Phytochemical Compounds and Antioxidant Capacity of Tucum-Do-Cerrado (Bactris setosa Mart), Brazil’s Native Fruit
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Standards
2.2. Sample Material
2.3. Moisture Content
2.4. Extraction and Isolation
2.5. HPLC-DAD Analysis of Phenolic Compounds
2.6. Antioxidant Activity
2.6.1. Ferric Reducing Antioxidant Power Assay (FRAP)
2.6.2. Carotene/Linoleic System
2.7. Determination of Phytochemical Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Tucum-Do-Cerrado Peel Has Higher Total Phenolic, Total Flavanol and Total Anthocyanin Content than the Pulp
3.2. Tucum-Do-Cerrado Peel has a High Antioxidant Activity via Ferric Reducing Antioxidant Power (FRAP) and β-Carotene/Linoleic Acid Assays in Relation to Pulp
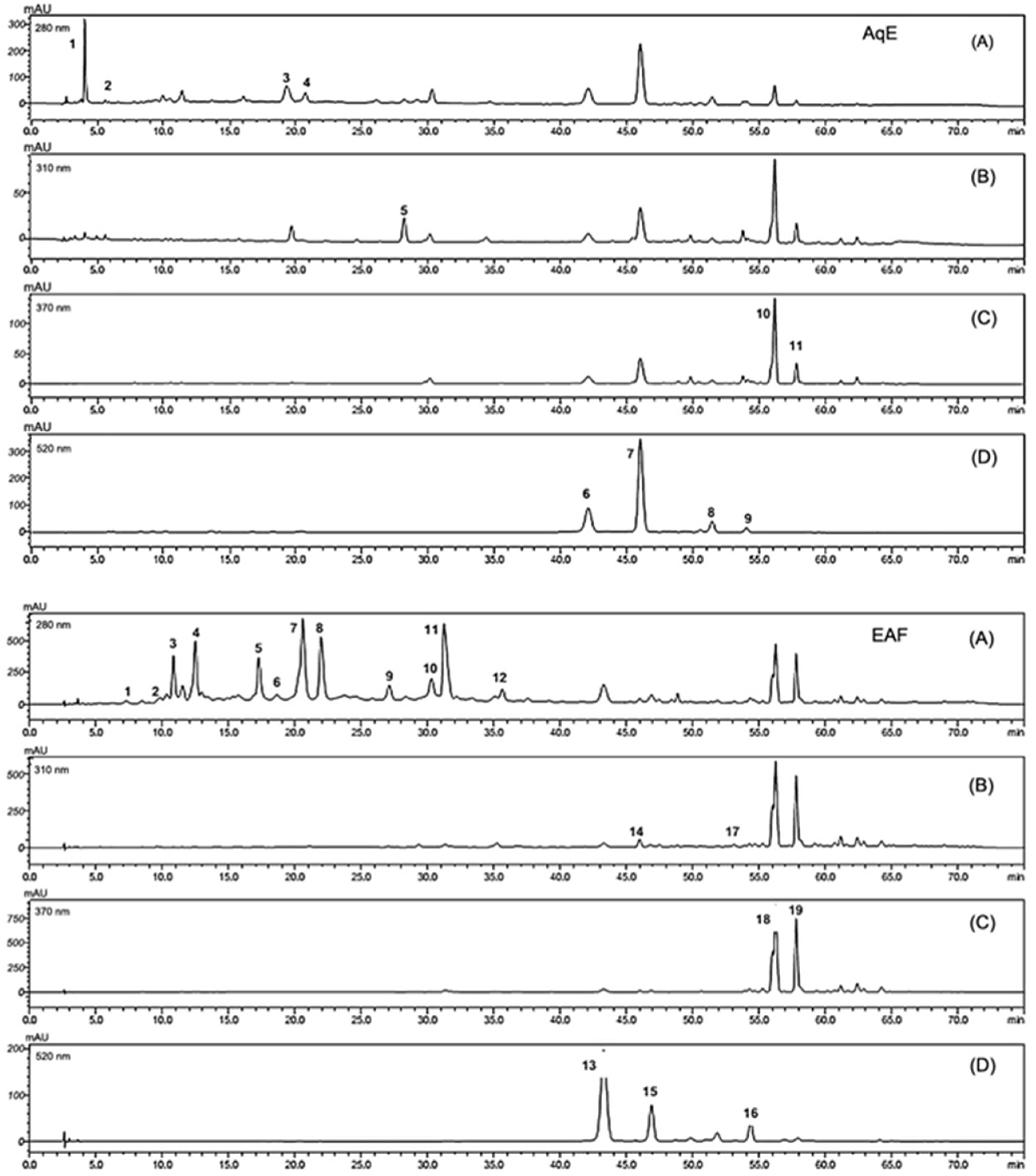
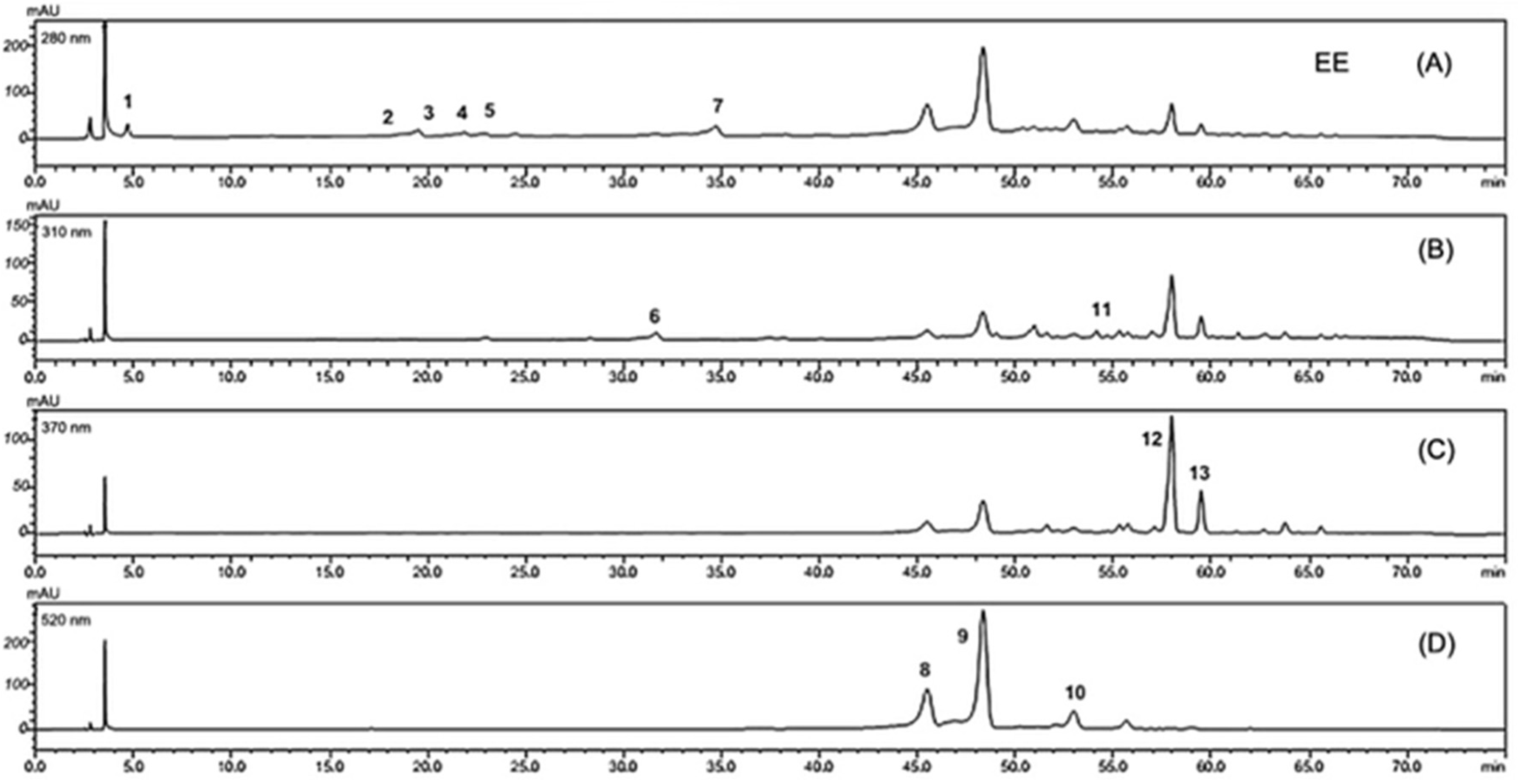
3.3. Flavanols, Anthocyanins, Hydroxybenzoic Acids and Flavones Are the Major Phenolics Compound Classes Identified in Tucum-Do-Cerrado Peel by HPLC-DAD
3.4. Main Classes of Phenolic Compounds Identified and Quantified in Aqueous, Methanolic and Ethanolic Extracts of Tucum-Do-Cerrado Peel by HPLC-DAD
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Lima, A.L.; Soares, J.J. Aspectos florísticos e ecológicos de palmeira (Arecaceae) da Reserva Biológica de Duas Bocas, Cariacica, Espírito Santo. Rev. Biol. Mello Leitão 2003, 16, 5–20. [Google Scholar]
- Duarte, A.Y.S.; Queiroz, R.S.D.; Sanches, R.A.; Garcia, C.R.; Dedini, F.G. Ethnobotany of Natural Fibres—Bactris setosa (tucum) in a Traditional Rural Community. Fibres Text. East. Eur. 2012, 20, 18–20. [Google Scholar]
- Siqueira, E.M.; Rosa, F.R.; Fustinoni, A.M.; Sant’Ana, L.P.D.; Arruda, S.F. Brazilian savanna fruits contain higher bioactive compounds content and higher antioxidant activity relative to the conventional Red Delicious apple. PLoS ONE 2013, 8, e72826. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Liu, J.; Chen, B. Apples prevent mammary tumors in rats. J. Agric. Food Chem. 2005, 53, 2341–2343. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Jabbar, Z.; Athar, M.; Alam, M.S. