Probiotics and Time to Achieve Full Enteral Feeding in Human Milk-Fed and Formula-Fed Preterm Infants: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Extraction and Meta-Analysis
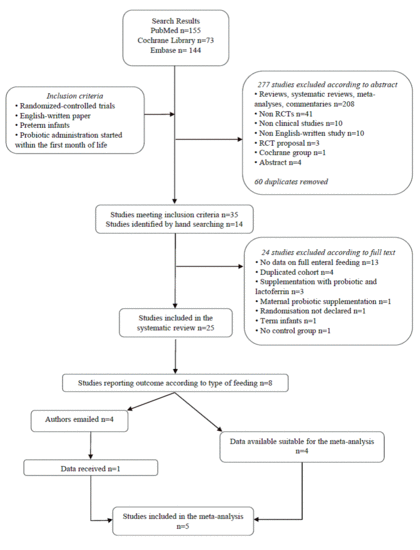
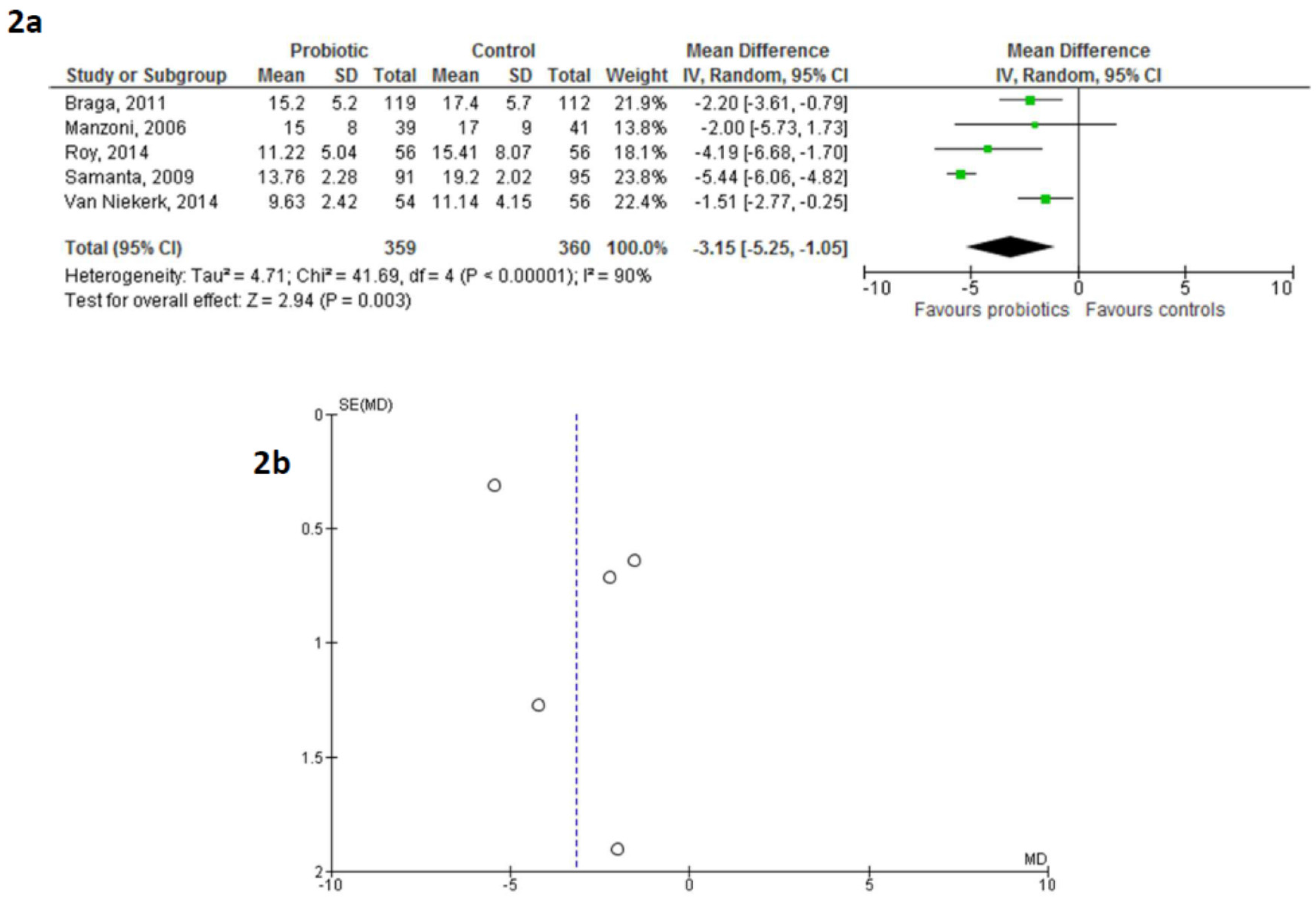
3. Results
Literature Search
4. Methodological Study Quality
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Faldella, G.; Aceti, A.; Corvaglia, L. Formula milk and neurodevelopmental and cognitive outcomes: Where are we now? Early Hum. Dev. 2011, 87S, S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-C.; Tsai, M.-L.; Chen, C.-C.; Lin, H.-C. Early optimal nutrition improves neurodevelopmental outcomes for very preterm infants. Nutr. Rev. 2014, 72, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Berrington, J.E.; Stewart, C.J.; Embleton, N.D.; Cummings, S.P. Gut microbiota in preterm infants: Assessment and relevance to health and disease. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F286–F290. [Google Scholar] [CrossRef] [PubMed]
- The SIFT Investigators Group. Early enteral feeding strategies for very preterm infants: Current evidence from Cochrane reviews. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F470–F472. [Google Scholar]
- Corvaglia, L.; Fantini, M.P.; Aceti, A.; Gibertoni, D.; Rucci, P.; Baronciani, D.; Faldella, G. Predictors of full enteral feeding achievement in very low birth weight infants. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Athalye-Jape, G.; Deshpande, G.; Rao, S.; Patole, S. Benefits of probiotics on enteral nutrition in preterm neonates: a systematic review. Am. J. Clin. Nutr. 2014, 100, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Gori, D.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Indrio, F.; Maggio, L.; Meneghin, F.; Morelli, L.; et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants: Systematic review and meta-analysis. Ital. J. Pediatr. 2015, 41, 89. