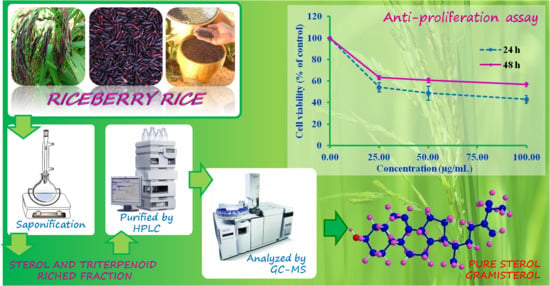
Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Extraction and Saponification
2.4. Fractionation and Purification of Sterols and Triterpenoids
2.5. GC-MS and LC-MS Analysis
2.6. NMR Characterization
2.7. Bioassay of the Rice Bran Sub-Fractions
3. Results
3.1. Sterols and Triterpenoids Found in Unsaponified Fraction and Sub-Fractions of the Rice Bran Extract
| Compounds | Yield mg/kg Bran | Percent (%) in Extract | Yield mg/kg Bran | Percent (%) in Fraction | Yield mg/kg Bran | Percent (%) in Fraction |
|---|---|---|---|---|---|---|
| RBD | RBDS | RBDS1 | ||||
| Sterols | ||||||
| 24-Methylene-ergosta-5-en-3β-ol | 298.79 | 58.13 | ||||
| 24-Methylene-ergosta-7-en-3β-ol | 197.98 | 41.88 | ||||
| Fucosterol | ||||||
| Gramisterol | 187.44 | 9.86 | ||||
| Campesterol | 34.36 | 0.38 | 200.31 | 10.57 | ||
| Stigmasterol | 39.02 | 0.41 | 114.30 | 5.82 | ||
| β-Sitosterol | 80.79 | 0.93 | 377.26 | 20.35 | ||
| Triterpenoids | ||||||
| Cycloeucalenol | 56.43 | 2.62 | ||||
| Lupenone | ||||||
| Lupeol | ||||||
| 24-Methylenecycloartanol | 83.92 | 0.51 | 830.11 | 45.38 | ||
| RBDS2 | RBDS3 | RBDS4 | ||||
| Sterols | ||||||
| 24-Methylene-ergosta-5-en-3β-ol | ||||||
| 24-Methylene-ergosta-7-en-3β-ol | ||||||
| Fucosterol | 398.90 | 15.80 | ||||
| Gramisterol | 1336.32 | 56.60 | ||||
| Campesterol | 2939.71 | 71.15 | ||||
| Stigmasterol | 1198.00 | 28.85 | ||||
| β-Sitosterol | 3803.12 | 53.28 | ||||
| Triterpenoids | ||||||
| Cycloeucalenol | 184.68 | 7.75 | ||||
| Lupenone | 83.69 | 3.12 | ||||
| Lupeol | 96.32 | 3.34 | ||||
| 24-Methylenecycloartanol | 3417.02 | 46.72 | ||||
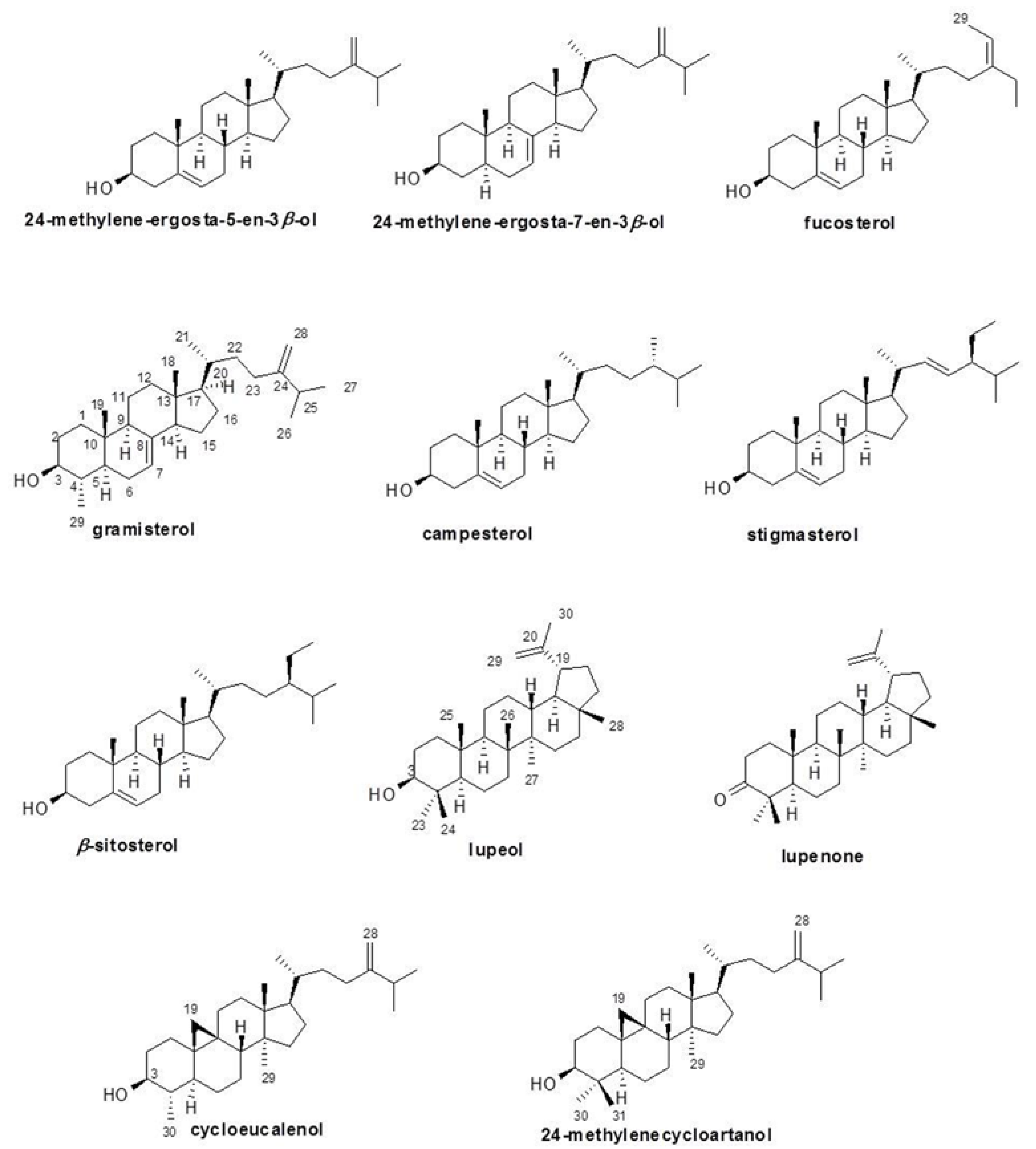
3.2. Identification and Characterization of the Black Rice Sterol and Triterpenoid Components
| Compound | Molecular Formula | Molecular Weight | Major Fragment Ion (m/z) | |||
|---|---|---|---|---|---|---|
