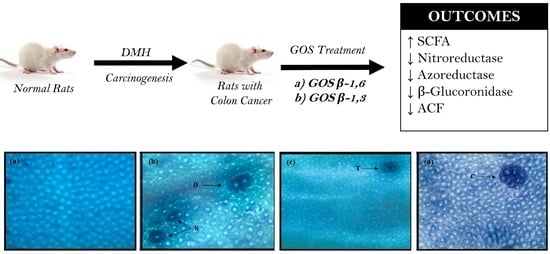
Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Carcinogenic Injection
2.5. Preparation of Prebiotic GOS
2.6. Measurement of Body Weight Changes
2.7. pH and Ammonia
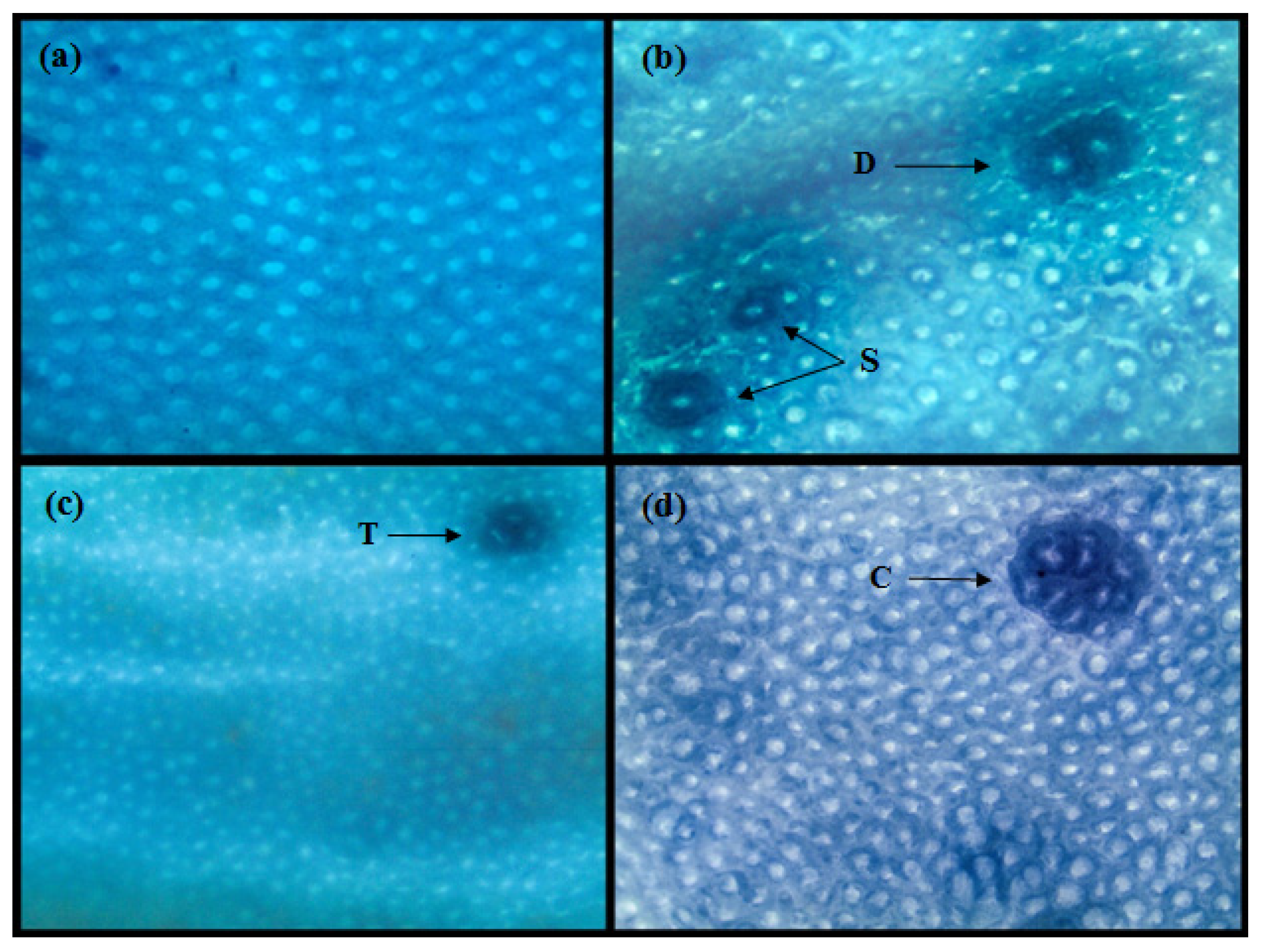
2.8. Aberrant Crypt Foci (ACF) Analysis
2.9. Samples Collection and Enzyme Analysis
2.9.1. Nitroreductase Assay
2.9.2. β-Glucuronidase Assay
2.9.3. β-Glucosidase Assay
2.10. Short Chain Fatty Acids
2.11. Statistical Analysis
3. Results
3.1. Body Weight and Food Intake
3.2. Individual Effect of GOS and Inulin on Aberrant Crypt Foci (ACF)
3.3. Combined Effect of Inulin and GOS on ACF
3.4. SCFA, pH, and Ammonia
3.5. Bacterial Enzymes
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflict of Interests
References
- Benson, A.L. Epidemiology, disease progression, and economic burden of colorectal cancer. J. Manag. Care Pharm. 2007, 13, S5–S18. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.; Collins, P.D. Colon cancer: A civilization disorder. Dig. Dis. 2011, 29, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Bastide, N.M.; Pierre, F.H.; Corpet, D.E. Heme iron from meat and risk of colorectal cancer: A meta-analysis and a review of the mechanisms involved. Cancer Prev. Res. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Mukhtar, H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett. 2010, 293, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Derks, S.; Smits, K.M.; Meijer, G.A.; Herman, J.G. Colorectal cancer epigenetics: Complex simplicity. J. Clin. Oncol. 2011, 29, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.; Mortensen, A.; Binderup, M.L.; Langkilde, S.; Markowski, J.; Dragsted, L.O. The effect of apple feeding on markers of colon carcinogenesis. Nutr. Cancer 2011, 63, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, R.S.; Siddiqui, I.A.; Mukhtar, H. Modulation of wnt/beta-catenin signaling pathway by bioactive food components. Carcinogenesis 2012, 33, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, C.I.; Stoidis, C.N.; Spyropoulos, B.G.; Zografos, E.D. Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer. World J. Gastroenterol. 2008, 14, 6453–6457. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011, 45, S120–S127. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Fahey, G.C., Jr.; Wolf, B.W. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [PubMed]