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, K.; Anabrees, J.; Bassler, D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, CD005496. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.C.; Athalye-jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic supplementation and late-onset sepsis in preterm infants: A meta-analysis. Pediatrics 2016, 137, e20153684. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Guarner, F.; Guerrant, R.; Holt, P.R.; Quigley, E.M.M.; Sartor, R.B.; Sherman, P.M.; Mayer, E.A. An update on the use and investigation of probiotics in health and disease. Gut 2013, 62, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Murguía-Peniche, T.; Mihatsch, W.A.; Zegarra, J.; Supapannachart, S.; Ding, Z.-Y.; Neu, J. Intestinal mucosal defense system, Part 2. Probiotics and prebiotics. J. Pediatr. 2013, 162, S64–S71. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Walker, W.A. Probiotics: Role in pathophysiology and prevention in necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M.; Morowitz, M.J. The intestinal microbiome and necrotizing enterocolitis. Curr. Opin. Pediatr. 2013, 25, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Repa, A.; Thanhaeuser, M.; Endress, D.; Weber, M.; Kreissl, A.; Binder, C.; Berger, A.; Haiden, N. Probiotics (Lactobacillus acidophilus and Bifidobacterium bifidum) prevent NEC in VLBW infants fed breast milk but not formula. Pediatr. Res. 2015, 77, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Available online: http://www.ncbi.nlm.nih.gov/pubmed (accessed on 28 July 2016).
- Cochrane Library. Available online: http://www.cochranelibrary.com/ (accessed on 28 July 2016).
- Embase. Available online: http://store.elsevier.com/en_US/info/30800006 (accessed on 28 July 2016).
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- RevMan Software. Available online: http://tech.cochrane.org/revman/download (accessed on 28 July 2016).
