Dietary Fiber Intake is Associated with Increased Colonic Mucosal GPR43+ Polymorphonuclear Infiltration in Active Crohn’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Ethical Considerations
| Group | N = | Activity of disease | Type of surgery | Enteral nutrition before surgery |
|---|---|---|---|---|
| ac-food | 7 | active | emergency | Normal food |
| ac-EN | 10 | active | selective | Peptison |
| ac-DF | 9 | active | selective | Nutrison |
| re-EN | 10 | remission | selective | Peptison |
| re-DF | 10 | remission | selective | Nutrison |
2.2. Preparation of Colon and White Blood Cells
2.3. Hematoxylin-Eosin Staining and Immunohistochemistry Staining
2.4. Western Blot
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Quantitative Real-Time Polymerase Chain Reaction Analysis
| Primer | Sequence |
|---|---|
| IL-6 forward | ACTCACCTCTTCAGAACGAATTG |
| IL-6 reverse | CCATCTTTGGAAGGTTCAGGTTG |
| TNF-α forward | CCTCTCTCTAATCAGCCCTCTG |
| TNF-α reverse | GAGGACCTGGGAGTAGATGAG |
| GAPDH forward | AGGCCGGTGCTGAGTATGTC |
| GAPDH reverse | TGCCTGCTTCACCACCTTCT |
2.7. Statistical Analysis
3. Results
3.1. Clinical Information
| Groups | can. | ac-food # | ac-EN | ac-DF | re-EN | re-DF |
|---|---|---|---|---|---|---|
| Female/Male | 5/5 | 2/5 | 7/3 | 4/5 | 4/6 | 6/4 |
| Age, years * | 48.6 ± 13.1 a | 34.2 ± 7.2 b | 28.4 ± 5.9 b | 27.8 ± 5.8 b | 35.6 ± 11.1 b | 30.0 ± 7.6 b |
| CRP, mg/L * | - | 74.0 ± 19.9 a | 38.0 ± 8.9 b | 36.2 ± 7.4 b | 11.2 ± 3.1 c | 11.6 ± 4.9 c |
| CDAI * | - | 365 ± 41 a | 237 ± 70 b | 215 ± 36 b | 113 ± 26 c | 106 ± 11 c |
| Medication history | ||||||
| Corticosteroid, n | - | 3 | 10 | 9 | 9 | 10 |
| 5-aminosalicylates, n | - | 5 | 10 | 9 | 8 | 9 |