| LC-APCI-MS | GC-MS | |||||
| Sterols | ||||||
| 24-Methylene-ergosta-5-en-3β-ol | C28H46O | 398 | 381 [M-H2O+H]+ | 398 [M]+, 383 [M-CH3]+ 365 [M-CH3-H2O]+ 314 [M-C5H9-CH3]+ 299 [M-C7H13-2H]+ 281 [M-C7H13-H2O-2H]+ 271 [M-side chain-2H] + | ||
| 24-Methylene-ergosta-7-en-3β-ol | C28H46O | 398 | 381 [M-H2O+H]+,399 [M+H]+ | 398 [M]+, 383 [M-CH3]+ 365 [M-CH3-H2O]+ 314 [M-C5H9-CH3]+ 299 [M-C7H13-2H]+ 271 [M-side chain-2H]+ | ||
| Fucosterol | C29H48O | 412 | 395 [M-H2O+H]+ | 412 [M]+, 397 [M-CH3]+ 379 [M-CH3-H2O]+ 314 [M-C6H11-CH3]+ 299 [M-C6H11-2CH3]+ 281 [M-C6H11-2CH3-H2O]+ 229 [M-side chain-ring D cleavage-CH3-2H]+ 213 [M-side chain-ring D cleavage-CH3-H2O]+ | ||
| Gramisterol | C29H48O | 412 | 395 [M-H2O+H]+ | 412 [M] +, 397 [M-CH3]+ 379 [M-CH3-H2O]+ 328 [M-C5H9-CH3]+ 285 [M-side chain-2H] + 269 [M- side chain -CH3-2H] + | ||
| Campesterol | C28H48O | 400 | 383 [M-H2O+H]+ | 400 [M]+, 385 [M-CH3]+ 382 [M-H2O]+ 367 [M-CH3-H2O]+ | ||
| Stigmasterol | C29H48O | 412 | 395 [M-H2O+H]+ | 412 [M]+, 397 [M-CH3]+ 394 [M-H2O]+ 369 [M-C3H5]+ 351 [M-C3H5-H2O]+ 314 [M-C7H14]+ 271 [M-side chain -2H]+ 255 [M-side chain-H2O]+ 213 [M-side chain-ring D cleavage-H2O]+ | ||
| β-Sitosterol | C29H50O | 414 | 397 [M-H2O+H]+ | 414 [M]+, 399 [M-CH3]+ 396 [M-H2O]+ 381 [M-CH3-H2O]+ 329 [M-C6H13]+ 303 [M-C7H11O]+ 273 [M-side chain]+ 255 [M-side chain -H2O]+ 231 [M-side chain-ring D cleavage-CH3]+ 213 [M-side chain-ring D cleavage-CH3-H2O]+ | ||
| Triterpenoids | ||||||
| Cycloeucalenol | C30H50O | 426 | 409 [M-H2O+H]+, 427 [M+H]+ | 426 [M]+, 411 [M-CH3]+ 408 [M-H2O]+ 393 [M-CH3-H2O]+ 353 [M-C3H7-2CH3-H2O]+ 300 [M-C7H13-CH2-CH3]+ | ||
| Lupenone | C30H48O | 424 | ND (minor compound) | 424 [M]+, 409 [M-CH3]+ 381 [M-side chain -2H]+ 368 [M-side chain-CH3]+ 342 [M-side chain-ring E cleavage]+ 313 [M-C7H11O]+ | ||