- Totsu, S.; Yamasaki, C.; Terahara, M.; Uchiyama, A.; Kusuda, S. Bifidobacterium and enteral feeding in preterm infants: Cluster-randomized trial. Pediatr. Int. 2014, 56, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, E.; Kirsten, G.F.; Nel, D.G.; Blaauw, R. Probiotics, feeding tolerance, and growth: A comparison between HIV-exposed and unexposed very low birth weight infants. Nutrition 2014, 30, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates—A randomised double blind placebo controlled trial. PLoS ONE 2014, 9, e89511. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.Y.; Sari, F.N.; Arayici, S.; Guzoglu, N.; Erdeve, O.; Uras, N.; Oguz, S.S.; Dilmen, U. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F110–F115. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Serce, O.; Benzer, D.; Gursoy, T.; Karatekin, G.; Ovali, F. Efficacy of saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: A randomised controlled trial. Early Hum. Dev. 2013, 89, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Erdeve, O.; Celik, I.H.; Dilmen, U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: A randomized, controlled study. Acta Paediatr. 2013, 102, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Lozano, J.M.; Rojas, M.X.; Rodriguez, V.A.; Rondon, M.A.; Bastidas, J.A.; Perez, L.A.; Rojas, C.; Ovalle, O.; Garcia-Harker, J.E.; et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012, 130, e1113–e1120. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.N.; Eras, Z.; Dizdar, E.A.; Erdeve, O.; Oguz, S.S.; Uras, N.; Dilmen, U. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am. J. Perinatol. 2012, 29, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carrocera, L.A.; Solis-Herrera, A.; Cabanillas-Ayón, M.; Gallardo-Sarmiento, R.B.; García-Pérez, C.S.; Montaño-Rodríguez, R.; Echániz-Aviles, M.O.L. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F5–F9. [Google Scholar] [CrossRef] [PubMed]
- Havranek, T.; Al-Hosni, M.; Armbrecht, E. Probiotics supplementation increases intestinal blood flow velocity in extremely low birth weight preterm infants. J. Perinatol. 2013, 33, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska-Liszewska, D.; Seliga-Siwecka, J.; Kornacka, M.K. The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Hum. Dev. 2012, 88, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.-J.; Soulaines, P.; Leroux, B.; Kalach, N.; Dupont, C.; Butel, M.-J. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.N.; Dizdar, E.A.; Oguz, S.; Erdeve, O.; Uras, N.; Dilmen, U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: A randomized, controlled trial. Eur. J. Clin. Nutr. 2011, 65, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Cavallo, L.; Francavilla, R. Effects of probiotic and prebiotic on gastrointestinal motility in newborns. J. Physiol. Pharmacol. 2009, 60, 27–31. [Google Scholar] [PubMed]
- Mihatsch, W.A.; Vossbeck, S.; Eikmanns, B.; Hoegel, J.; Pohlandt, F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: A randomized controlled trial. Neonatology 2010, 98, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Rougé, C.; Piloquet, H.; Butel, M.-J.; Berger, B.; Rochat, F.; Ferraris, L.; Des Robert, C.; Legrand, A.; de la Cochetiere, M.-F.; N’Guyen, J.-M.; et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009, 89, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Samanta, M.; Sarkar, M.; Ghosh, P.; Ghosh, J.K.; Sinha, M.K.; Chatterjee, S. Prophylactic probiotics for prevention of necrotizing enetrocolitis in very low birth weight newborns. J. Trop. Pediatr. 