| Immunosuppressive drugs or infliximab, n | - | 3 | 6 | 7 | 10 | 9 |
3.2. Histological Features
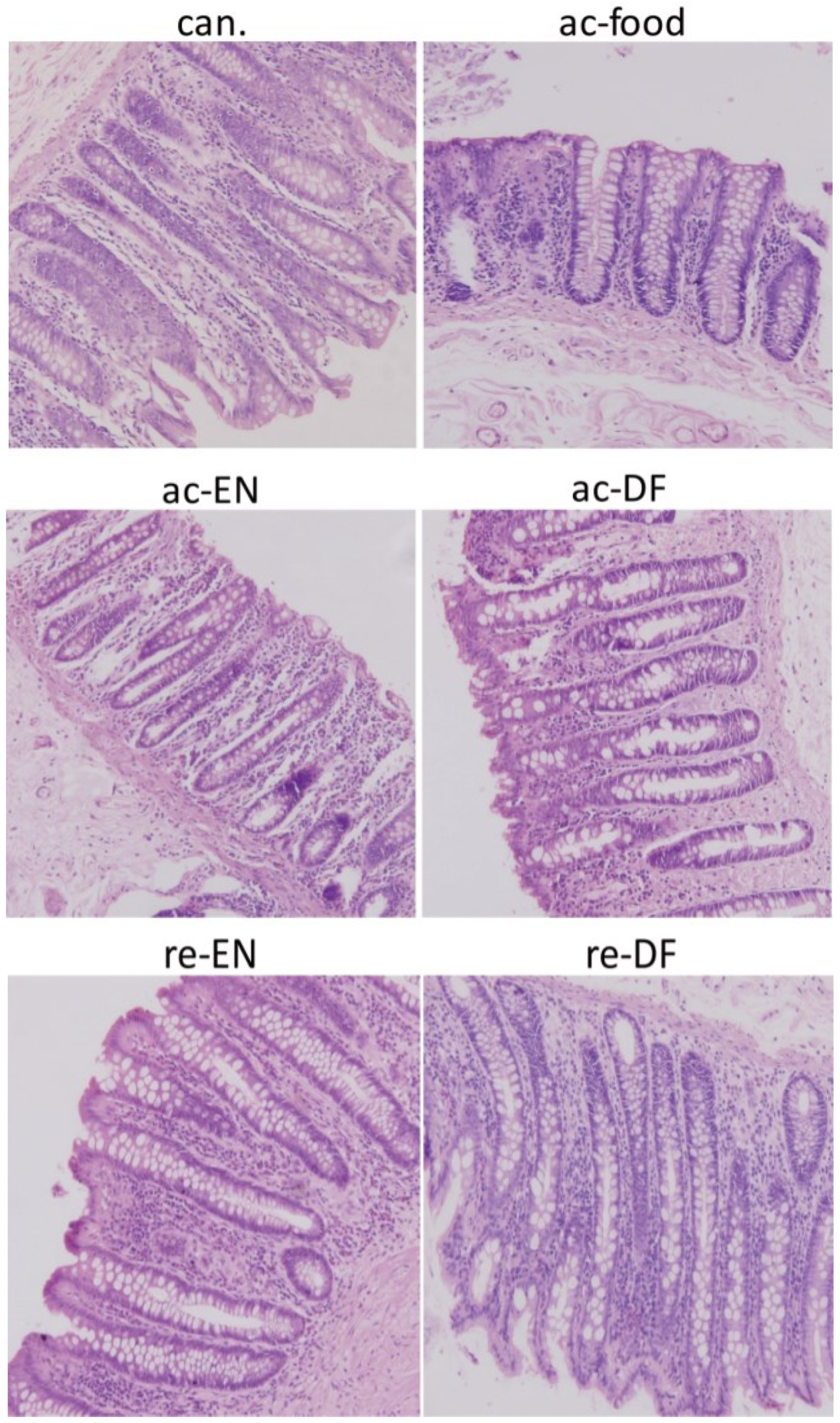

3.3. Cytokines

3.4. Expression and Location of GPR43

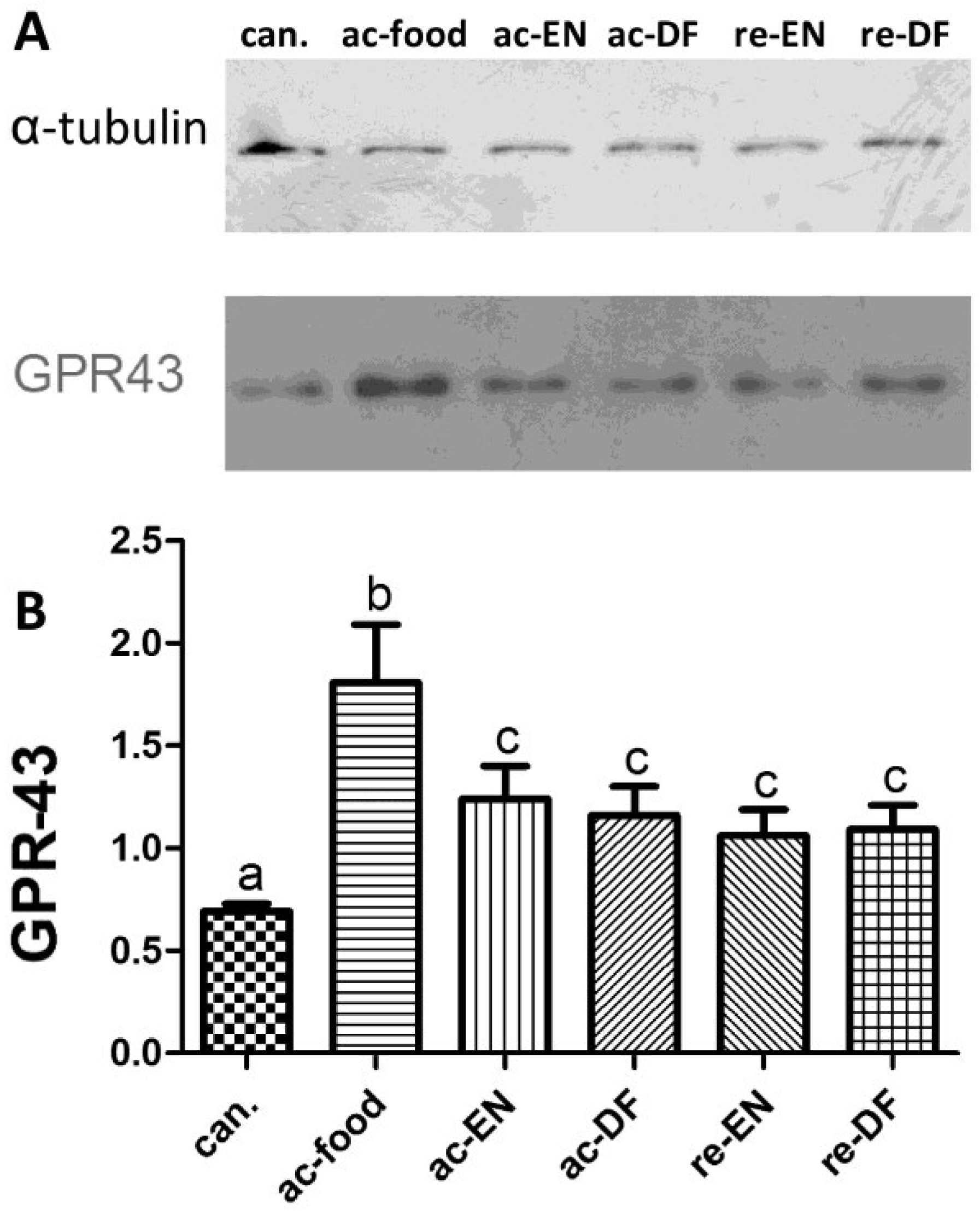

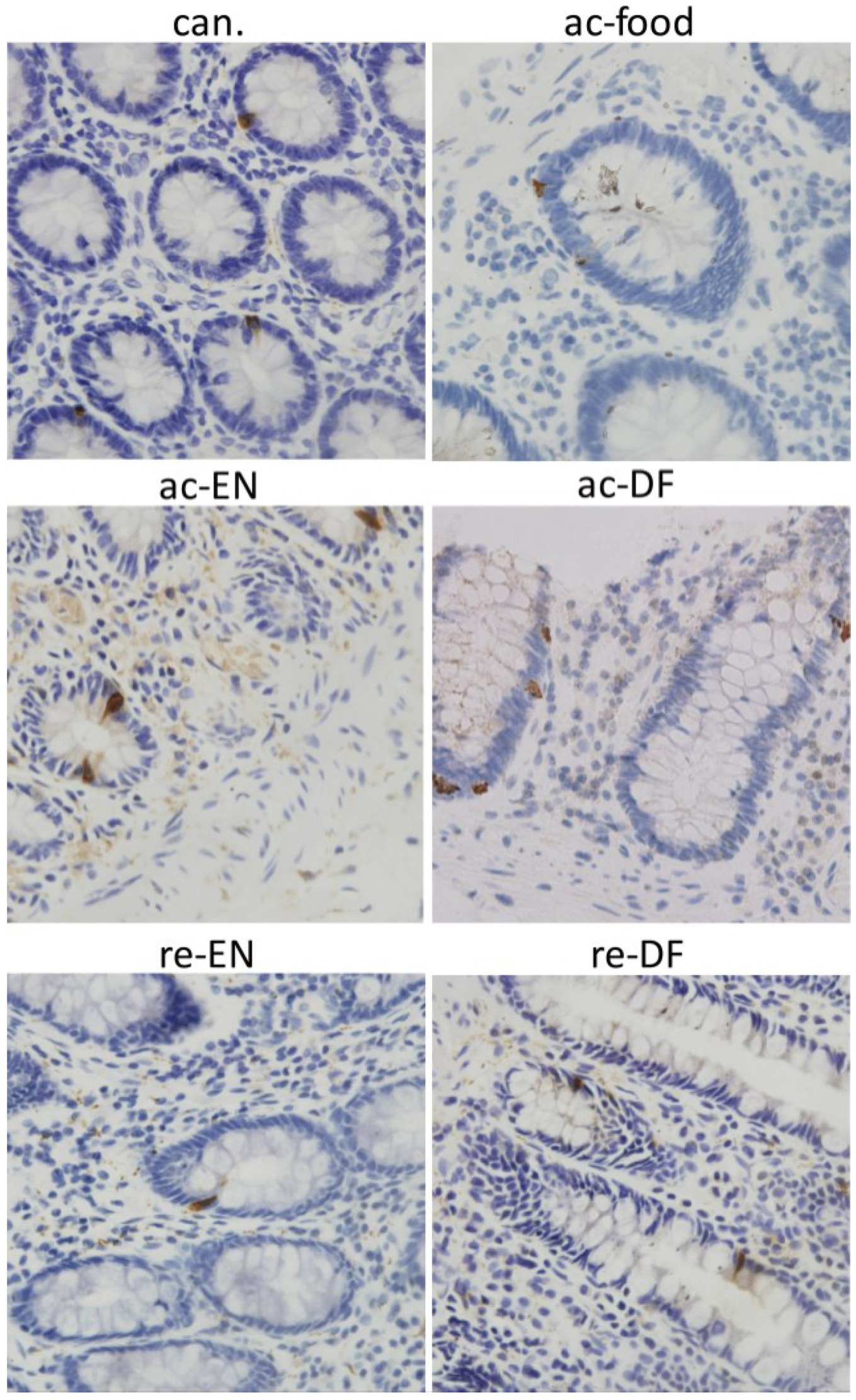
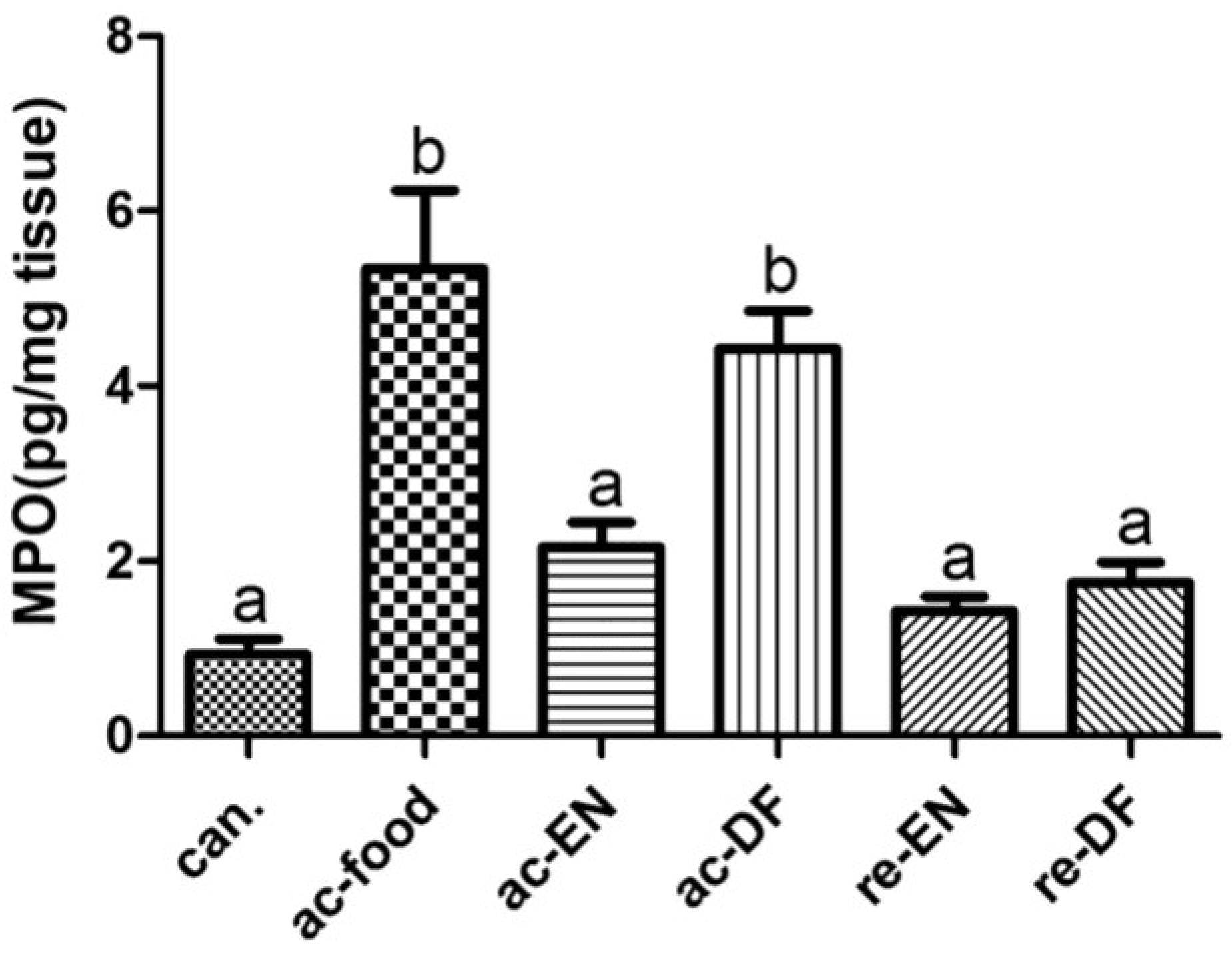
3.5. Expression of MPO
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ford, W.D.; Boelhouwer, R.U.; King, W.W.; de Vries, J.E.; Ross, J.S.; Malt, R.A. Total parenteral nutrition inhibits intestinal adaptive hyperplasia in young rats: Reversal by feeding. Surgery 1984, 96, 527–534. [Google Scholar] [PubMed]
- Jacobs, L.R.; Lupton, J.R. Effect of dietary fibers on rat large bowel mucosal growth and cell proliferation. Am. J. Physiol. 1984, 246, G378–385. [Google Scholar] [PubMed]
- Harig, J.M.; Soergel, K.H.; Komorowski, R.A.; Wood, C.M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N. Engl. J. Med. 1989, 320, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, R.A. Histologic spectrum of diversion colitis. Am. J. Surg. Pathol. 1990, 14, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mao, X.; He, J.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Chen, D. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Kaldma, M.; Petersson, B.G. Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand. J. Gastroenterol. 1991, 26, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Banares, F.; Hinojosa, J.; Sanchez-Lombrana, J.L.; Navarro, E.; Martínez-Salmerón, J.F.; García-Pugés, A.; González-Huix, F.; Riera, J.; González-Lara, V.; Domínguez-Abascal, F.; et al. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Am. J. Gastroenterol. 