| Lupeol | C30H50O | 426 | ND (minor compound) | 426 [M]+, 411 [M-CH3]+ 393 [M-CH3-H2O]+ 383 [M-side chain-2H]+ 370 [M-side chain -CH3]+ 329 [M-side chain-ring E clevage-CH3]+ | ||
| 24-Methylenecycloartanol | C31H52O | 440 | 423 [M-H2O+H]+,441 [M+H]+ | 440 [M]+, 425 [M-CH3]+ 422 [M-H2O]+ 407 [M-CH3-H2O]+ 397 [M-C3H7]+ 379 [M-C3H7-H2O]+ 315 [M-C9H17]+ 300 [M-C9H17-CH3]+ 285 [M-C9H17-2CH3]+ 203 [M-C9H17-5CH3]+ | ||
| Compound | Selected 1H-NMR Data δ (Multiplicity/Hz) | Selected 13C-NMR Data δ |
|---|---|---|
| Sterols | ||
| 24-Methylene-ergosta-5-en-3β-ol | 0.68 (s, H18), 0.95 (d, 6.6, H21), 1.00 (s, H19), 1.02, 1.03 (d, 6.8, H26, H27), 3.52 (m, H3), 4.65 (s, H28), 4.71 (s, H28), 5.35 (d, 4.9, H6) | 11.86 (C18), 18.71 (C21), 19.40 (C19), 21.87, 22.00 (C26, C27), 71.82 (C3), 105.93 (C28), 121.72 (C6), 140.77 (C5) |
| 24-Methylene-ergosta-7-en-3β-ol | 0.54 (s, H18), 0.80 (s, H19), 0.94 (d, 6.6, H21), 1.02, 1.03 (d, 6.8, H26, H27), 3.62 (m, H3), 4.65 (s, H28), 4.71 (s, H28), 5.15 (d, 2.3, H7) | 11.86 (C18), 13.04 (C19), 18.84 (C21), 21.87, 22.00 (C26, C27), 71.08 (C3), 105.93 (C28), 120.00 (C7), 139.57 (C8) |
| Fucosterol | 0.68 (s, H18), 0.98 (d, 6.4, H21), 1.01, 1.02 (d, 6.8, H26, H27), 1.00 (s, H19), 1.57(d, 7.2, H29), 3.53 (m, H3), 5.18 (q, 6.7, H28), 5.35 (d, 5.2, H6). | 11.85 (C18), 13.05 (C29), 18.81 (C21), 19.40 (C19), 21.87 (C27), 22.01 (C26), 71.82 (C3), 116.46 (C28), 121.72 (C6), 140.77 (C5), 146.80 (C24). |
| Gramisterol | 0.54 (s, H18), 0.79 (s, H19), 0.89 (d, 6.5, H21), 0.95 (d, 6.4, H29), 1.01, 1.02 (d, 6.8, H26, H27), 3.11 (dt, 4.3, 10.9, H3), 4.65 (s, H28), 4.71 (s, H28), 5.12 (d, 3.9, H7). | 11.85 (C18), 14.14 (C19), 15.15 (C29), 18.84 (C21), 21.37 (C11), 21.87 (C27), 22.01 (C26), 76.23 (C3), 105.95 (C28), 117.51 (C7), 139.10 (C8), 156.87 (C24). |
| Campesterol | 0.67 (s, H18), 0.77 (d, 6.6, H28), 0.79 (d, 6.8, H27), 0.84 (d, 6.8, H26), 0.90 (d, 6.6, H21), 1.00 (s, H19), 3.51 (m, H3), 5.33 (d, 5.2, H6). | 11.87 (C18), 15.38 (C28), 18.26 (C27), 18.71 (C21), 19.40 (C19), 20.21 (C26), 71.81 (C3), 121.72 (C6), 140.77 (C5). |