- Ebert, M.N.; Beyer-Sehlmeyer, G.; Liegibel, U.M.; Kautenburger, T.; Becker, T.W.; Pool-Zobel, B.L. Butyrate induces glutathione s-transferase in human colon cells and protects from genetic damage by 4-hydroxy-2-nonenal. Nutr. Cancer 2001, 41, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef]
- Laparra, J.M.; Diez-Municio, M.; Herrero, M.; Moreno, F.J. Structural differences of prebiotic oligosaccharides influence their capability to enhance iron absorption in deficient rats. Food Funct. 2014, 5, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; Spinardi-Barbisan, A.L.T.; Barbisan, L.F.; Bedani, R.; Pauly, N.D.; Carlos, I.Z.; Benzatti, F.; Vendramini, R.C.; Rossi, E.A. Probiotic Enterococcus faecium CRL 183 inhibit chemically induced colon cancer in male Wistar rats. Eur. Food Res. Technol. 2008, 228, 231–237. [Google Scholar] [CrossRef]
- Gallaher, D.D.; Khil, J. The effect of synbiotics on colon carcinogenesis in rats. J. Nutr. 1999, 129, 1483S–1487S. [Google Scholar] [PubMed]
- Femia, A.P.; Luceri, C.; Dolara, P.; Giannini, A.; Biggeri, A.; Salvadori, M.; Clune, Y.; Collins, K.J.; Paglierani, M.; Caderni, G. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics lactobacillus rhamnosus and bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis 2002, 23, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.; van Loo, J.; Rowland, I.; Roberfroid, M.B. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br. J. Nutr. 2002, 87 (Suppl. S2), S273–S281. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S. Prevention of colon cancer by pre- and probiotics: Evidence from laboratory studies. Br. J. Nutr. 1998, 80, S219–S223. [Google Scholar] [PubMed]
- Wijnands, M.V.; Appel, M.J.; Hollanders, V.M.; Woutersen, R.A. A comparison of the effects of dietary cellulose and fermentable galacto-oligosaccharide, in a rat model of colorectal carcinogenesis: Fermentable fibre confers greater protection than non-fermentable fibre in both high and low fat backgrounds. Carcinogenesis 1999, 20, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, M.V.; Schoterman, H.C.; Bruijntjes, J.B.; Hollanders, V.M.; Woutersen, R.A. Effect of dietary galacto-oligosaccharides