1999, 94, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Galvez, J.; Rodriguez-Cabezas, M.E.; Zarzuelo, A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Sawzdargo, M.; George, S.R.; Nguyen, T.; Xu, S.; Kolakowski, L.F.; O’Dowd, B.F. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene onchromosome19q13.1. Biochem. Biophys. Res. Commun. 1997, 239, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N.M. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ulven, T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. (Lausanne) 2012, 3, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermanowicz, A.; Gibson, P.R.; Jewell, D.P. The role of phagocytes in inflammatory bowel disease. Clin. Sci. (Lond.) 1985, 69, 241–249. [Google Scholar] [PubMed]
- Hayee, B.; Rahman, F.Z.; Sewell, G.; Smith, A.M.; Segal, A.W. Crohn’s disease as an immunodeficiency. Expert Rev. Clin. Immunol. 2010, 6, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Hayee, B.H.; Rahman, F.Z.; Tempero, J.; McCartney, S.; Bloom, S.L.; Segal, A.W.; Smith, A.M. The neutrophil respiratory burst and bacterial digestion in Crohn’s disease. Dig. Dis. Sci. 2011, 56, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W.; Loewi, G. Neutrophil dysfunction in Crohn’s disease. Lancet 1976, 2, 219–221. [Google Scholar] [CrossRef]
- Marks, D.J.B.; Radulovic, M.; McCartney, S.; Bloom, S.; Segal, A.W. Modified skin window technique for the extended characterisation of acute inflammation in humans. Inflamm. Res. 2007, 56, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.J.B.; Harbord, M.W.N.; MacAllister, R.; Rahman, F.Z.; Young, J.; Al-Lazikani, B.; Lees, W.; Novelli, M.; Bloom, S.; Segal, A.W. Defective acute inflammation in Crohn’s disease: A clinical investigation. Lancet 2006, 367, 668–678. [Google Scholar] [CrossRef]