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Nicholson, R.L. Families of Phenolic Compounds and Means of Classification. In Phenolic Compound Biochemistry; Vermerris, W., Nicholson, R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; Volume 12, pp. 1–34. [Google Scholar]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell Longev. 2015, 2015, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Rad. Biol. Med. 2012, 15, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A.; Ibrahim, A.S.; Badria, A.F.; Elmarakby, A.A. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Rani, N.; Velan, L.P.; Vijaykumar, S.; Arunachalam, A. An insight into the potentially old-wonder molecule-quercetin: the perspectives in foresee. Chin. J. Integr. Med. 2015, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Campos, D.; Betalleluz, I.; Giusti, M.M.; Schwartz, S.J.; Tian, Q.; Larondelle, Y. High-performance liquid chromatography with photodiode array detection (HPLC-DAD)/HPLC-mass spectrometry (MS) profiling of anthocyanins from Andean Mashua Tubers (Tropaeolum tuberosum Ruiz and Pavon) and their contribution to the overall antioxidant activity. J. Agric. Food Chem. 2006, 54, 7089–7097. [Google Scholar] [PubMed]
- Simirgiotis, M.J.; Silva, M.; Becerra, J.; Schmeda-Hirschmann, G. Direct characterisation of phenolic antioxidants in infusions from four Mapuche medicinal plants by liquid chromatography with diode array detection (HPLC-DAD) and electrospray ionisation tandem mass spectrometry (HPLC-ESI-MS). Food Chem. 2012, 131, 318–327. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘Antioxidant Power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compost. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Francis, F.J. Analysis of Anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 181–207. [Google Scholar]
- Association of Official Agricultural Chemists. Assay for vitamin C. In Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed.; AOAC, Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1997; Volume 2, pp. 16–17. [Google Scholar]
- Rodriguez-Amaya, D.B. A Guide to Carotenoids Analysis in Foods, 1st ed.; ILSI Press: Washington, DC, USA, 1999; pp. 1–64. [Google Scholar]
- Leite-Legatti, A.V.; Batista, Â.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Júnior, M.R.M. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef]
