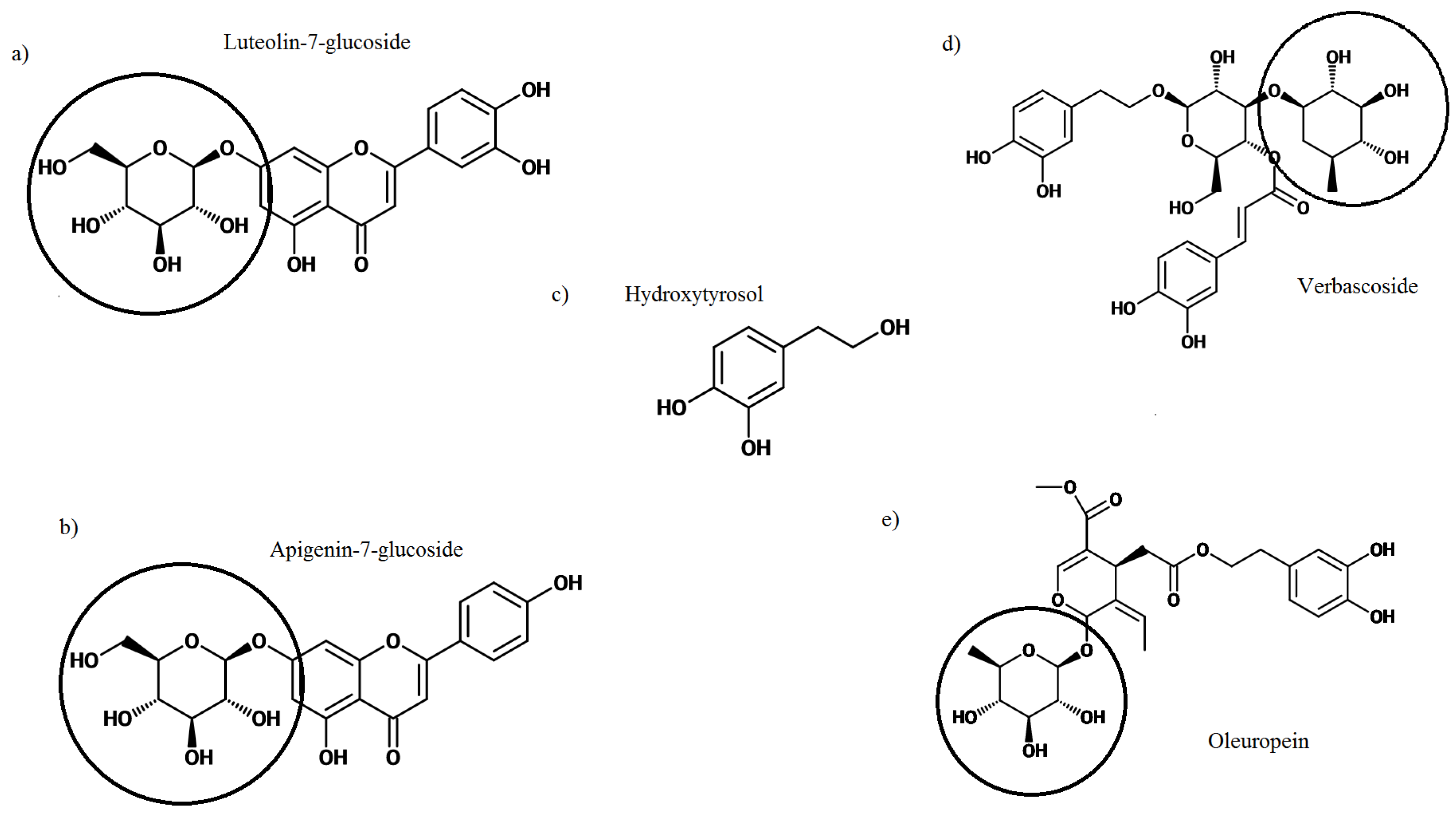
2. Olive Leaf Polyphenols
3. Bioavailability of Olive Leaf Polyphenols
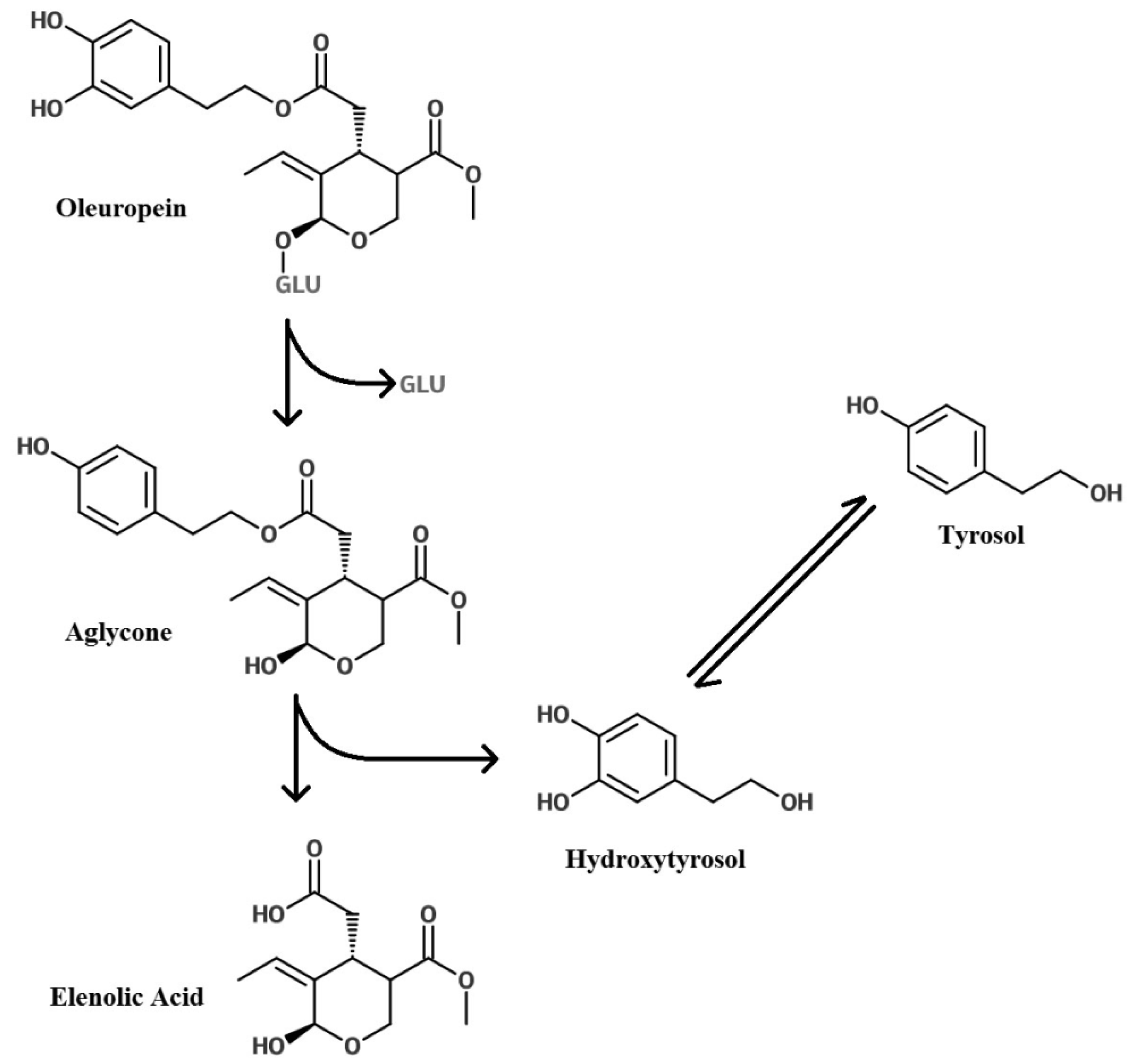
Glycosylation of Polyphenols
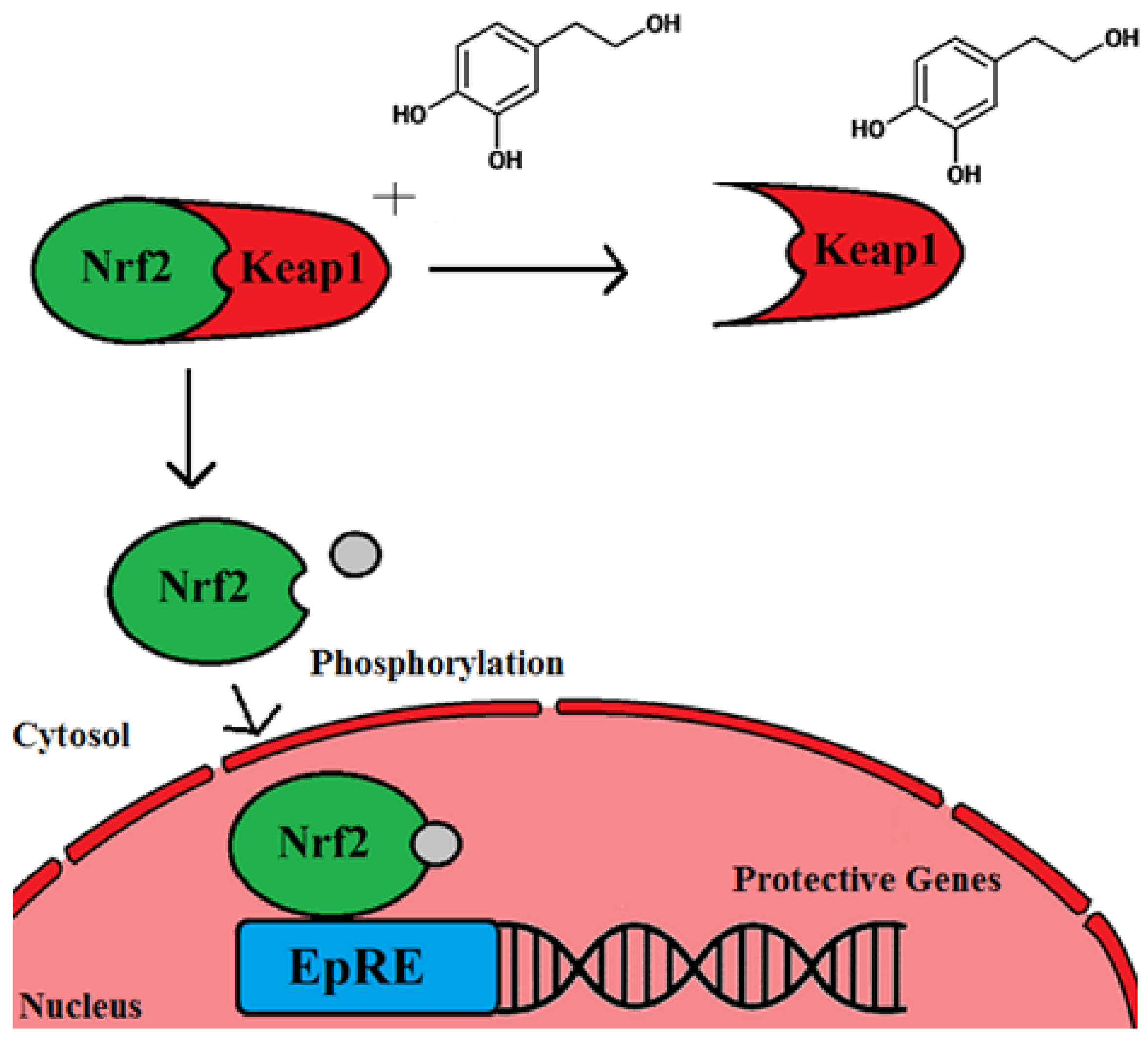
4. OLE and Evidence of the Ability of Olive Leaf Polyphenols to Scavenge Nitric Oxide and Quench Reactive Oxygen Species
5. Olive Leaf Properties That Protect against Development and Progression of Cancer
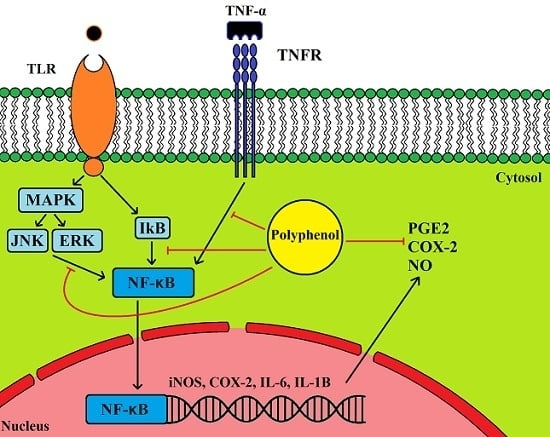
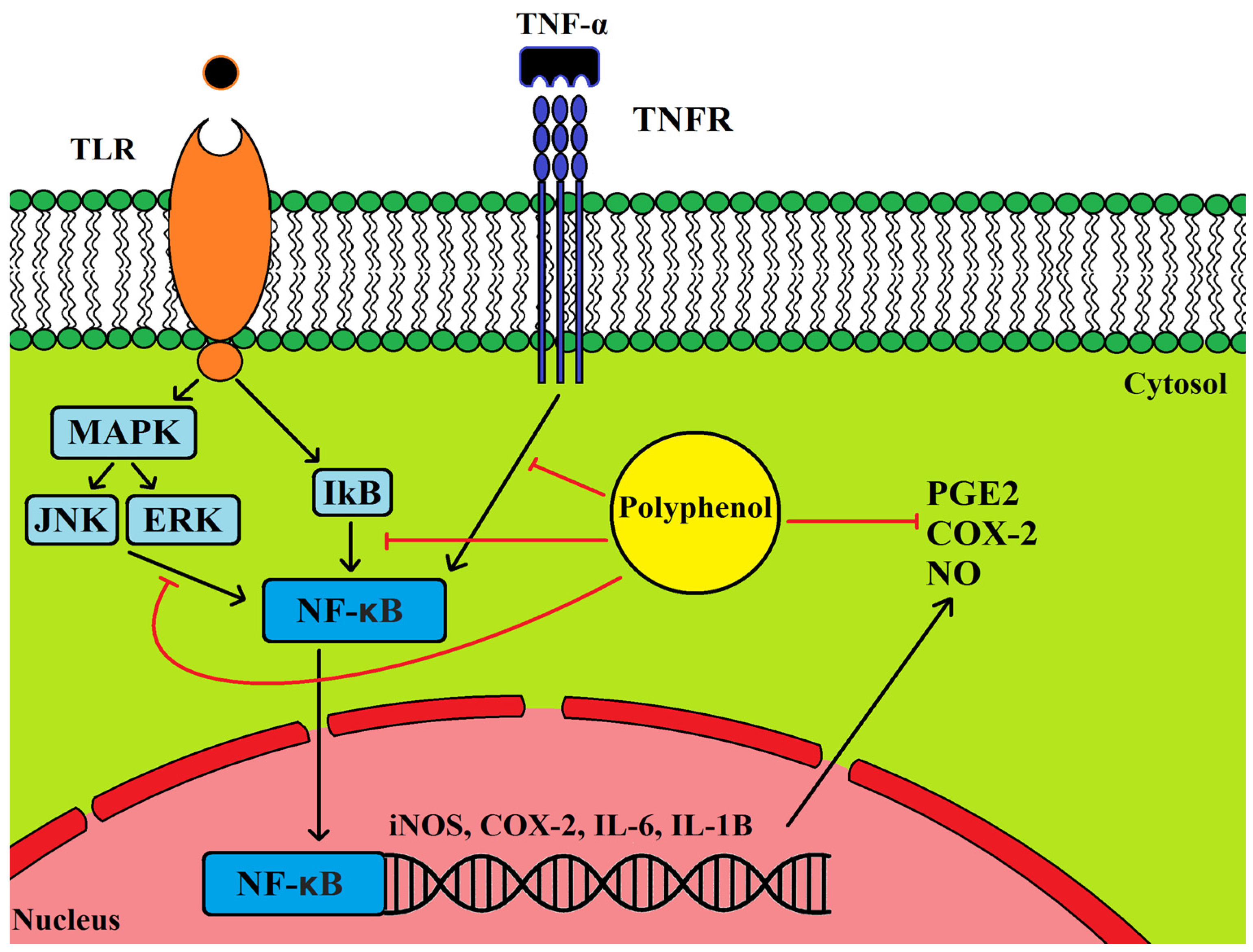
5.1. Anti-Inflammatory Properties of Olive Leaf Polyphenols and Their Effects on Cancer
5.2. Cancer, Inflammation and COX2 Expression
5.3. Quinone Hypothesis for Anti-Cancer Properties of Olive Leaf
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| AP1 | Activator protein-1 |
| EVOO | Extra Virgin Olive oil |
| HT | Hydroxytyrosol |
| JNK | c-Jun N-terminal kinase |
| MD | Mediterranean diet |
| MAPK | Mitogen-activated protein kinase |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| NO | Nitric oxide |
| OLE | Olive leaf extract |
| OO | Olive oil |
| ROS | Reactive oxygen species |
| TLR | Toll-like receptor |
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136. [Google Scholar] [CrossRef] [PubMed]