- Smith, A.M.; Rahman, F.Z.; Hayee, B.H.; Graham, S.J.; Marks, D.J.; Sewell, G.W.; Palmer, C.D.; Wilde, J.; Foxwell, B.M.; Gloger, I.S.; et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J. Exp. Med. 2009, 206, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Sina, C.; Gavrilova, O.; Forster, M.; Till, A.; Derer, S.; Hildebrand, F.; Raabe, B.; Chalaris, A.; Scheller, J.; Rehmann, A.; et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009, 183, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Ferguson, G.J.; Kulkarni, S.; Damoulakis, G.; Anderson, K.; Bohlooly-Y, M.; Stephens, L.; Hawkins, P.T.; Curi, R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE 2011, 6, e21205. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Masui, R.; Sasaki, M.; Funaki, Y.; Ogasawara, N.; Mizuno, M.; Iida, A.; Ogasawara, N.; Mizuno, M.; Iida, A.; Izawa, S.; et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm. Bowel Dis. 2013, 19, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.; Tazoe, H.; Hayashi, H.; Kashiwabara, H.; Tooyama, K.; Suzuki, Y.; Kuwahara, A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 2008, 39, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Sartory, N.; Zahn, N.; Geisslinger, G.; Radeke, H.H.; Stein, J.M. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4 + CD25 + regulatory T cells. J. Immunol. 2007, 178, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhao, D.H.; Jiang, M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int. J. Mol. Med. 2012, 29, 202–208. [Google Scholar] [PubMed]