on azoxymethane-induced aberrant crypt foci and colorectal cancer in fischer 344 rats. Carcinogenesis 2001, 22, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Rossow, K.L.; Lindlauf, J. Development and testing of the ain-93 purified diets for rodents: Results on growth, kidney calcification and bone mineralization in rats and mice. J. Nutr. 1993, 123, 1923–1931. [Google Scholar] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Spinardi-Barbisan, A.L.; Rodrigues, M.A.; de Camargo, J.L.; Teran, E.; Barbisan, L.F. Lack of chemopreventive effects of ginger on colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Food Chem. Toxicol. 2006, 44, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Vieiralves, N.F.; Gomes, M.I.; Salvadori, D.M.; Rodrigues, M.A.; Barbisan, L.F. Effects of lycopene, synbiotic and their association on early biomarkers of rat colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Nguyen, T.H.; Nguyen, T.T.; Maischberger, T.; Haltrich, D. Beta-galactosidase from Lactobacillus plantarum WCFS1: Biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydr. Res. 2010, 345, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Visek, W.J. Large intestinal ph and ammonia in rats: Dietary fat and protein interactions. J. Nutr. 1991, 121, 832–843. [Google Scholar] [PubMed]
- Bird, R.P. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995, 93, 55–71. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J. Natl. Cancer Inst. 1976, 57, 371–375. [Google Scholar] [PubMed]
- Zhao, G.; Nyman, M.; Åke Jönsson, J. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shukla, G. Administration of prebiotic inulin suppresses 1,2 dimethylhydrazine dihydrochloride induced procarcinogenic biomarkers fecal enzymes and preneoplastic lesions in early colon carcinogenesis in sprague dawley rats. J. Funct. Foods 2013, 5, 991–996. [Google Scholar] [CrossRef]
- Aachary, A.A.; Gobinath, D.; Srinivasan, K.; Prapulla, S.G. Protective effect of xylooligosaccharides from corncob on 1,2-dimethylhydrazine induced colon cancer in rats. Bioact. Carbohydr. Diet. Fibre 2015, 5, 146–152. [Google Scholar] [CrossRef]
- Pool-Zobel, B.L.; Sauer, J. Overview of experimental data on reduction of colorectal cancer risk by inulin-type fructans. J. Nutr. 2007, 137, 2580S–2584S. [Google Scholar] [PubMed]