- Filomeno, M.; Bosetti, C.; Bidoli, E.; Levi, F.; Serraino, D.; Montella, M.; La Vecchia, C.; Tavani, A. Mediterranean diet and risk of endometrial cancer: A pooled analysis of three Italian case-control studies. Br. J. Cancer 2015, 112, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Does a Mediterranean-Type Diet Reduce Cancer Risk? Curr. Nutr. Rep. 2015, 5, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Lanzarini, C.; Pini, E.; Scurti, M.; Vianello, D.; Bertarelli, C.; Fabbri, C.; Izzi, M.; Palmas, G.; Biondi, F.; et al. Inflammaging and cancer: A challenge for the Mediterranean diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Panunzio, M.F.; Caporizzi, R.; Antoniciello, A.; Cela, E.P.; Ferguson, L.R.; D’Ambrosio, P. Randomized, controlled nutrition education trial promotes a Mediterranean diet and improves anthropometric, dietary, and metabolic parameters in adults. Ann. Ig. Med. Prev. Comunità 2010, 23, 13–25. [Google Scholar]
- Yubero-Serrano, E.M.; Delgado-Casado, N.; Delgado-Lista, J.; Perez-Martinez, P.; Tasset-Cuevas, I.; Santos-Gonzalez, M.; Caballero, J.; Garcia-Rios, A.; Marin, C.; Gutierrez-Mariscal, F.M.; et al. Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age (Dordr) 2011, 33, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Delgado-Lista, J.; Garcia-Rios, A.; Cruz-Teno, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Lora-Aguilar, P.; Rodriguez-Cantalejo, F.; Fuentes-Jimenez, F.; et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br. J. Nutr. 