| Stigmasterol | 0.69 (s, H18), 0.76 (d, 7.8, H29), 0.80 (d, 6.8, H27), 0.85 (d, 6.8, H26), 1.00 (s, H19), 1.01 (d, 6.6, H21), 3.51 (m, H3), 5.00, 5.15 (dd, 8.6, 5.2, H22, H23), 5.33 (d, 5.2, H6). | 12.05 (C18), 12.25 (C27), 15.15 (C29), 18.90 (C21), 19.40 (C19), 20.52(C26), 71.81 (C3), 121.72 (C6), 129.29, 138.32 (C22, C23), 140.77 (C5). |
| β-Sitosterol | 0.67 (s, H18), 0.80, 0.82 (d, 6.8, H26, H27), 0.84 (d, 7.8, H29), 0.92 (d, 6.6, H21), 1.00 (s, H19), 3.52 (m, H3), 5.35 (d, 5.2, H6). | 11.87 (C18), 11.99 (C29), 18.79 (C21), 19.05, 19.83 (C26, C27), 19.41 (C19), 70.79 (C3), 121.72 (C6), 140.77 (C5). |
| Triterpenoids | ||
| Cycloeucalenol | 0.15 (d, H19), 0.38 (d, H19), 3.20 (m, H3), 4.64 (s, H28), 4.73 (s, H28). | ND (minor compound) |
| Lupenone | 4.57 (m, H29), 4.68 (d, 2.2, H29) | ND (minor compound) |
| Lupeol | 3.20 (m, H3), 4.57 (m, H29), 4.68 (d, 2.2, H29) | ND (minor compound) |
| 24-Methylenecycloartanol | 0.33 (d, 4.2, H19), 0.55 (d, 4.2, H19), 0.81 (s, H18), 0.89 (d, 4.3, H21), 0.90 (s, H29), 0.97 (s, H30, H31), 1.02 (d, 2.2, H26), 1.04 (d, 2.2, H27), 3.28 (dd, 4.3, 11.1, H3), 4.66 (s, H28), 4.71 (s, H28). | 14.15, 18.04, 18.32, 19.33, 21.88, 22.01, 25.24 (7Me), 29.72 (C19), 78.85 (C3), 105.94 (C28), 156.92 (C24). |
3.3. Anti-Proliferation (MTT) Activity
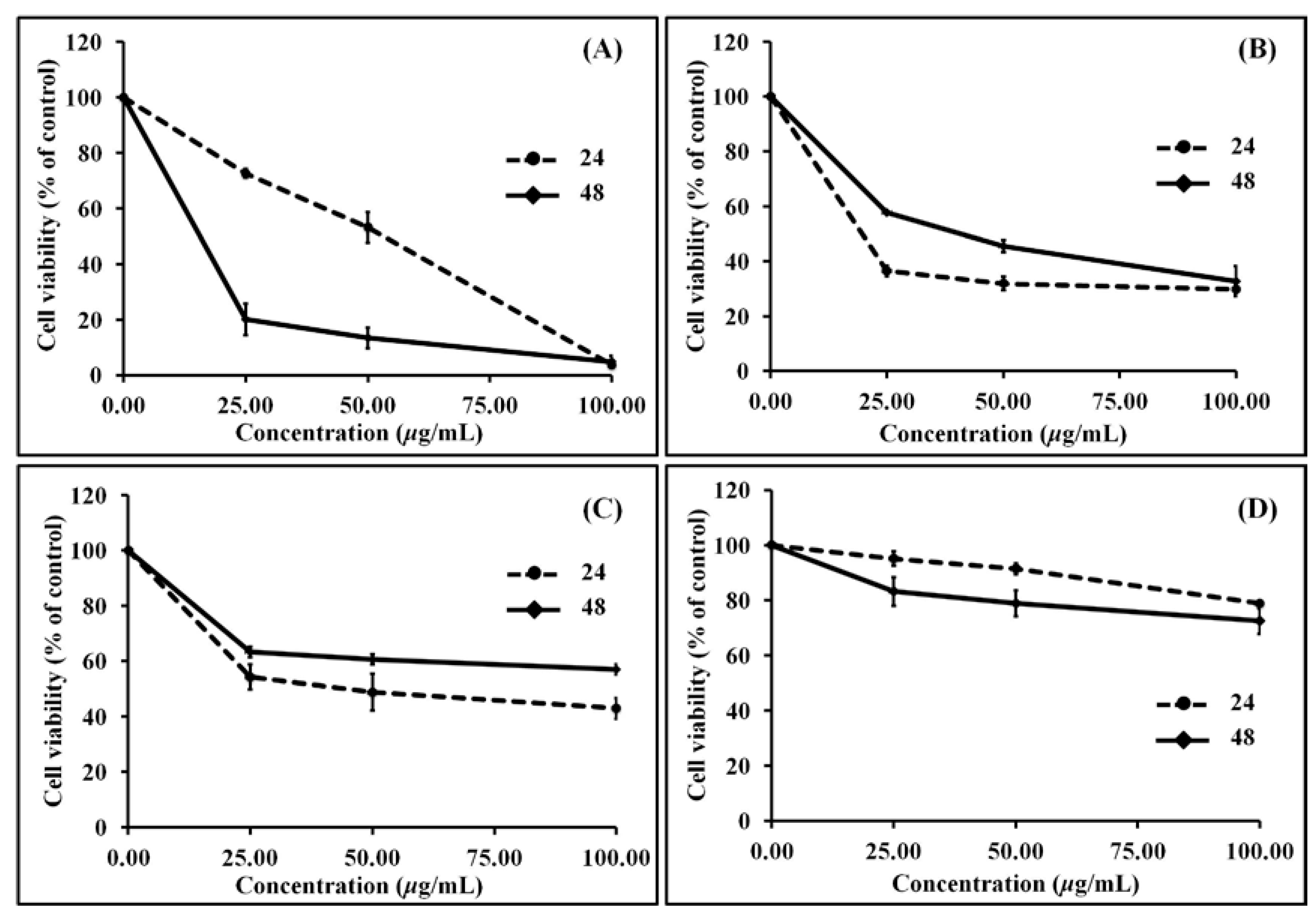
| Extracts | IC50 WEHI-3 (μg/mL) | |
|---|---|---|
| 24 h | 48 h | |
| RBDS1 | 32.89 | 3.32 |
| RBDS2 | 2.80 | 36.30 |
| RBDS3 | 47.59 | 376.87 |
| RBDS4 | 467.11 | 615.24 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S.; Suda, I.; Sato, T. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J. Agric. Food Chem. 2002, 50, 7524–7529. [Google Scholar]