- Barros, H.R.D.M.; Ferreira, T.A.P.D.C.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.D.S.M.; Alves, R.E.; Brito, E.S.D.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1003. [Google Scholar] [CrossRef]
- Souza, V.R.D.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; Carneiro, J.D.D.S. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- Faria, A.F.; Marques, M.C.; Mercadante, A.Z. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. 2011, 126, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.; Kimura, M.; Amaya-Farfan, J. Fontes Brasileiras de Carotenóides: Tabela Brasileira de Composição de carOtenóides em Alimentos; Ministério do Meio Ambiente/Secretaria de Biodiversidades e Florestas: Brasília, Brasil, 2008; pp. 1–99. [Google Scholar]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An Alternative Use of Horticultural Crops: Stressed Plants as Biofactories of Bioactive Phenolic Compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Mason, M.; Scampicchio, M.; Andreotti, C.; Cesco, S.; Mimmo, T. Enhancement of the bioactive compound content in strawberry fruits grown under iron and phosphorus deficiency. J. Sci. Food Agric. 2015, 95, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, I.; Nikolova, I.; Danchev, N.; Nikolov, S. Phytochemical analysis of ethyl acetate extract from Astragalus corniculatus Bieb. and brain antihypoxic activity. Acta Pharm. 2004, 54, 151–156. [Google Scholar] [PubMed]
- Rezaire, A.; Robinson, J.C.; Bereau, B.; Verbaere, A.; Sommerer, N.; Khan, M.K.; Fils-Lycaon, B. Amazonian palm Oenocarpus bataua (“patawa”): chemical and biological antioxidant activity—Phytochemical composition. Food Chem. 2014, 149, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.D.S.C.; Vieira, F.G.K.; Copetti, C.; Gonzaga, L.V.; Zambiazi, R.C.; Filho, J.M.; Fett, R. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Res. Int. 2011, 44, 2128–2133. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; Segura-Carretero, A.; Guerra-Hernandez, E.; Garcia-Villanova, B.; Fernandez-Gutierrez, A. Influence of technological processes on phenolic compounds, organic acids, furanic derivatives, and antioxidant activity of whole-lemon powder. Food Chem. 2013, 141, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Zhao, Y. Effect of Different Drying Methods and Storage Time on the Retention of Bioactive Compounds and Antibacterial Activity of Wine Grape Pomace (Pinot Noir and Merlot). J. Food Sci. 2012, 77, H192–H201. [Google Scholar] [CrossRef] [PubMed]