2008, 55, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Hsu, C.-H.; Chen, H.-L.; Chung, M.-Y.; Hsu, J.-F.; Lien, R.; Tsao, L.-Y.; Chen, C.-H.; Su, B.-H. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A multicenter, randomized, controlled trial. Pediatrics 2008, 122, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Bin-Nun, A.; Bromiker, R.; Wilschanski, M.; Kaplan, M.; Rudensky, B.; Caplan, M.; Hammerman, C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J. Pediatr. 2005, 147, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Costalos, C.; Skouteri, V.; Gounaris, A.; Sevastiadou, S.; Triandafilidou, A.; Ekonomidou, C.; Kontaxaki, F.; Petrochilou, V. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum. Dev. 2003, 74, 89–96. [Google Scholar] [CrossRef]
- Dani, C.; Biadaioli, R.; Bertini, G.; Martelli, E.; Rubaltelli, F.F. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. Biol. Neonate 2002, 82, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Stansbridge, E.M.; Walker, V.; Hall, M.A.; Smith, S.L.; Millar, M.R.; Bacon, C.; Chen, S. Effects of feeding premature infants with Lactobacillus GG on gut fermentation. Arch. Dis. Child. 1993, 69, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Millar, M.R.; Bacon, C.; Smith, S.L.; Walker, V.; Hall, M.A. Enteral feeding of premature infants with Lactobacillus GG. Arch. Dis. Child. 1993, 69, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Cavallo, L.; Francavilla, R. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J. Pediatr. 2008, 152, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Al-Hosni, M.; Duenas, M.; Hawk, M.; Stewart, L.A.; Borghese, R.A.; Cahoon, M.; Atwood, L.; Howard, D.; Ferrelli, K.; Soll, R. Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 2012, 32, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Ceratto, S.; Poggi, E.; Cartosio, M.E.; Cordero di Montezemolo, L.; Giannattasio, A. Preventive effects of oral probiotic on infantile colic: A prospective, randomised, blinded, controlled trial using Lactobacillus reuteri DSM 17938. Benef. Microbes 2014. [Google Scholar] [CrossRef]
- Saengtawesin, V.; Tangpolkaiwalsak, R.; Kanjanapattankul, W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J. Med. Assoc. Thail. 2014, 97, S20–S25. [Google Scholar]
- Dilli, D.; Aydin, B.; Fettah, N.; Özyazıcı, E.; Beken, S.; Zenciroğlu, A.; Okumuş, N.; Özyurt, B.; İpek, M.; Akdağ, A.; et al. The propre-save study: Effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J. Pediatr. 2015, 28, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Chaudhuri, J.; Sarkar, D.; Ghosh, P.; Chakraborty, S. Role of enteric supplementation of Probiotics on late-onset sepsis by Candida species in preterm low birth weight neonates: A randomized, double blind, placebo-controlled trial. N. Am. J. Med. Sci. 2014, 6, 50–57. [Google Scholar] [PubMed]
- Oncel, M.Y.; Arayici, S.; Sari, F.N.; Simsek, G.K.; Yurttutan, S.; Erdeve, O.; Saygan, S.; Uras, N.; Oguz, S.S.; Dilmen, U. Comparison of Lactobacillus reuteri and nystatin prophylaxis on Candida colonization and infection in very low birth weight infants. J. Matern. Neonatal Med. 2014, 1–5. [Google Scholar] [CrossRef]
- Millar, M.; Wilks, M.; Fleming, P.; Costeloe, K. Should the use of probiotics in the preterm be routine? Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F70–F74. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Manzoni, P.; Meyer, M.; Casa, E.D.; Pugni, L.; Mosca, F.; Stolfi, I.; Messner, H.; Memo, L.; Laforgia, N.; et al. Bovine lactoferrin supplementation for prevention of necrotising enterocolitis in preterm very-low-birth-weight neonates: A randomised trial. Early Hum. Dev. 2012, 88, S102. [Google Scholar] [CrossRef]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgia, N.; et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial. Early Hum. Dev. 2014, 90, S60–S65. [Google Scholar] [CrossRef]