- Pitija, K.; Nakornriab, M.; Sriseadka, T.; Vanavichit, A.; Wongpornchai, S. Anthocyanin content and antioxidant capacity in bran extracts of some Thai black rice varieties. Int. J. Food Sci. Tech. 2013, 48, 300–308. [Google Scholar]
- Sriseadka, T.; Wongpornchai, S.; Rayanakorn, M. Quantification of flavonoids in black rice by liquid chromatography-negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2012, 60, 11723–11732. [Google Scholar]
- Sookwong, P.; Nakagawa, K.; Murata, K.; Kojima, Y.; Miyazawa, T. Quantitation of tocotrienol and tocopherol in various rice brans. J. Agric. Food Chem. 2007, 55, 461–466. [Google Scholar]
- Pereira-Caro, G.; Watanabe, S.; Crozier, A.; Fujimura, T.; Yokota, T.; Ashihara, H. Phytochemical profile of a Japanese black-purple rice. Food Chem. 2013, 141, 2821–2827. [Google Scholar]
- Afinisha Deepam, L.S.; Sundaresan, A.; Arumughan, C. Stability of rice bran oil in terms of oryzanol, tocopherols, tocotrienols and sterols. J. Am. Oil. Chem. Soc. 2011, 88, 1001–1009. [Google Scholar]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar]
- Choi, S.P.; Kang, M.Y.; Koh, H.J.; Nam, S.H.; Friedman, M. Antiallergic activities of pigmented rice bran extracts in cell assays. J. Food Sci. 2007, 72, 719–726. [Google Scholar]
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Kozukue, N.; Friedman, M. Antioxidative, antimutagenic, and anticarcinogenic activities of rice bran extracts in chemical and cell assays. J. Agric. Food Chem. 2005, 53, 816–822. [Google Scholar]
- Leardkamolkarn, V.; Thongthep, W.; Suttiarporn, P.; Kongkachuichai, R.; Wongpornchai, S.; Wanavijitr, A. Chemopreventive properties of the bran extracted from a newly-developed Thai rice: The Riceberry. Food Chem. 2011, 125, 978–985. [Google Scholar]
- Prangthip, P.; Surasiang, R.; Charoensiri, R.; Leardkamolkarn, V.; Komindr, S.; Yamborisut, U.; Vanavichit, A.; Kongkachuichai, R. Amelioration of hyperglycemia, hyperlipidemia, oxidative stress and inflammation in steptozotocin-induced diabetic rats fed a high fat diet by Riceberry supplement. J. Funct. Food. 2013, 5, 195–203. [Google Scholar]