2012, 108, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Rinaldi, V.A.; Gonnella, M. The Mediterranean Diet between traditional foods and human health: The culinary example of Puglia (Southern Italy). Int. J. Gastron. Food Sci. 2015, 2, 63–71. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Portarena, S.; Baldacchini, C.; Brugnoli, E. Geographical discrimination of extra-virgin olive oils from the Italian coasts by combining stable isotope data and carotenoid content within a multivariate analysis. Food Chem. 2017, 215, 1–6. [Google Scholar] [CrossRef]
- Jabeur, H.; Zribi, A.; Bouaziz, M. Changes in chemical and sensory characteristics of Chemlali extra-virgin olive oil as depending on filtration. Eur. J. Lipid Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Aparicio, R.; Harwood, J. Handbook of Olive Oil: Analysis and Properties. Springer Science & Business Media, 2013. Available online: https://books.google.com/books?hl=en&lr=&id=gQrkBwAAQBAJ&pgis=1 (accessed on 8 March 2016).
- EFSA Panel on Dietetic Products, N. and A. (NDA). Scientific Opinion on the Substantiation of health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Mainte. EFSA J. 2011 2011, 9. [Google Scholar] [CrossRef]
- Farràs, M.; Valls, R.M.; Fernández-Castillejo, S.; Giralt, M.; Solà, R.; Subirana, I.; Motilva, M.-J.; Konstantinidou, V.; Covas, M.-I.; Fitó, M. Olive oil polyphenols enhance the expression of cholesterol efflux related genes in vivo in humans. A randomized controlled trial. J. Nutr. Biochem. 2013, 24, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Corella, D.; Covas, M.I.; Sorlí, J.V.; Subirana, I.; Flores-Mateo, G.; Nonell, L.; Bulló, M.; de la Torre, R.; Portolés, O.; et al. In vivo transcriptomic profile after a Mediterranean diet in high-cardiovascular risk patients: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Olive leaf phenolics and cardiovascular risk reduction: Physiological effects and mechanisms of action. Nutr. Aging 2012, 1, 125–140. [Google Scholar]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Wren, R. Potter’s New Cyclopaedia of Botanical Drugs and Preparations. 1994. Available online: https://scholar.google.co.nz/scholar?hl=en&q=R.C.+Wren+%28Ed.%29%2C+Potter%27s+New+Cyclopaedia+of+Botanical+Drugs+and+Preparations%2C+The+C.W.+Daniel%2C+Essex%2C+UK+%281994%29%2C+p.+20&btnG=&as_sdt=1%2C5&as_sdtp=#0 (accessed on 21 October 2015).
- Ye, J.; Wang, C.; Chen, H.; Zhou, H. Variation Rule of Hydroxytyrosol Content in Olive Leaves. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-LCHX201102015.htm (accessed on 15 May 2015).