- Benor, S.; Marom, R.; Tov, A.B.; Domany, K.A.; Zaidenberg-Israeli, G.; Dollberg, S. Probiotic supplementation in mothers of very low birth weight infants. Am. J. Perinatol. 2014, 31, 497–504. [Google Scholar] [PubMed]
- Manzoni, P.; Mostert, M.; Leonessa, M.L.; Priolo, C.; Farina, D.; Monetti, C.; Latino, M.A.; Gomirato, G. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: A randomized study. Clin. Infect. Dis. 2006, 42, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, H.; Sumida, Y.; Tanaka, R.; Yuki, N.; Takayama, H.; Fujimura, M. Early administration of Bifidobacterium breve to preterm infants: Randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 76, F101–F107. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Koebnick, C.; Schildt, J.; Schmidt, S.; Mueller, M.; Possner, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: A double-blind, placebo-controlled, randomized study. J. Clin. Microbiol. 2006, 44, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Su, B.-H.; Chen, A.-C.; Lin, T.-W.; Tsai, C.-H.; Yeh, T.-F.; Oh, W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005, 115, 1–4. [Google Scholar] [PubMed]
- Braga, T.D.; da Silva, G.A.P.; de Lira, P.I.; de Carvalho Lima, M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: A double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 2011, 93, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Stratiki, Z.; Costalos, C.; Sevastiadou, S.; Kastanidou, O.; Skouroliakou, M.; Giakoumatou, A.; Petrohilou, V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 2007, 83, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shimizu, T.; Hosaka, A.; Kaneko, N.; Ohtsuka, Y.; Yamashiro, Y. Effects of bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr. Int. 2004, 46, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2015, 387, 649–660. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, P.; Narang, A. Comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: A randomized controlled trial. Am. J. Perinatol. 2015, 32, 733–740. [Google Scholar] [PubMed]
- Hays, S.; Jacquot, A.; Gauthier, H.; Kempf, C.; Beissel, A.; Pidoux, O.; Jumas-Bilak, E.; Decullier, E.; Lachambre, E.; Beck, L.; et al. Probiotics and growth in preterm infants: A randomized controlled trial, PREMAPRO study. Clin. Nutr. 2014. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.G.; Romeo, D.M.; Trovato, L.; Oliveri, S.; Palermo, F.; Cota, F.; Betta, P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2011, 31, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hikaru, U.; Koichi, S.; Yayoi, S.; Hiromici, S.; Hiroaki, S.; Yoshkazu, O.; Seigo, A.; Satoru, N.; Toshiaki, S.; Yuichiro, Y. Bifidobacteria prevents preterm infants from developing infection and sepsis. Int. J. Probiotics Prebiotics 2010, 5, 33–36. [Google Scholar]