- Van de Wiele, T.; Boon, N.; Possemiers, S.; Jacobs, H.; Verstraete, W. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J. Appl. Microbiol. 2007, 102, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Cuff, M.A.; Fung, F.; Shirazi-Beechey, S.P. The importance of colonic butyrate transport to the regulation of genes associated with colonic tissue homoeostasis. Biochem. Soc. Trans. 2005, 33, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Lupton, J.R.; Marchant, L.J. Independent effects of fiber and protein on colonic luminal ammonia concentration. J. Nutr. 1989, 119, 235–241. [Google Scholar] [PubMed]
- De Preter, V.; Vanhoutte, T.; Huys, G.; Swings, J.; de Vuyst, L.; Rutgeerts, P.; Verbeke, K. Effects of Lactobacillus casei shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G358–G368. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.P.; de Hertogh, G.; de Preter, V.; Luypaerts, A.; Bammens, B.; Evenepoel, P.; Ghoos, Y.; Geboes, K.; Rutgeerts, P.; Verbeke, K. The influence of inulin on the absorption of nitrogen and the production of metabolites of protein fermentation in the colon. Br. J. Nutr. 2006, 96, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A.; Deutsch, A.S. The physiological relevance of the intestinal microbiota—Contributions to human health. J. Am. Coll. Nutr. 2007, 26, 679S–683S. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E.; Zduńczyk, Z.; Juśkiewicz, J. Effect of lactobacillus fermented beetroot juice on composition and activity of cecal microflora of rats. Eur. Food Res. Technol. 2009, 229, 153–157. [Google Scholar] [CrossRef]
- Ayesh, R.; Weststrate, J.A.; Drewitt, P.N.; Hepburn, P.A. Safety evaluation of phytosterol esters. Part 5. Faecal short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem. Toxicol. 1999, 37, 1127–1138. [Google Scholar] [PubMed]
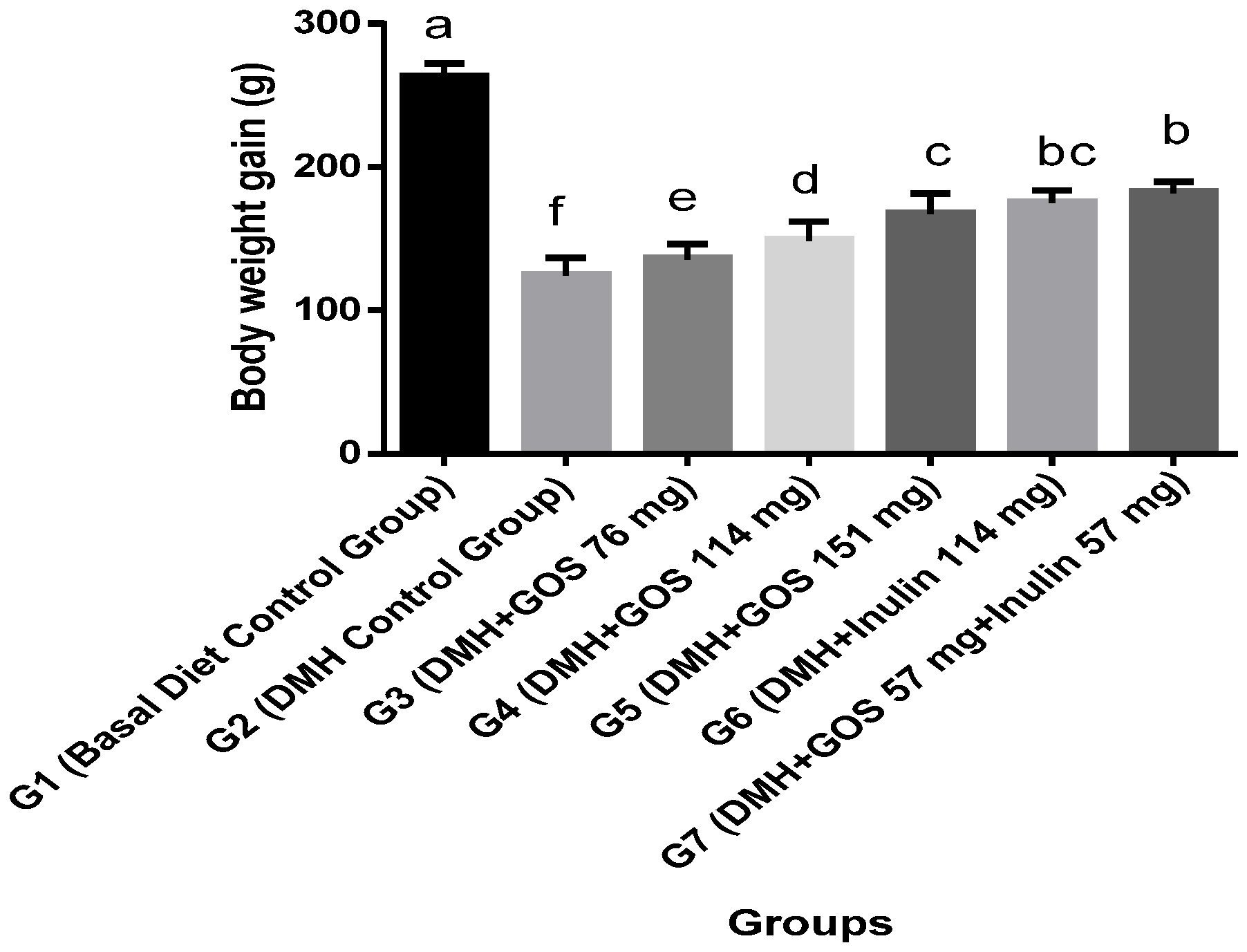
| Groups | Food Intake (g/Rat/Day) |
|---|---|
| G1 (Basal Diet Control Group) | 20.0 ± 0.5 a |
| G2 (DMH Control Group) | 20.6 ± 0.6 a |
| G3 (DMH + GOS 76 mg) | 21.0 ± 0.6 a |
| G4 (DMH + GOS 114 mg) | 20.3 ± 0.5 a |
| G5 (DMH + GOS 151 mg) | 20.1 ± 0.5 a |
| G6 (DMH + Inulin 114 mg) | 21.0 ± 0.6 a |
| G7 (DMH + GOS 57 mg + Inulin 57 mg) | 20.7 ± 0.6 a |