- Caunii, A.; Pribac, G.; Grozea, I.; Gaitin, D.; Samfira, I. Design of optimal solvent for extraction of bio-active ingredients from six varieties of Medicago sativa. Chem. Central J. 2012, 6, 123–130. [Google Scholar] [CrossRef] [PubMed]

| Components | Tucum-Do-Cerrado | ||
|---|---|---|---|
| Peel | Pulp | Whole Fruit | |
| Moisture | 71.4 ± 0.8 **;## | 91.2 ± 1.3 *** | 77.9 ± 1.0 |
| Total phenolics § | 28,287.6 ± 614.8 ***;## | 230.5 ± 0.9 *** | 5214.3 ± 132.5 |
| Total flavanols § | 1068.5 ± 10.2 **;## | 79.2 ± 2.0 *** | 746.7 ± 4.6 |
| Total anthocyanins | 638.5 ± 3.3 ***;## | 2.4 ± 0.1 ** | 83.2 ± 6.5 |
| Yellow flavonoids | 323.0 ± 7.9 **;## | 5.3 ± 0.1 *** | 42.2 ± 2.1 |
| Vitamin C | 181.2 ± 23.2 *;# | 53.7 ± 0.0 * | 100.6 ± 20.3 |
| Total carotenoids ¥ | 290.4 ± 20.7 **;## | 56.5 ± 4.7 ** | 147.8 ± 20.5 |
| Extracts | FRAP (μmol Fe2SO4/g Fresh Matter) | β-Carotene/Linoleic Acid (%) g Fresh Matter |
|---|---|---|
| Peel | ||
| Aqueous extract (AqE) | 160.36 ± 9.48 | 3.13 ± 0.10 |
| Methanol/water fraction (MAqF) | 68.02 ± 0.74 * | 1.12 ± 0.11 *** |
| Ethyl acetate fraction (EAF) | 37.09 ± 0.55 *;§§§ | 0.61 ± 0.04 ***;§§ |
| Hexane fraction (HF) | 2.69 ± 0.09 *;§§§ | nd |
| Methanolic extract (ME) | 237.95 ± 6.50 ** | 3.41 ± 0.10 * |
| Ethanolic extract (EE) | 303.90 ± 12.52 ** | 7.04 ± 0.42 *** |
| Pulp | ||
| Aqueous extract (AqE) | 9.66 ± 0.06 ## | 0.33 ± 0.08 ### |
| Methanol/water fraction (MAqF) | 3.01 ± 0.06 **;### | 0.15 ± 0.06 *;### |
| Ethyl acetate fraction (EAF) | 2.39 ± 0.07 **;###;§§§ | 0.08 ± 0.03 **;### |
| Hexane fraction (HF) | 0.22 ± 0.01 **;###;§§§ | nd |
| Methanolic extract (ME) | 26.92 ± 0.43 **;### | 0.67 ± 0.04 **;### |
| Ethanolic extract (EE) | 22.81 ± 1.16 *;### | 0.91 ± 0.14 **;### |
| Peak Number | Retention Time (min) | λ Max. (nm) | Compound Class | Hypothesis | Amount (mg/100 g FW) | |
|---|---|---|---|---|---|---|
| Aqueous extract (AqE) | ||||||
| 1 | 4.05 | 226, 272 | Hydroxybenzoic acid | Gallic acid derivative | 61.46 | |