- Patole, S.K.; Keil, A.D.; Nathan, E.; Doherty, D.; Esvaran, M.; Simmer, K.N.; Conway, P. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in growth restricted very preterm infants -analysis from a randomised trial. J. Matern. Fetal Neonatal Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tewari, V.V.; Dubey, S.K.; Gupta, G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: A randomized controlled trial. J. Trop. Pediatr. 2015, 61, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Giuliani, F.; Baricco, M.; Di Nicola, P.; Peila, C.; Vassia, C.; Chiale, F.; Pirra, A.; Cresi, F.; Martano, C.; Coscia, A. Benefits of donor milk in the feeding of preterm infants. Early Hum. Dev. 2013, 89, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 4653–4658. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Pillonel, T.; Torregrossa, A.; Prod’hom, G.; Fischer, C.J.; Greub, G.; Giannoni, E. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clin. Infect. Dis. 2015, 60, 924–927. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Study Details | Study Population | Intervention Specie | Milk | Placebo | FEF Definition |
|---|---|---|---|---|---|---|
| Dose (D) | ||||||
| Start of Treatment (S) | ||||||
| End of Treatment (E) | ||||||
| Bin-Nun, 2005 [40] | P | Preterm infants with BW < 1500 g, who began enteral feeding on a weekday | B. infantis, Str. thermophilus, B. bifidus | OMM, PFM | HM or FM | 100 mL/kg/day |
| B | D: 0.35 × 109 CFU each, OD | |||||
| R | S: start of enteral feeding | |||||
| C | E: 36 w postconceptual age | |||||
| Braga, 2011 [60] | P | Inborn infants with BW 750–1499 g | L. casei, B. Breve | HM (± PFM from w3) | Extra HM | 150 mL/kg/day |
| DB | D: 3.5 × 107 CFU to 3.5 × 109 CFU OD | |||||
| R | S: day 2 | |||||
| C | E: day 30, NEC diagnosis, discharge, death, whichever occurred first | |||||
| Costalos, 2003 [41] | P | GA 28–32 w | Saccharomyces boulardii | PFM | MDX | Not defined |
| R | No major GI problem | D: 1 × 109 CFU BD | ||||
| C | Not receiving antibiotics | S: non-specified | ||||
| Not receiving breast milk | Median duration of probiotic supplementation: 30 days | |||||
| Costeloe, 2015 [64] | P | Preterm infants with GA 23–30 + 6 weeks, without any lethal malformation or any malformation of the GI tract | Bifidobacterium breve BBG-001 | OMM, DHM, FM | Corn starch powder | 150 mL/kg/day |
| DB | D: 8 · 3–8 · 8 log10 CFU/day | |||||
| R | S: as soon as possible after randomisation | |||||
| C | E: 36 w PMA or discharge if sooner | |||||
| Multic. | ||||||
| Demirel, 2013 [27] | P | Preterm infants with GA ≤ 32 weeks and BW ≤ 1500 g, who survived to feed enterally | S. boulardii | HM, FM | None | Not defined |
| B | D: 5 × 109 CFU OD | |||||
| R | S: first feed | |||||
| C | E: discharge | |||||
| Dilli, 2015 [49] | P | Preterm infants with GA < 32 weeks and BW < 1500 g, born at or transferred to the NICU within the first week of life and fed enterally before inclusion | B. lactis | HM, FM | MDX powder | 100 mL/kg/day (FEF for hydration) |
| DB | D: 5 × 109 CFU | 150 mL/kg/day (FEF for growth) | ||||
| R | S: beyond d7 after birth | |||||
| C | E: death or discharge (max 8 weeks) | |||||
| Multic. | ||||||
| Fernández-Carrocera, 2013 [30] | P | Preterm infants with | L. acidophilus 1 × 109 CFU/g, L. rhamnosus 4.4 × 108 CFU/g, L. casei 1 × 109 CFU/g, L. plantarum 1.76 × 108 CFU/g, B. infantis 2.76 × 107 CFU/g, Str. thermophilus 6.6 × 105 CFU/g | OMM, PFM | None | Not defined |
| DB | BW < 1500 g | Total D: 1 g powder OD | ||||
| R | Infants with NEC stage IA and stage IB were excluded | S: start of enteral feeding | ||||
| C | E: non specified | |||||
| Hays, 2014 [66] | P | Preterm infants with GA 25–31 weeks, BW 700–1600 g, AGA, enteral feeding initiated before day 5 | Probiotic group composed of 3 subgroups: | OMM, DM or PFM | MDX | Not defined |
| DB | Infants with NEC stage ≥ IB, malformations or severe medical or surgical conditions were excluded | P1 B. lactis | ||||
| R | P2 B. longum | |||||
| C | P3 B. lactis + longum | |||||
| Multic. | D: 1 × 109 CFU each probiotic daily | |||||