| ACF | G1 (Basal Diet Control Group) | G2 (DMH Control Group) | G3 (DMH + GOS 76 mg) | G4 (DMH + GOS 114 mg) | G5 (DMH + GOS 151 mg) | G6 (DMH + Inulin 114 mg) | G7 (DMH + GOS 57 mg + Inulin 57 mg) |
|---|---|---|---|---|---|---|---|
| ACF/proximal colon | ND | 24.6 ± 2.73 a | 23.8 ± 2.87 a | 12.6 ± 2.13 b | 8.7 ± 1.47 b,c | 7.5 ± 1.05 b,c | 5.3 ± 0.63 c |
| ACF/middle colon | ND | 39.6 ± 4.03 a | 33.3 ± 3.09 a | 23.8 ± 2.82 b | 15.2 ± 1. 86 c | 16.2 ± 1.92 b,c | 18.8 ± 2.72 b,c |
| ACF/distal colon | ND | 106.3 ± 6.77 a | 92.3 ± 5.50 a | 95.2 ± 5.63 a | 76.0 ± 5.32 b | 59.6 ± 4.98 c | 51.2 ± 4.14 c |
| Total ACF/colon | ND | 170.4 ± 7.34 a | 153.1 ± 9.23 a | 131.6 ± 9.77 b | 99.8 ± 8.93 c | 83.3 ± 5.52 c,d | 75.3 ± 6.95 d |
| % of total ACF inhibition | - | - | 10.2 | 22.8 | 41.4 | 51.2 | 55.8 |
| Parameters | G1 (Basal Diet Control Group) | G2 (DMH Control Group) | G3 (DMH + GOS 76 mg) | G4 (DMH + GOS 114 mg) | G5 (DMH + GOS 151 mg) | G6 (DMH + Inulin 114 mg) | G7 (DMH + GOS 57 mg + Inulin 57 mg) | |
|---|---|---|---|---|---|---|---|---|
| CECUM | Acetate | 82.6 ± 2.73 c,d | 80.5 ± 3.30 e | 86.8 ± 3.66 c,d | 91.4 ± 3.08 b,c | 97.1 ± 2.89 a,b | 99.3 ± 3.55 a,b | 104.0 ± 3.95 a |
| Propionate | 23.3 ± 1.89 c | 22.7 ± 1.96 c | 24.7 ± 2.21 b,c | 27.8 ± 1.41 b,c | 28.2 ± 1.54 b,c | 29.3 ± 1.58 a,b | 33.6 ± 1.79 a | |
| Butyrate | 15.7 ± 0.79 c,d | 15.4 ± 1.08 d | 16.3 ± 1.13 c,d | 18.8 ± 1.08 b,c | 19.7 ± 1.19 a,b | 20.2 ± 0.99 a,b | 22.7 ± 1.44 a | |
| pH | 6.6 ± 0.15 | 6.7 ± 0.14 | 6.5 ± 0.12 | 6.5 ± 0.07 | 6.4 ± 0.11 | 6.4 ± 0.13 | 6.3 ± 0.18 | |
| Ammonia | 15.3 ± 1.48 a | 15.6 ± 1.69 a | 14.9 ± 2.05 a | 12.8 ± 1.62 a,b | 11.1 ± 1.06 a,b,c | 10.3 ± 1.15 b,c | 7.7 ± 0.64 c | |
| FECAL | Acetate | 55.4 ± 2.60 c | 53.8 ± 2.84 c | 57.5 ± 3.08 b,c | 63.7 ± 2.38 a,b | 67.1 ± 3.16 a | 69.6 ± 2.58 a | 70.4 ± 2.64 a |
| Propionate | 15.4 ± 0.83 b,c | 15.3 ± 0.78 c | 16.8 ± 0.98 b,c | 18.4 ± 1.05 b | 21.3 ± 1.04 a | 22.3 ± 1.07 a | 24.3 ± 1.27 a | |
| Butyrate | 5.5 ± 0.51 c | 5.3 ± 0.53 c | 6.2 ± 0.67 c | 7.3 ± 0.66 b,c | 8.4 ± 0.73 a,b | 8.8 ± 0.73 a,b | 9.8 ± 0.94 a | |
| pH | 6.6 ± 0.16 | 6.6 ± 0.14 | 6.5 ± 0.10 | 6.4 ± 0.08 | 6.4 ± 0.11 | 6.4 ± 0.13 | 6.3 ± 0.11 | |
| Ammonia | 10.1 ± 1.13 a | 10.6 ± 1.34 a | 8.8 ± 0.84 a,b | 6.8 ± 0.87 b,c | 5.9 ± 0.95 b,c | 5.5 ± 0.92 c | 4.2 ± 0.37 c | |
