Suppression of Endogenous Glucose Production by Isoleucine and Valine and Impact of Diet Composition
Abstract
:1. Introduction
2. Experimental Section
2.1. Animal Studies
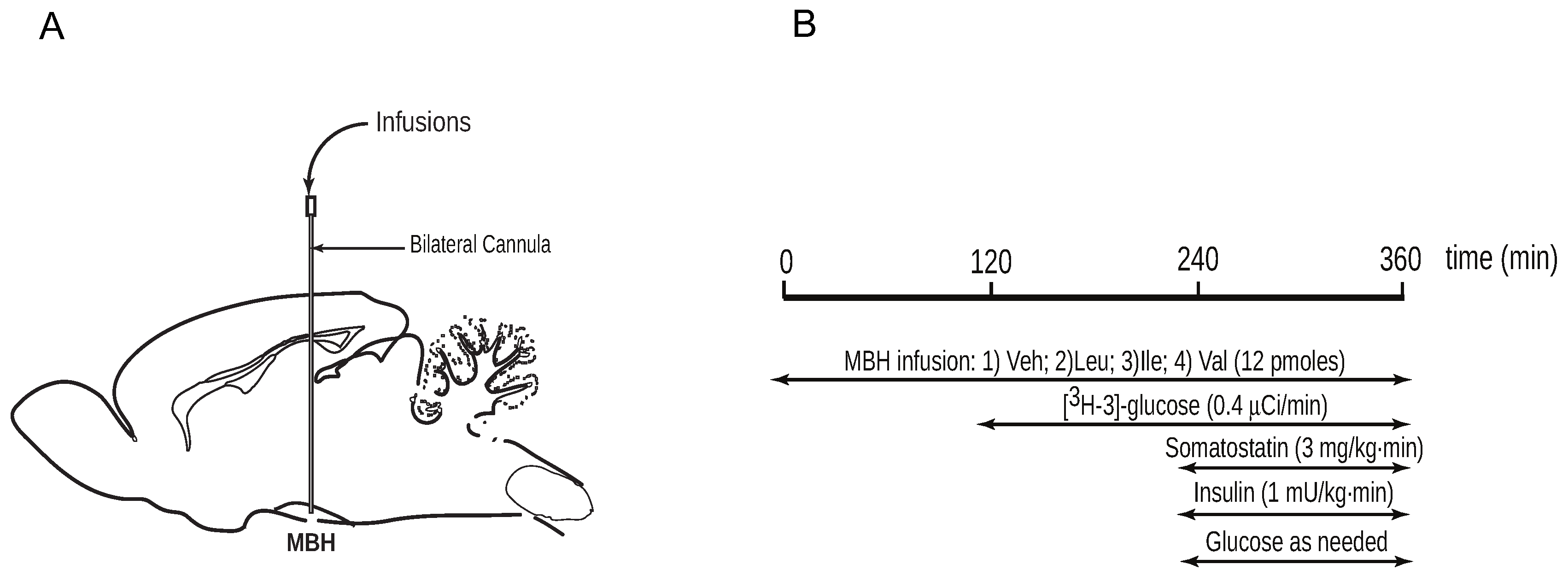
2.1.1. Animal Surgeries
2.1.2. Diets
2.1.3. Intrahypothalamic Infusions
2.1.4. Pancreatic Clamp Studies and Glucose Kinetics Measurements
2.2. Analytical Procedures and Calculations
2.3. Statistical Analysis
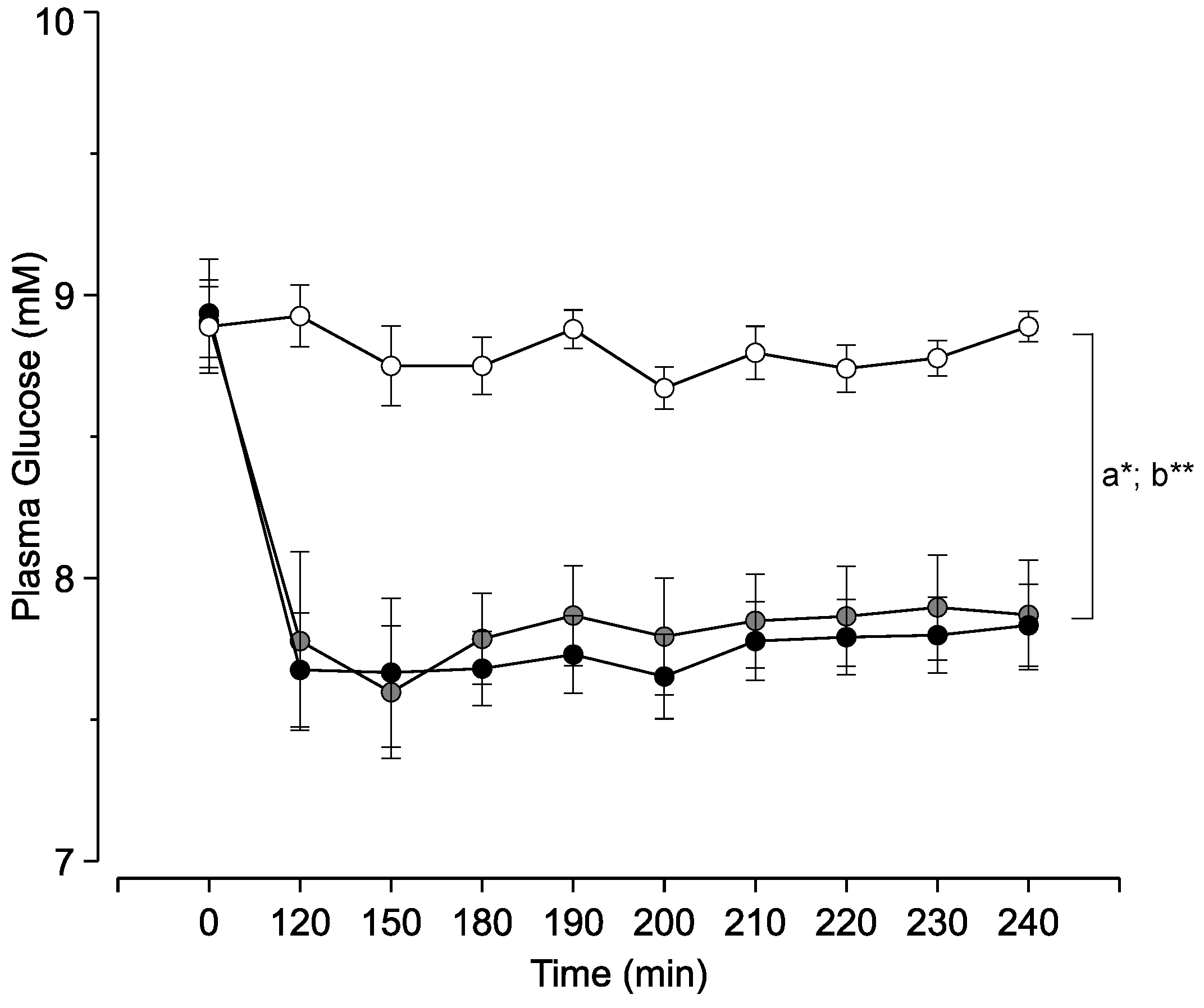
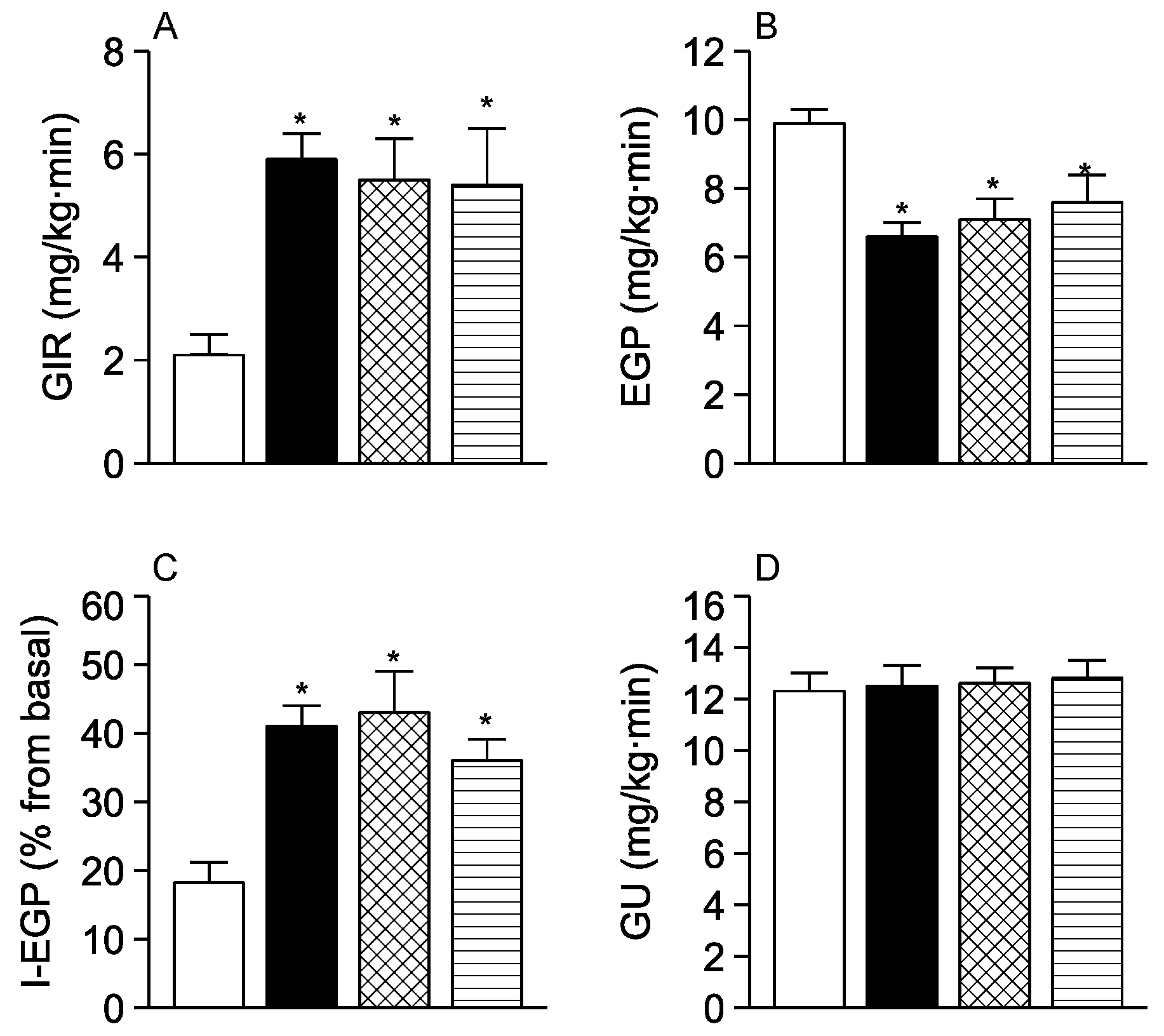
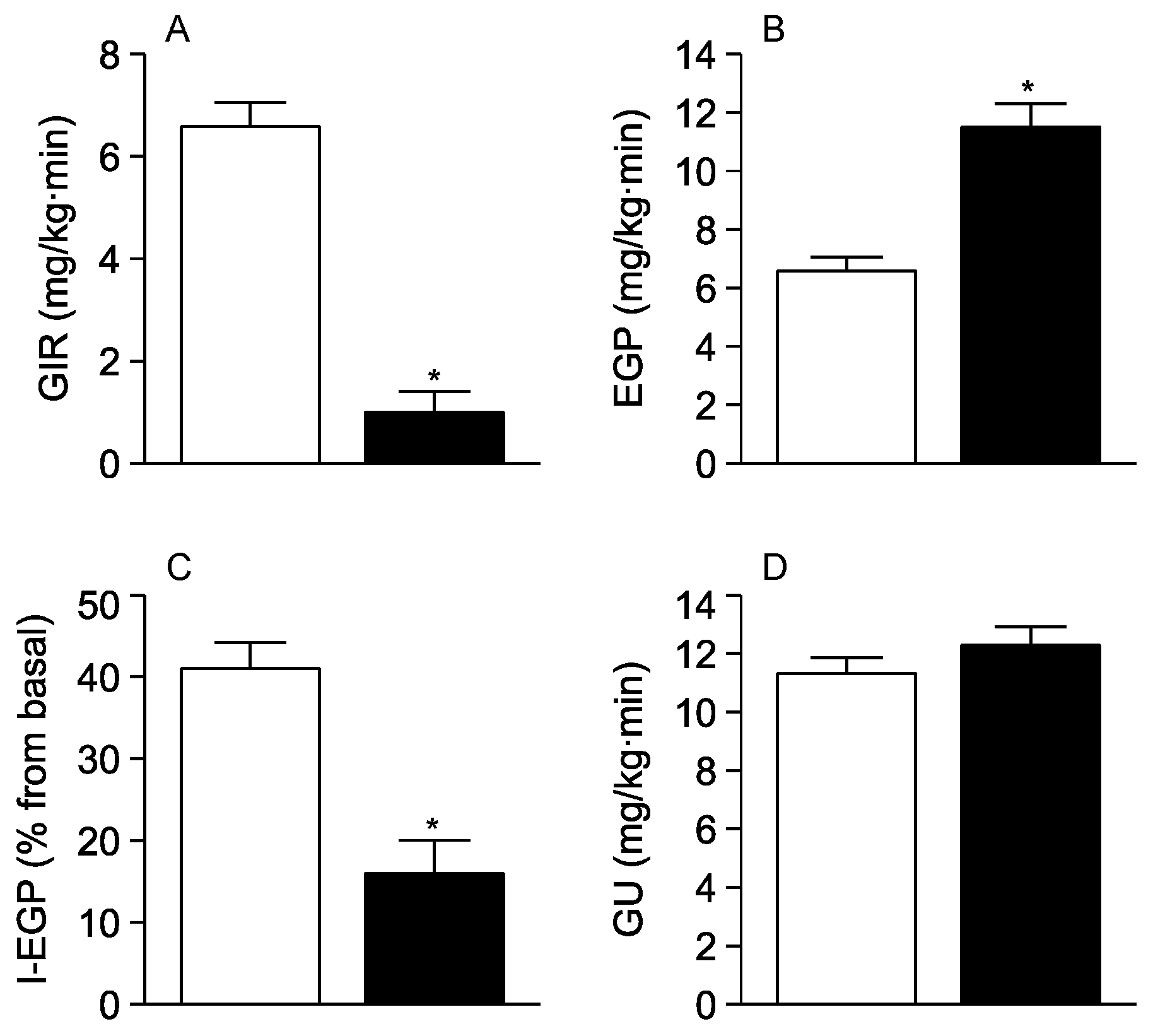
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eaton, S.B.; Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Peters, J.C. Environmental contributions to the obesity epidemic. Science 1998, 280, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Ukropec, J.; Sebokova, E.; Klimes, I. Nutrient sensing, leptin and insulin action. Arch. Physiol. Biochem. 2001, 109, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Obici, S.; Rossetti, L. Minireview: Nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 2003, 144, 5172–5178. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, J.E.; Rutter, J. Nutrient sensing and metabolic decisions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J. Biology of eating behavior in obesity. Obes. Res. 2004, 12 (Suppl. 