- Jenkins, D.; Balsitis, M.; Gallivan, S.; Dixon, M.F.; Gilmour, H.M.; Shepherd, N.A.; Theodossi, A.; Williams, G.T. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. J. Clin. Pathol. 1997, 50, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.M.; Paintin, M.; Arnaud-Battandier, F.; Beattie, R.M.; Hollis, A.; Kitching, P.; Donnet-Hughes, A.; MacDonald, T.T.; Walker-Smith, J.A. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2000, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Maathuis, A.J.; Venema, K.; Michael, R.L.; Roland, J.G.; Wood, S. Effect of the novel polysaccharide PolyGlycopleX® on short-chain fatty acid production in a computer-controlled in vitro model of the human large intestine. Nutrients 2014, 6, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Bamias, G.; Rivera-Nieves, J.; Moskaluk, C.A.; Hoang, S.B.; Ross, W.G.; Pizarro, T.T.; Cominelli, F. TNF-α neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8366–8371. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Nalle, S.C.; Shen, L.; Singh, G.; Breskin, L.A.; Khramtsova, E.A.; Khramtsova, G.; Tsai, P.Y.; Fu, Y.X.; Abraham, C.; et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013, 145, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Nasef, N.A.; Mehta, S.; Murray, P.; Marlow, G.; Ferguson, L.R. Anti-inflammatory activity of fruit fractions in vitro, mediated through toll-like receptor 4 and 2 in the context of inflammatory bowel disease. Nutrients 2014, 6, 5265–5279. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Hurtado, I.; Santacruz, A.; Peiro, G.; Zapater, P.; Gutiérrez, A.; Pérez-Mateo, M.; Sanz, Y.; Francés, R. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PLoS ONE 2011, 6, e23037. [Google Scholar] [CrossRef] [PubMed]