- Mihailova, A.; Abbado, D.; Pedentchouk, N. Differences in n-alkane profiles between olives and olive leaves as potential indicators for the assessment of olive leaf presence in virgin olive oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1480–1485. [Google Scholar] [CrossRef]
- Nenadis, N.; Moutafidou, A.; Gerasopoulos, D.; Tsimidou, M.Z. Quality characteristics of olive leaf-olive oil preparations. Eur. J. Lipid Sci. Technol. 2010, 112, 1337–1344. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.-O.; Budhraja, A.; Wang, X.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; Kim, D.; Divya, S.P.; et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE 2012, 7, e52279. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, L.; Lin, S.; Leung, L.K. Dietary flavones and flavonones display differential effects on aromatase (CYP19) transcription in the breast cancer cells MCF-7. Mol. Cell. Endocrinol. 2011, 344, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Choi, H.-S.; Kim, S.-R.; Choi, Y.K.; Woo, S.-M.; Shin, I.; Woo, J.-K.; Park, S.-Y.; Shin, Y.C.; Ko, S.-G.; et al. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Taamalli, A.; Quirantes-Piné, R.; Roldan-Segura, C.; Arráez-Román, D.; Segura-Carretero, A.; Micol, V.; Zarrouk, M. Differential metabolomic analysis of the potential antiproliferative mechanism of olive leaf extract on the JIMT-1 breast cancer cell line. J. Pharm. Biomed. Anal. 2015, 105, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Menendez, P.; Hevia, D.; Rodriguez-Garcia, A.; Mayo, J.C.; Sainz, R.M. Regulation of GLUT transporters by flavonoids in androgen-sensitive and -insensitive prostate cancer cells. Endocrinology 2014, 155, 3238–3250. [Google Scholar] [CrossRef] [PubMed]
- Artajo, L.S.; Romero, M.P.; Motilva, M.J. Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J. Sci. Food Agric. 2006, 86, 518–527. [Google Scholar] [CrossRef]
- Luján, R.J.; Capote, F.P.; Marinas, A.; de Castro, M.D.L. Liquid chromatography/triple quadrupole tandem mass spectrometry with multiple reaction monitoring for optimal selection of transitions to evaluate nutraceuticals from olive-tree materials. Rapid Commun. Mass Spectrom. 2008, 22, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Isabel, M.; Romero-González, R.; Frenich, G.; Vidal, A.; Martínez, L.J. Analysis of phenolic compounds in olive oil by solid-phase extraction and ultra high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2012, 134, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Šarolić, M.; Gugić, M.; Friganović, E.; Tuberoso, C.; Jerković, I. Phytochemicals and Other Characteristics of Croatian Monovarietal Extra Virgin Olive Oils from Oblica, Lastovka and Levantinka Varieties. Molecules 2015, 20, 4395–4409. [Google Scholar] [CrossRef] [PubMed]
- Rigane, G.; Ayadi, M.; Boukhris, M.; Sayadi, S.; Bouaziz, M. Characterisation and phenolic profiles of two rare olive oils from southern Tunisia: Dhokar and Gemri-Dhokar cultivars. J. Sci. Food Agric. 2013, 93, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Aouidi, F.; Ayari, S.; Ferhi, H.; Roussos, S.; Hamdi, M. Gamma irradiation of air-dried olive leaves: Effective decontamination and impact on the antioxidative properties and on phenolic compounds. Food Chem. 2011, 127, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Japón-Luján, L.; Luque-Rodríguez, J.; Luque de Castro, M. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Japón-Luján, R.; Luque de Castro, M.D. Liquid-liquid extraction for the enrichment of edible oils with phenols from olive leaf extracts. J. Agric. Food Chem. 2008, 56, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-López, M.-J.; Berná, G.; Jurado-Ruiz, E.; López-García de la Serrana, H.; Martín, F. Consumption of extra-virgin olive oil rich in phenolic compounds has beneficial antioxidant effects in healthy human adults. J. Funct. Foods 2014, 10, 475–484. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: implications of human gut microbiota. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.K.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef] [PubMed]
- López de las Hazas, M.-C.; Piñol, C.; Macià, A.; Romero, M.-P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.-J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical Properties and Anti-Proliferative Activity of Olea europaea L. Leaf Extracts against Pancreatic Cancer Cells. Molecules 2015, 20, 12992–3004. [Google Scholar] [CrossRef] [PubMed]
- Samet, I.; Han, J.; Jlaiel, L.; Sayadi, S.; Isoda, H. Olive (Olea europaea) Leaf Extract Induces Apoptosis and Monocyte/Macrophage Differentiation in Human Chronic Myelogenous Leukemia K562 Cells: Insight into the Underlying Mechanism. Oxid. Med. Cell. Longev. 2014, 2014, 927619. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Zurek, G.; Barrajón-Catalán, E.; Bäßmann, C.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A metabolite-profiling approach to assess the uptake and metabolism of phenolic compounds from olive leaves in SKBR3 cells by HPLC-ESI-QTOF-MS. J. Pharm. Biomed. Anal. 2013, 72, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G.; Vanella, L. Antiproliferative effect of oleuropein in prostate cell lines. Int. J. Oncol. 2012, 41, 31–38. [Google Scholar] [PubMed]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, R.; Chimento, A.; De Luca, A.; Casaburi, I.; Rizza, P.; Onofrio, A.; Iacopetta, D.; Puoci, F.; Andò, S.; Maggiolini, M.; et al. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Mol. Nutr. Food Res. 2010, 54, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; et al. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Erbay, Z.; Icier, F. A review of thin layer drying of foods: Theory, modeling, and experimental results. Crit. Rev. Food Sci. Nutr. 2010, 50, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Rizvi, S. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Kuem, N.; Song, S.J.; Yu, R.; Yun, J.W.; Park, T. Oleuropein attenuates visceral adiposity in high-fat diet-induced obese mice through the modulation of WNT10b- and galanin-mediated signalings. Mol. Nutr. Food Res. 2014, 58, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Nekooeian, A.A.; Khalili, A.; Khosravi, M.B. Oleuropein offers cardioprotection in rats with simultaneous type 2 diabetes and renal hypertension. Indian J. Pharmacol. 2015, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.H.; Al-Maliki, S.S. Leishmanicidal and apoptotic activities of oleuropein on Leishmania major. Int. J. Clin. Pharmacol. Ther. 2014, 52, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; Alarcón de la Lastra, C. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.K.; Elamin, M.H.; Omer, S.A.; Daghestani, M.H.; Al-Olayan, E.S.; Elobeid, M.A.; Virk, P. Oleuropein Induces Apoptosis Via the p53 Pathway in Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2013, 14, 6739–6742. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Al-Rimawi, F.; Odeh, I.; Bisher, A.; Abbadi, J.; Qabbajeh, M. Effect of Geographical Region and Harvesting Date on Antioxidant Activity, Phenolic and Flavonoid Content of Olive Leaves. J. Food Nutr. Res. 2014, 2, 925–930. [Google Scholar] [CrossRef]
- Ando, C.; Takahashi, N.; Hirai, S.; Nishimura, K.; Lin, S.; Uemura, T.; Goto, T.; Yu, R.; Nakagami, J.; Murakami, S.; et al. Luteolin, a food-derived flavonoid, suppresses adipocyte-dependent activation of macrophages by inhibiting JNK activation. FEBS Lett. 2009, 583, 3649–3654. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Guinda, Á.; Pérez-Camino, M.C.; Lanzón, A. Supplementation of oils with oleanolic acid from the olive leaf (Olea europaea). Eur. J. Lipid Sci. Technol. 2004, 106, 22–26. [Google Scholar] [CrossRef]
- Tabera, J.; Guinda, A.; Ruiz-Rodríguez, A.; Señoráns, F.J.; Ibáñez, E.; Albi, T.; Reglero, G. Countercurrent supercritical fluid extraction and fractionation of high-added-value compounds from a hexane extract of olive leaves. J. Agric. Food Chem. 2004, 52, 4774–4779. [Google Scholar] [CrossRef] [PubMed]
- Virtamo, J.; Taylor, P.R.; Kontto, J.; Männistö, S.; Utriainen, M.; Weinstein, S.J.; Huttunen, J.; Albanes, D. Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Int. J. Cancer 2014, 135, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Borrás-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufí, S.; Fernández-Arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A. Polyphenols and the modulation of gene expression pathways: Can we eat our way out of the danger of chronic disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E. The search for a new paradigm to study micronutrient and phytochemical bioavailability: From reductionism to holism. Med. Hypotheses. 2014, 82, 181–186. [Google Scholar] [CrossRef] [PubMed]
- De Marino, S.; Festa, C.; Zollo, F.; Nini, A.; Antenucci, L.; Raimo, G.; Iorizzi, M. Antioxidant Activity and Chemical Components as Potential Anticancer Agents in the Olive Leaf (Olea europaea L. cv Leccino.) Decoction. Anticancer. Agents Med. Chem. 2014, 14, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Benavente-garcia, O.; Castillo, J.; Lorente, J.; Ortun, A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Etcheverry, E.P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.; Batterham, M.; Callahan, D.L.; Jardine, D.; Prenzler, P.D.; Robards, K.; Ryan, D. Randomized controlled study of the urinary excretion of biophenols following acute and chronic intake of olive leaf supplements. Food Chem. 2012, 130, 651–659. [Google Scholar] [CrossRef]
- Lin, P.; Qian, W.; Wang, X.; Cao, L.; Li, S.; Qian, T. The biotransformation of oleuropein in rats. Biomed. Chromatogr. 2013, 27, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tzounis, X.; Assunta DessÌ, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Curiel, J.A.; Rodríguez, H.; de las Rivas, B.; Muñoz, R. Study of the inhibitory activity of phenolic compounds found in olive products and their degradation by Lactobacillus plantarum strains. Food Chem. 2008, 107, 320–326. [Google Scholar] [CrossRef]
- Ramírez, E.; Medina, E.; Brenes, M.; Romero, C. Endogenous enzymes involved in the transformation of oleuropein in Spanish table olive varieties. J. Agric. Food Chem. 2014, 62, 9569–9575. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Macciola, V.; Cuomo, F.; Lopez, F. Evidence of oleuropein degradation by olive leaf protein extract. Food Chem. 2015, 175, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic hydrolysis of oleuropein from Olea europea (olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta 2013, 1835, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Carrasco-Pancorbo, A.; Garcia-Villalba, R.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Olive oil’s bitter principle reverses acquired autoresistance to trastuzumab (Herceptin) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.-G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- de la Puerta, R.; Domı́nguez, M.E.M.; Ruı́z-Gutı́errez, V.; Flavill, J.A.; Hoult, J.R.S. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001, 69, 1213–1222. [Google Scholar] [CrossRef]