| Duration: 4 weeks for infants ≥29 w/6 weeks for infants ≤28 w GA | ||||||
| Hikaru, 2010 [68] | P | Extremely low birth weight and very low birth weight infants | B. breve | OMM, PFM | None | Not defined |
| R | D: 0.5 × 109 CFU BD | |||||
| C | S: birth | |||||
| E: discharge from NICU | ||||||
| Jacobs, 2013 [25] | P | Preterm infants with GA <32 weeks and BW < 1500 g | B. infantis BB-02 300 CFU × 106, Str. thermophilus Th-4 350 CFU × 106, B. lactis BB-12 350 CFU × 106 | HM, FM | MDX powder | Enteral feeds of 120 mL/kg for ≥3 days |
| DB | Total D: 1 × 109 CFU × 1.5 g MDX powder OD | |||||
| R | S: enteral feed ≥ 1 mL every 4 h | |||||
| C | E: discharge or term corrected age | |||||
| Multic. | ||||||
| Lin, 2008 [39] | P | Preterm infants with GA < 34 weeks and BW ≤ 1500 g, who survived to feed enterally | L. acidophilus NCDO 1746, B. bifidum NCDO 1453 109 CFU | HM, FM | None | Oral intake of 100 mL/kg/day |
| B | D: 1 × 109 CFU each probiotic (= 125 mg/kg) BD | |||||
| R | S: day 2 of age | |||||
| C | Duration: 6 weeks | |||||
| Multic. | ||||||
| Manzoni, 2006 [56] | P | Infants with BW < 1500 g, ≥3 day of life, who started enteral feeding with HM | L. casei subspecies rhamnosus LGG | OMM, DM | None | Not defined |
| DB | D: 6 × 109 CFU/day | |||||
| R | S: day 3 of life | |||||
| C | E: end of the 6th week or discharge | |||||
| Mihatsch, 2010 [36] | P | Preterm infants with GA < 30 weeks and BW ≤ 1500 g | B. lactis BB12 | OMM, PFM | Indistinguishable powder | 150 mL/kg/day |
| R | D: 2 × 109 CFU/kg 6 times a day | |||||
| C | S: start of enteral feeding | |||||
| E: non specified | ||||||
| Oncel, 2014 [24] | P | Preterm infants with GA ≤ 32 weeks and BW ≤ 1500 g, who survived to feed enterally | L. reuteri DSM 17938 | HM, FM | Oil base | Not defined |
| DB | D: 1 × 108 CFU OD | |||||
| R | S: first feed | |||||
| C | E: death or discharge | |||||
| Patole, 2014 [23] | P | Preterm infants with GA < 33 weeks and BW < 1500 g | B. breve | HM, FM | Dextrin | 150 mL/kg/day enteral feeding |
| DB | D: 3 × 109 CFU OD (1.5 × 109 CFU OD for newborn ≤ 27 w until they reached 50 mL/kg/day enteral feeds) | |||||
| R | S: start of enteral feed | |||||
| C | E: corrected age of 37 w | |||||
| Rougé, 2009 [37] | P | Preterm infants with GA < 32 weeks and BW < 1500 g, ≤2 weeks of age, without any disease other than those linked to prematurity, who started enteral feeding before inclusion | B. longum BB536, L. rhamnosus GG BB536-LGG | OMM, DM or PFM | MDX | Not defined |
| DB | Total D: 1 × 108 CFU/day | |||||
| R | S: start of enteral feeding | |||||
| C | E: discharge | |||||
| Bic. | ||||||
| Roy, 2014 [50] | P | Preterm infants (GA < 37 weeks) and BW < 2500 g, with stable enteral feeding within 72 h of birth | L. acidophilus 1.25 × 109 CFU × 1 g, B. longum 0.125 × 109 CFU × 1 g, B. bifidum 0.125 × 109 CFU × 1 g, B. lactis 1 × 109 CFU × 1 g | HM | Sterile water | 120 mL/kg/day for ≥3 d |
| DB | D: half a 1 g sachet | |||||
| R | S: from 72 h of life | |||||
| C | E: after 6 w or at discharge | |||||
| Saengtawesin, 2014 [48] | P | Preterm infants with GA ≤ 34 weeks and BW ≤ 1500 g | L. acidophilus 1 × 109 CFU, B. bifidum 1 × 109 CFU | HM, PFM | None | 150 mL/kg/day |
| R | D: 125 mg/kg BD | |||||
| C | S: start of feeding | |||||
| E: 6 w of age or discharge. | ||||||
| Samanta, 2008 [38] | P | Preterm infants with GA < 32 weeks and BW < 1500 g, who started enteral feeding and survived beyond 48 h of age | B. infantis, B. bifidum, B. longum, L. acidophilus | HM | None | Not defined |
| DB | ||||||
| R | D: 2.5 × 109 CFU each probiotic, BD | |||||