| Enzymes | G1 (Basal Diet Control Group) | G2 (DMH Control Group) | G3 (DMH + GOS 76 mg) | G4 (DMH + GOS 114 mg) | G5 (DMH + GOS 151 mg) | G6 (DMH + Inulin 114 mg) | G7 (DMH + GOS 57 mg + Inulin 57 mg) | |
|---|---|---|---|---|---|---|---|---|
| CECUM | β-Glucosidase | 0.97 ± 0.12 a,b | 1.13 ± 0.21 a | 1.09 ± 0.11 a | 0.83 ± 0.11 a,b,c | 0.77 ± 0.11 a,b,c | 0.64 ± 0.12 b,c | 0.52 ± 0.13 c |
| β-Glucoronidase | 3.15 ± 0.19 a | 3.19 ± 0.19 a | 2.94 ± 0.18 a | 2.57 ± 0.18 a,b | 2.19 ± 0.27 b | 2.27 ± 0.25 b | 1.94 ± 0.19 b | |
| Nitroreductase | 4.17 ± 0.65 a | 4.33 ± 0.53 a | 4.16 ± 0.82 a | 2.58 ± 0.54 b | 2.17 ± 0.27 b | 2.02 ± 0.21 b | 1.68 ± 0.19 b | |
| Azoreductase | 10.75 ± 1.14 a | 10.58 ± 1.06 a | 8.42 ± 0.97 a,b | 7.25 ± 0.93 b | 5.67 ± 0.69 b,c | 6.08 ± 0.86 b,c | 4.33 ± 0.58 c | |
| FECAL | β-Glucosidase | 0.80 ± 0.12 a,b | 1.12 ± 0.13 a | 0.73 ± 0.08 b,c | 0.81 ± 0.10 a,b | 0.57 ± 0.10 b,c | 0.47 ± 0.10 b,c | 0.43 ± 0.11 c |
| β-Glucoronidase | 2.56 ± 0.33 a | 2.64 ± 0.35 a | 2.14 ± 0.26 a,b | 1.71 ± 0.19 b,c | 1.50 ± 0.25 b,c | 1.37 ± 0.12 c | 1.17 ± 0.11 c | |
| Nitroreductase | 2.67 ± 0.36 a,b | 2.92 ± 0.45 a | 2.66 ± 0.62 a,b | 1.75 ± 0.18 b,c | 1.27 ± 0.16 c | 1.14 ± 0.17 c | 0.97 ± 0.16 c | |
| Azoreductase | 6.58 ± 0.85 a | 6.83 ± 0.81 a | 5.75 ± 0.79 a,b | 4.33 ± 0.54 b,c | 3.67 ± 0.33 c,d | 3.42 ± 0.23 c,d | 2.17 ± 0.28 d | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qamar, T.R.; Syed, F.; Nasir, M.; Rehman, H.; Zahid, M.N.; Liu, R.H.; Iqbal, S. Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats. Nutrients 2016, 8, 465. https://doi.org/10.3390/nu8080465
Qamar TR, Syed F, Nasir M, Rehman H, Zahid MN, Liu RH, Iqbal S. Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats. Nutrients. 2016; 8(8):465. https://doi.org/10.3390/nu8080465
Chicago/Turabian StyleQamar, Tahir Rasool, Fatima Syed, Muhammad Nasir, Habib Rehman, Muhammad Nauman Zahid, Rui Hai Liu, and Sanaullah Iqbal. 2016. "Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats" Nutrients 8, no. 8: 465. https://doi.org/10.3390/nu8080465
APA StyleQamar, T. R., Syed, F., Nasir, M., Rehman, H., Zahid, M. N., Liu, R. H., & Iqbal, S. (2016). Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats. Nutrients, 8(8), 465. https://doi.org/10.3390/nu8080465