2), 102S–106S. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Rothman, D.L.; DeFronzo, R.A.; Shulman, G.I. Effect of dietary protein on in vivo insulin action and liver glycogen repletion. Am. J. Physiol. 1989, 257, E212–E219. [Google Scholar] [PubMed]
- Patti, M.E.; Brambilla, E.; Luzi, L.; Landaker, E.J.; Kahn, C.R. Bidirectional modulation of insulin action by amino acids. J. Clin. Investig. 1998, 101, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Saeed, A.; Jordan, K.; Hoover, H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 734–741. [Google Scholar] [PubMed]
- Nuttall, F.Q.; Gannon, M.C.; Saeed, A.; Jordan, K.; Hoover, H. The metabolic response of subjects with type 2 diabetes to a high-protein, weight-maintenance diet. J. Clin. Endocrinol. Metab. 2003, 88, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Krebs, M.; Dombrowski, L.; Brehm, A.; Bernroider, E.; Roth, E.; Nowotny, P.; Waldhausl, W.; Marette, A.; Roden, M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005, 54, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Promintzer, M.; Krebs, M. Effects of dietary protein on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Blouet, C.; Jo, Y.H.; Li, X.; Schwartz, G.J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 2009, 29, 8302–8311. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lam, T.K.; He, W.; Pocai, A.; Bryan, J.; Aguilar-Bryan, L.; Gutierrez-Juarez, R. Hypothalamic leucine metabolism regulates liver glucose production. Diabetes 2012, 61, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Cruz, I.; Su, Y.; Knight, C.M.; Lam, T.K.T.; Gutiérrez-Juárez, R. Evidence for a role of proline and hypothalamic astrocytes in the regulation of glucose metabolism in rats. Diabetes 2013, 62, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Hawes, J.W.; Harris, R.A.; Shimomura, Y.; Jenkins, A.E.; Hutson, S.M. A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 1998, 68, 72–81. [Google Scholar] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Branched-chain amino acids: Enzyme and substrate regulation. J. Nutr. 2006, 136, 207S–211S. [Google Scholar] [PubMed]
- Morgan, K.; Obici, S.; Rossetti, L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J. Biol. Chem. 2004, 279, 31139–31148. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Gutierrez-Juarez, R.; Pocai, A.; Rossetti, L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 2005, 309, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Pocai, A.; Lam, T.K.; Obici, S.; Gutierrez-Juarez, R.; Muse, E.D.; Arduini, A.; Rossetti, L. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J. Clin. Investig. 2006, 116, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Gutierrez-Juarez, R.; Pocai, A.; Bhanot, S.; Tso, P.; Schwartz, G.J.; Rossetti, L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat. Med. 2007, 13, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Gutierrez-Juarez, R.; Obici, S.; Rossetti, L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J. Biol. Chem. 2004, 279, 49704–49715. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Karkanias, G.B.; Morales, J.C.; Hawkins, M.; Barzilai, N.; Wang, J.; Rossetti, L. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J. Biol. Chem. 1998, 273, 31160–31167. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; Clark, P.W.; Jenkins, A.B.; Daley, E.A.; Chisholm, D.J.; Storlien, L.H. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 1991, 40, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Obici, S.; Morgan, K.; Barzilai, N.; Feng, Z.; Rossetti, L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 2001, 50, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Nakamura, Y.; Inaba, Y.; Matsumoto, M.; Kido, Y.; Asahara, S.I.; Matsuda, T.; Watanabe, H.; Maeda, A.; Inagaki, F.; et al. Histidine augments the suppression of hepatic glucose production by central insulin action. Diabetes 2013, 62, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Yu, Y.H.; Hou, J.; Zhang, Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr. Metab. (Lond.) 2010, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Reid, T.M.; Bronson, S.K.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutson, S.M. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007, 6, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta-Cruz, I.; Su, Y.; Gutiérrez-Juárez, R. Suppression of Endogenous Glucose Production by Isoleucine and Valine and Impact of Diet Composition. Nutrients 2016, 8, 79. https://doi.org/10.3390/nu8020079
Arrieta-Cruz I, Su Y, Gutiérrez-Juárez R. Suppression of Endogenous Glucose Production by Isoleucine and Valine and Impact of Diet Composition. Nutrients. 2016; 8(2):79. https://doi.org/10.3390/nu8020079
Chicago/Turabian StyleArrieta-Cruz, Isabel, Ya Su, and Roger Gutiérrez-Juárez. 2016. "Suppression of Endogenous Glucose Production by Isoleucine and Valine and Impact of Diet Composition" Nutrients 8, no. 2: 79. https://doi.org/10.3390/nu8020079
APA StyleArrieta-Cruz, I., Su, Y., & Gutiérrez-Juárez, R. (2016). Suppression of Endogenous Glucose Production by Isoleucine and Valine and Impact of Diet Composition. Nutrients, 8(2), 79. https://doi.org/10.3390/nu8020079