| 2 | 5.60 | 224, 276 | Hydroxybenzoic acid | Gallic acid | 0.65 | |
| 3 | 19.28 | 224, 278 | Flavanol | Catechin derivative a | 214.61 | |
| 4 | 20.70 | 224, 278 | Flavanol | Catechin derivative a | 88.97 | |
| 5 | 28.16 | 222, 319 | Hydroxycinnamic acid | Caffeic acid | 1.95 | |
| 6 | 42.05 | 279, 516 | Anthocyanin | Cyanidin derivative b | 25.13 | |
| 7 | 45.99 | 280, 520 | Anthocyanin | Cyanidin derivative b | 71.44 | |
| 8 | 51.41 | 279, 520 | Anthocyanin | Cyanidin derivative b | 6.52 | |
| 9 | 54.03 | 279, 520 | Anthocyanin | Cyanidin derivative b | 1.74 | |
| 10 | 56.13 | 234, 355 | Flavone | Rutin | 60.56 | |
| 11 | 57.77 | 257, 355 | Flavone | Rutin derivative d | 8.19 | |
| EAF | ||||||
| 1 | 7.25 | 226, 278 | Hydroxybenzoic acid | Gallic acid derivative | 0.93 | |
| 2 | 10.81 | 224, 278 | Flavanol | Catechin derivative a | 17.22 | |
| 3 | 11.50 | 225, 278 | Flavanol | Catechin derivative a | 6.65 | |
| 4 | 12.46 | 226, 278 | Flavanol | Catechin derivative a | 29.09 | |
| 5 | 17.23 | 225, 278 | Flavanol | Catechin derivative a | 26.31 | |
| 6 | 18.62 | 225, 278 | Flavanol | Catechin derivative a | 3.93 | |
| 7 | 20.56 | 227, 278 | Flavanol | Catechin derivative a | 62.85 | |
| 8 | 21.94 | 226, 278 | Flavanol | Catechin derivative a | 38.72 | |
| 9 | 27.08 | 225, 278 | Flavanol | Catechin derivative a | 9.44 | |
| 10 | 30.27 | 225, 278 | Flavanol | Catechin derivative a | 14.49 | |
| 11 | 31.21 | 226, 278 | Flavanol | Catechin derivative a | 50.26 | |
| 12 | 35.61 | 225, 278 | Flavanol | Catechin derivative a | 4.95 | |
| 13 | 43.26 | 279, 516 | Anthocyanin | Cyanidin derivative b | 1.56 | |
| 14 | 45.97 | 258, 324 | Hydroxycinnamic acid | Ferulic acid derivative | 0.25 | |
| 15 | 46.86 | 279, 519 | Anthocyanin | Cyanidin derivative b | 0.43 | |
| 16 | 51.82 | 277, 521 | Anthocyanin | Cyanidin derivative b | 0.06 | |
| 17 | 53.08 | 300, 314 | Stilbene | Resveratrol derivative c | 0.04 | |
| 18 | 56.22 | 255, 354 | Flavone | Rutin | 13.80 | |
| 19 | 57.77 | 255, 354 | Flavone | Rutin derivative d | 8.11 | |
| MAqF | ||||||
| 1 | 4.50 | 225, 272 | Hydroxybenzoic acid | Gallic acid derivative | 27.79 | |
| 2 | 6.54 | 224, 276 | Hydroxybenzoic acid | Gallic acid derivative | 0.63 | |