- de la Puerta, R.; Ruiz Gutierrez, V.; Hoult, J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.C.; Tomé-Carneiro, J.; Burgos-Ramos, E.; Loria Kohen, V.; Espinosa, M.I.; Herranz, J.; Visioli, F. One-week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacol. Res. 2015, 95, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.-S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Parzonko, A.; Czerwińska, M.E.; Kiss, A.K.; Naruszewicz, M. Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine 2013, 20, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Alfano, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Burger, R.A.; Chlebowski, R.T.; Fabian, C.J.; Gucalp, A.; Hershman, D.L.; Hudson, M.M.; et al. American Society of Clinical Oncology position statement on obesity and cancer. J. Clin. Oncol. 2014, 32, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, S.A.; Timotijevic, G.S.; Miljkovic, D.M.; Radovic, J.M.; Maksimovic-Ivanic, D.D.; Dekanski, D.P.; Stosic-Grujicic, S.D. Multiple antimelanoma potential of dry olive leaf extract. Int. J. Cancer 2011, 128, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Fares, R.; Bazzi, S.; Baydoun, S.E.; Abdel-Massih, R.M. The antioxidant and anti-proliferative activity of the Lebanese Olea europaea extract. Plant Foods Hum. Nutr. 2011, 66, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R. Endogenous hormone metabolism as an exposure marker in breast cancer chemoprevention studies. IARC Sci. Publ. 2001, 154, 149–162. [Google Scholar] [PubMed]
- Key, T.; Appleby, P.; Barnes, I.; Reeves, G. Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002, 94, 606–616. [Google Scholar] [PubMed]
- Bartlett, J.M.S.; Brookes, C.L.; Robson, T.; van de Velde, C.J.H.; Billingham, L.J.; Campbell, F.M.; Grant, M.; Hasenburg, A.; Hille, E.T.M.; Kay, C.; et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: A prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J. Clin. Oncol. 2011, 29, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Santolla, M.F.; Maggiolini, M.; Pezzi, V.; et al. Oleuropein and hydroxytyrosol activate GPER/GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-W.; Lee, E.-R.; Min, H.-M.; Jeong, H.-S.; Ahn, J.-Y.; Kim, J.-H.; Choi, H.-Y.; Choi, H.; Kim, E.Y.; Park, S.P.; et al. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol. Ther. 2014, 7, 1080–1089. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Morozzi, G.; Fabiani, R. Oleuropein inhibits tumour growth and metastases dissemination in ovariectomised nude mice with MCF-7 human breast tumour xenografts. J. Funct. Foods. 2014, 8, 269–273. [Google Scholar] [CrossRef]
- Milanizadeh, S.; Bigdeli, M.R.; Rasoulian, B.; Amani, D. The Effects of Olive Leaf Extract on Antioxidant Enzymes Activity and Tumor Growth in Breast Cancer. Thrita 2014, 3. [Google Scholar] [CrossRef]
- Osborne, C.; Tripathy, D. Aromatase inhibitors: Rationale and use in breast cancer. Annu. Rev. Med. 2005, 56, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Knox, J.; Cawthorn, S.; Saunders, C.; Roche, N.; Mansel, R.E.; von Minckwitz, G.; et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet 2014, 383, 1041–1048. [Google Scholar] [CrossRef]
- Amakura, Y.; Tsutsumi, T.; Sasaki, K.; Nakamura, M.; Yoshida, T.; Maitani, T. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry 2008, 69, 3117–3130. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Boivin, G.P.; Knudsen, E.S.; Nebert, D.W.; Xia, Y.; Puga, A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010, 70, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Karin, M. Roles of tumor suppressors in regulating tumor-associated inflammation. Cell Death Differ. 2014, 21, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Killeen, M.J.; Linder, M.; Pontoniere, P.; Crea, R. NF-κβ signaling and chronic inflammatory diseases: Exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov. Today 2014, 19, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-J.; Choi, H.-S.; Yoon, K.-Y.; Lee, O.-H.; Kim, K.-J.; Lee, B.-Y. Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmied. Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Sepporta, M.V.; Rosignoli, P. The hydroxytyrosol-dependent increase of TNF-α in LPS-activated human monocytes is mediated by PGE2 and adenylate cyclase activation. Toxicol. Vitro 2015, 29, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Ben Saad, A.; Zgheib, A.; Annabi, B. Olive oil compounds inhibit the paracrine regulation of TNF-α-induced endothelial cell migration through reduced glioblastoma cell cyclooxygenase-2 expression. J. Nutr. Biochem. 2015, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Moldovan, P.L.; Ben Saad, A.; Annabi, B. Biphasic effects of luteolin on interleukin-1β-induced cyclooxygenase-2 expression in glioblastoma cells. Biochim. Biophys. Acta 2015, 1853, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Ievolella, M.; Autelli, R.; Motta, M.; Mosso, L.; Torchio, B.; Bozzo, F.; Cannito, S.; Paternostro, C.; Colombatto, S.; et al. Expression of COX-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasiveness. Expert Opin. Ther. Targets 2014, 18, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Casto, B.C.; Harris, Z.M. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J. Clin. Oncol. 2014, 5, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, J.; Childress, C.; Swatski, M.D.; Yang, W. COX-2 inhibitors arrest prostate cancer cell cycle progression by down-regulation of kinetochore/centromere proteins. Prostate 2014, 74, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhou, Y.; Wang, Y.; Mou, H.; Zhao, Q. Prognostic significance of COX-2 immunohistochemical expression in colorectal cancer: A meta-analysis of the literature. PLoS ONE 2013, 8, e58891. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; Bonner, M.R.; Moysich, K.B.; Ambrosone, C.B.; Nie, J.; Tao, M.H.; Edge, S.B.; Kallakury, B.V.S.; Marian, C.; Trevisan, M.; et al. Non-steroidal anti-inflammatory drug (NSAID) use and breast cancer risk in the Western New York Exposures and Breast Cancer (WEB) Study. Cancer Causes Control 2010, 21, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.L.P.; Warnberg, F.; Farnie, G.; White, D.; Jiang, W.; Anderson, E.; Bundred, N.J. Cyclooxygenase-2 inhibition: Effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br. J. Cancer 2007, 96, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Katkoori, V.R.; Manne, K.; Vital-Reyes, V.S.; Rodríguez-Burford, C.; Shanmugam, C.; Sthanam, M.; Manne, U.; Chatla, C.; Abdulkadir, S.A.; Grizzle, W.E. Selective COX-2 inhibitor (celecoxib) decreases cellular growth in prostate cancer cell lines independent of p53. Biotech. Histochem. 2013, 88, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.W.; Ahn, Y.E.; Chung, W.S.; Choi, H.J.; Suh, Y.J. Synergistic effect between celecoxib and luteolin is dependent on estrogen receptor in human breast cancer cells. Tumor Biol. 2015, 36, 6349–6359. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Howe, L.R.; Port, E.R.; Brogi, E.; Fishman, J.; Liu, C.H.; Hla, T.; Hudis, C.; Dannenberg, A.J. HER-2/neu status is a determinant of mammary aromatase activity in vivo: Evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006, 66, 5504–5511. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Morris, P.G.; Zhou, X.K.; Morrow, M.; Du, B.; Giri, D.; Kopelovich, L.; Hudis, C.A.; Dannenberg, A.J. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012, 2, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-M.; Heo, J.-I.; Oh, J.-Y.; Kim, Y.-M.; Ha, K.-S.; Kim, J.-I.; Han, J.A. COX-2 regulates p53 activity and inhibits DNA damage-induced apoptosis. Biochem. Biophys. Res. Commun. 2005, 328, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Docanto, M.M.; Sasano, H.; Lo, C.; Simpson, E.R.; Brown, K.A. Prostaglandin E2 inhibits p53 in human breast adipose stromal cells: A novel mechanism for the regulation of aromatase in obesity and breast cancer. Cancer Res. 2015, 75, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wu, J.; Yang, X.; Yang, J.; Zhang, Y.; Du, L. Oleuropein Induced Apoptosis in HeLa Cells via a Mitochondrial Apoptotic Cascade Associated With Activation of the c-Jun NH2-Terminal Kinase. J. Pharmacol. Sci. 2014, 125, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.P.; Markosyan, N.; Connolly, E.; Lawson, J.A.; Li, X.; Grant, G.R.; Grosser, T.; FitzGerald, G.A.; Smyth, E.M. Myeloid Cell COX-2 deletion reduces mammary tumor growth through enhanced cytotoxic T-lymphocyte function. Carcinogenesis 2014, 35, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, B.; Huang, J.; Lin, Y.; Xiang, T.; Wan, J.; Li, H.; Chouaib, S.; Ren, G. Cyclooxygenase-2 in tumor-associated macrophages promotes breast cancer cell survival by triggering a positive-feedback loop between macrophages and cancer cells. Oncotarget 2015, 6, 29637–29650. [Google Scholar] [PubMed]
- Llorente-Cortés, V.; Estruch, R.; Mena, M.P.; Ros, E.; González, M.A.M.; Fitó, M.; Lamuela-Raventós, R.M.; Badimon, L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010, 208, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrera, P.; Delgado-Lista, J.; Torres-Sanchez, L.; Rangel-Zuñiga, O.; Camargo, A.; Moreno-Navarrete, J.; Garcia-Olid, B.; Quintana-Navarro, G.; Alcala-Diaz, J.; Muñoz-Lopez, C.; et al. The postprandial inflammatory response after ingestion of heated oils in obese persons is reduced by the presence of phenol compounds. Mol. Nutr. Food Res. 2012, 56, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Rangel-Zuñiga, O.A.; Haro, C.; Meza-Miranda, E.R.; Peña-Orihuela, P.; Meneses, M.E.; Marin, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chem. 2014, 162, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Vann, K.R.; Sedgeman, C.A.; Gopas, J.; Golan-Goldhirsh, A.; Osheroff, N. Effects of Olive Metabolites on DNA Cleavage Mediated by Human Type II Topoisomerases. Biochemistry 2015, 54, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, S.; Zhou, M.; Yu, W.; Zhang, Y.; He, X. Phytoestrogens and risk of prostate cancer: A meta-analysis of observational studies. World J. Surg. Oncol. 2015, 13, 231. [Google Scholar] [CrossRef] [PubMed]
| Hydroxytyrosol | Oleuropein | Luteolin-7-Glucoside | Apigenin-7-Glucoside | Verbascoside | Oleuropein Aglycone | Reference | |
|---|---|---|---|---|---|---|---|
| Olive oil mg/Kg | 131.77 ± 32 | ND | ND | ND | ND | 17.24 ± 1.15 | [30] |
| 3.0 ± 0.2 | ND | ND | ND | 0.08 ± 0.02 | NM | [31] | |
| 12.5 | ND | NM | NM | NM | NM | [32] | |
| 4.3–9.9 | ND | 4.0–7.6 | 1.5–2.6 | ND | 67.7–136.4 | [33] | |
| 0.15–1.53 | ND | ND | ND | ND | 0.35–6.43 | [34] | |
| Olive leaf mg/Kg | NM | 26,471.4 ± 1760.2 | 4208.9 ± 97.8 | 2333.1 ± 74.7 | 966.1 ± 18.1 | NM | [35] |
| ND | 19,050 ± 880 | 155 ± 10 | 207 ± 10 | 1428 ± 46 | NM | [31] | |
| NM | 19,860 ± 54 | NM | NM | 200 ± 40 | NM | [36] | |
| NM | 22,610 ± 632 | 970 ± 43 | 1072 ± 38 | 488 ± 21 | NM | [37] | |
| NM | 5173–12,921 | 219–444 | 192–488 | 213–501 | NM | [38] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.G.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. https://doi.org/10.3390/nu8080513
Boss A, Bishop KS, Marlow G, Barnett MPG, Ferguson LR. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients. 2016; 8(8):513. https://doi.org/10.3390/nu8080513
Chicago/Turabian StyleBoss, Anna, Karen S. Bishop, Gareth Marlow, Matthew P. G. Barnett, and Lynnette R. Ferguson. 2016. "Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions" Nutrients 8, no. 8: 513. https://doi.org/10.3390/nu8080513
APA StyleBoss, A., Bishop, K. S., Marlow, G., Barnett, M. P. G., & Ferguson, L. R. (2016). Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients, 8(8), 513. https://doi.org/10.3390/nu8080513