| C | S: start of enteral feeding | |||||
| E: discharge | ||||||
| Sari, 2011 [34] | P | Preterm infants with GA < 32 weeks or BW < 1500 g, who survived to feed enterally | L. sporogenes | HM, FM | None | Not defined |
| B | D: 0.35 × 109 CFU OD | |||||
| R | S: first feed | |||||
| C | E: discharge | |||||
| Serce, 2013 [26] | P | Preterm infants with GA ≤ 32 weeks and BW ≤ 1500 g, who survived to feed enterally | S. boulardii | HM, FM | Distilled water | 100 mL/kg/day |
| M | D: 0.5 × 109 CFU/kg BD | enteral feeding | ||||
| R | S: non specified | |||||
| C | E: non specified | |||||
| Stratiki, 2007 [61] | P | Preterm infants with GA 27–32 weeks, formula-fed, without major congenital anomalies | Bifidobacterium lactis | FM | None | 150 mL/kg/day |
| B | D: 2 × 107 CFU/g of milk powder | |||||
| R | S: start of enteral feeding | |||||
| C | E: not specified | |||||
| Tewari, 2015 [70] | P | Preterm infants with GA < 34 weeks | Bacillus clausii | OMM, DHM | Sterile water | 180 mL/kg/day |
| DB | Excluded if: NEC, congenital anomaly , outborn and >10 days of with sepsis | D: 2.4 × 109 CFU/day | ||||
| R | Stratified as extreme preterm (GA 27–30 + 6) and very preterm (GA 31–33 + 6) | S: by day 5 in asymptomatic and by day 10 in symptomatic infants | ||||
| C | E: 6 weeks of age, discharge or death (whichever occurred first) | |||||
| Totsu, 2014 [21] | P | Infants with BW < 1500 g | B. bifidum | HM, FM | Dextrin | Postnatal day at which the amount of enteral feeding exceeded 100 mL/kg/day |
| DB | D: 2.5 × 109 CFU, divided in two doses | |||||
| CLR | S: within 48 h after birth | |||||
| C | E: body weight 2000 g | |||||
| Multic. | ||||||
| Van Niekerk, 2014 [22] | P | Preterm infants with GA < 34 weeks and BW < 1250 g, exposed and non-exposed to HIV (only infants unexposed to HIV are included in the meta-analysis) | L. rhamnosus, B. infantis | HM | MCT oil | “when infants no longer required the use of IV fluids” |
| DB | D: 0.35 × 109 CFU each probiotic | |||||
| R | S: start of enteral feeding | |||||
| C | E: day 28 postconceptual age |
| Study | Random Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|
| Braga, 2011 [60] | Low | Low | Low | Low | Unclear | Low |
| Manzoni, 2006 [56] | Low | Low | Low | Unclear | Unclear | Low |
| Roy, 2014 [50] | Low | Unclear | Low | Low | Unclear | Unclear |
| Samanta, 2008 [38] | Low | Low | Low | Unclear | Unclear | Unclear |
| Van Niekerk, 2014 [22] | Low | Unclear | Low | Unclear | Unclear | Unclear |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aceti, A.; Gori, D.; Barone, G.; Callegari, M.L.; Fantini, M.P.; Indrio, F.; Maggio, L.; Meneghin, F.; Morelli, L.; Zuccotti, G.; et al. Probiotics and Time to Achieve Full Enteral Feeding in Human Milk-Fed and Formula-Fed Preterm Infants: Systematic Review and Meta-Analysis. Nutrients 2016, 8, 471. https://doi.org/10.3390/nu8080471
Aceti A, Gori D, Barone G, Callegari ML, Fantini MP, Indrio F, Maggio L, Meneghin F, Morelli L, Zuccotti G, et al. Probiotics and Time to Achieve Full Enteral Feeding in Human Milk-Fed and Formula-Fed Preterm Infants: Systematic Review and Meta-Analysis. Nutrients. 2016; 8(8):471. https://doi.org/10.3390/nu8080471
Chicago/Turabian StyleAceti, Arianna, Davide Gori, Giovanni Barone, Maria Luisa Callegari, Maria Pia Fantini, Flavia Indrio, Luca Maggio, Fabio Meneghin, Lorenzo Morelli, Gianvincenzo Zuccotti, and et al. 2016. "Probiotics and Time to Achieve Full Enteral Feeding in Human Milk-Fed and Formula-Fed Preterm Infants: Systematic Review and Meta-Analysis" Nutrients 8, no. 8: 471. https://doi.org/10.3390/nu8080471