| 3 | 11.87 | 224, 279 | Flavanol | Catechin derivative a | 11.07 | |
| 4 | 12.71 | 224, 279 | Flavanol | Catechin derivative a | 5.15 | |
| 5 | 13.72 | 224, 278 | Flavanol | Catechin derivative a | 12.17 | |
| 6 | 21.66 | 224, 272 | Flavanol | Catechin derivative a | 28.32 | |
| 7 | 22.60 | 224, 280 | Flavanol | Catechin derivative a | 12.30 | |
| 8 | 24.11 | 224, 278 | Flavanol | Catechin derivative a | 6.88 | |
| 9 | 31.31 | 305, 317 | Stilbene | Resveratrol derivative c | 0.73 | |
| 10 | 45.15 | 279, 517 | Anthocyanin | Cyanidin derivative b | 8.52 | |
| 11 | 48.03 | 280, 520 | Anthocyanin | Cyanidin derivative b | 29.34 | |
| 12 | 52.76 | 279, 521 | Anthocyanin | Cyanidin derivative b | 2.62 | |
| 13 | 55.47 | 268, 519 | Anthocyanin | Cyanidin derivative b | 0.45 | |
| 14 | 57.69 | 255, 355 | Flavone | Rutin derivative d | 10.98 | |
| 15 | 67.73 | 258, 367 | Flavonol | Quercetin | 1.26 | |
| Methanolic extract (ME) | ||||||
| 1 | 4.25 | 224, 272 | Hydroxybenzoic acid | Gallic acid derivative | 74.30 | |
| 2 | 11.23 | 224, 278 | Flavanol | Catechin derivative a | 36.16 | |
| 3 | 12.94 | 224, 278 | Flavanol | Catechin derivative a | 27.77 | |
| 4 | 17.96 | 224, 278 | Flavanol | Catechin derivative a | 182.32 | |
| 5 | 20.30 | 224, 278 | Flavanol | Catechin derivative a | 82.59 | |
| 6 | 21.39 | 224, 278 | Flavanol | Catechin derivative a | 57.15 | |
| 7 | 22.79 | 224, 278 | Flavanol | Catechin derivative a | 57.44 | |
| 8 | 32.41 | 224, 278 | Flavanol | Catechin derivative a | 243.89 | |
| 9 | 35.99 | 224, 320 | Hydroxycinnamic acid | Caffeic acid derivative | 7.15 | |
| 10 | 38.29 | 224, 279 | Flavanol | Catechin derivative a | 41.63 | |
| 11 | 43.88 | 279, 516 | Anthocyanin | Cyanidin derivative b | 37.50 | |
| 12 | 47.13 | 280, 519 | Anthocyanin | Cyanidin derivative b | 105.38 | |
| 13 | 49.04 | 224, 321 | Hydroxycinnamic acid | Ferulic acid derivative | 4.11 | |
| 14 | 51.21 | 279, 519 | Anthocyanin | Cyanidin derivative b | 1.59 | |
| 15 | 52,10 | 279, 519 | Anthocyanin | Cyanidin derivative b | 14.85 | |
| 16 | 52.89 | 307 | Stilbene | Resveratrol derivative c | 0.83 | |
| 17 | 53.67 | 225, 323 | Hydroxycinnamic acid | Ferulic acid derivative | 0.58 | |