- Vinh, D.C.; Behr, M.A. Crohn’s as an immune deficiency: From apparent paradox to evolving paradigm. Expert Rev. Clin. Immunol. 2013, 9, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.P.; Segal, A.W. What is wrong with granulocytes in inflammatory bowel diseases? Dig. Dis. 2013, 31, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.J.B.; Miyagi, K.; Rahman, F.Z.; Novelli, M.; Bloom, S.L.; Segal, A.W. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 117–124. [Google Scholar] [CrossRef] [PubMed]
- O’Morain, C.A.; Segal, A.W.; Walker, D.; Levi, A.J. Abnormalities of neutrophil function do not cause the migration defect in Crohn’s disease. Gut 1981, 22, 817–822. [Google Scholar]
- Sewell, G.W.; Marks, D.J.; Segal, A.W. The immunopathogenesis of Crohn’s disease: A three-stage model. Curr. Opin. Immunol. 2009, 21, 506–513. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhu, W.; Gong, J.; Zuo, L.; Zhao, J.; Sun, J.; Li, N.; Li, J. Dietary Fiber Intake is Associated with Increased Colonic Mucosal GPR43+ Polymorphonuclear Infiltration in Active Crohn’s Disease. Nutrients 2015, 7, 5327-5346. https://doi.org/10.3390/nu7075223
Zhao M, Zhu W, Gong J, Zuo L, Zhao J, Sun J, Li N, Li J. Dietary Fiber Intake is Associated with Increased Colonic Mucosal GPR43+ Polymorphonuclear Infiltration in Active Crohn’s Disease. Nutrients. 2015; 7(7):5327-5346. https://doi.org/10.3390/nu7075223
Chicago/Turabian StyleZhao, Mingli, Weiming Zhu, Jianfeng Gong, Lugen Zuo, Jie Zhao, Jing Sun, Ning Li, and Jieshou Li. 2015. "Dietary Fiber Intake is Associated with Increased Colonic Mucosal GPR43+ Polymorphonuclear Infiltration in Active Crohn’s Disease" Nutrients 7, no. 7: 5327-5346. https://doi.org/10.3390/nu7075223