| 18 | 54.68 | 280, 519 | Anthocyanin | Cyanidin derivative | 5.98 | |
| 19 | 56.02 | 253, 330 | Hydroxycinnamic acid | Ferulic acid derivative | 0.94 | |
| 20 | 56.76 | 255, 354 | Flavone | Rutin | 95.82 | |
| 21 | 58.29 | 256, 354 | Flavone | Rutin derivative d | 26.03 | |
| Ethanolic Extract (EE) | ||||||
| 1 | 4.68 | 224, 273 | Hydroxybenzoic acid | Gallic acid derivative | 8.37 | |
| 2 | 19.52 | 224, 278 | Flavanol | Catechin derivative a | 106.84 | |
| 2 | 21.88 | 224, 278 | Flavanol | Catechin derivative a | 45.95 | |
| 4 | 22.90 | 224, 279 | Flavanol | Catechin derivative a | 18.96 | |
| 5 | 24.48 | 224, 278 | Flavanol | Catechin derivative a | 13.67 | |
| 6 | 31.66 | 305 | Stilbene | Resveratrol derivative c | 1.72 | |
| 7 | 34.70 | 224, 278 | Flavanol | Catechin derivative a | 126.40 | |
| 8 | 45.50 | 279, 517 | Anthocyanin | Cyanidin derivative b | 32.73 | |
| 9 | 48.35 | 280, 520 | Anthocyanin | Cyanidin derivative b | 83.25 | |
| 10 | 52.97 | 279, 519 | Anthocyanin | Cyanidin derivative b | 9.22 | |
| 11 | 54.14 | 303 | Stilbene | Resveratrol derivative c | 0.62 | |
| 12 | 57.98 | 255, 355 | Flavone | Rutin derivative d | 78.74 | |
| 13 | 59.47 | 255, 355 | Flavone | Rutin derivative d | 16.97 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, F.R.; Arruda, A.F.; Siqueira, E.M.A.; Arruda, S.F. Phytochemical Compounds and Antioxidant Capacity of Tucum-Do-Cerrado (Bactris setosa Mart), Brazil’s Native Fruit. Nutrients 2016, 8, 110. https://doi.org/10.3390/nu8030110
Rosa FR, Arruda AF, Siqueira EMA, Arruda SF. Phytochemical Compounds and Antioxidant Capacity of Tucum-Do-Cerrado (Bactris setosa Mart), Brazil’s Native Fruit. Nutrients. 2016; 8(3):110. https://doi.org/10.3390/nu8030110
Chicago/Turabian StyleRosa, Fernanda R., Andréa F. Arruda, Egle M. A. Siqueira, and Sandra F. Arruda. 2016. "Phytochemical Compounds and Antioxidant Capacity of Tucum-Do-Cerrado (Bactris setosa Mart), Brazil’s Native Fruit" Nutrients 8, no. 3: 110. https://doi.org/10.3390/nu8030110
APA StyleRosa, F. R., Arruda, A. F., Siqueira, E. M. A., & Arruda, S. F. (2016). Phytochemical Compounds and Antioxidant Capacity of Tucum-Do-Cerrado (Bactris setosa Mart), Brazil’s Native Fruit. Nutrients, 8(3), 110. https://doi.org/10.3390/nu8030110