- Posuwan, J.; Prangthip, P.; Leardkamolkarn, V.; Yamborisut, U.; Surasiang, R.; Charoensiri, R.; Kongkachuichai, R. Long-term supplementation of high pigmented rice bran oil (Oryza sativa L.) on amelioration of oxidative stress and histological changes in streptozotocin-induced diabetic rats fed a high fat diet; Riceberry bran oil. Food Chem. 2013, 138, 501–508. [Google Scholar]
- Ohta, G.; Shimizu, M. A new triterpenoid alcohol, 24-methylenecycloartanol, as its ferulate, from rice bran oil. Chem. Pharm. Bull (Tokyo) 1958, 6, 325–326. [Google Scholar]
- Saito, S.; Matsumoto, T. Arousal effect of plant triterpenes on the central nervous system. Ikagaku Oyo Kenkyu Zaidan Kenkyu Hokoku 1987, 5, 127–132. [Google Scholar]
- Akihisa, T.; Yasukawa, K.; Yamaura, M.; Ukiya, M.; Kimura, Y.; Shimizu, N.; Arai, K. Triterpene alcohol and sterol ferulates from rice bran and their anti-inflammatory effects. J. Agric. Food Chem. 2000, 48, 2313–2319. [Google Scholar]
- Kpoviessi, D.S.S.; Accrombessi, G.C.; Gbenou, J.D.; Gbaguidi, F.A.; Kossou, D.K.; Moudachirou, M.; Quetin-Leclercq, J. Cytotoxic activities of sterols and triterpenes identified by GC-MS in Justicia anselliana (NEES) T. anders active fractions and allelopathic effects on cowpea (Vigna unguiculata (L.) Walp) plant. J. Soc. Ouest-Afr. Chim. 2008, 13, 59–67. [Google Scholar]
- Vissers, M.N.; Zock, P.L.; Meijer, G.W.; Katan, M.B. Effect of plant sterols from rice bran oil and triterpene alcohols from sheanut oil on serum lipoprotein concentrations in humans. Am. J. Clin. Nutr . 2000, 72, 1510–1515. [Google Scholar]
- Abdul, M.S.N.; Shin, S.K.; Abdul, W.N.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1724. [Google Scholar]
- Ding, Y.; Nguyen, H.T.; Kim, S.I.; Kim, H.W.; Kim, Y.H. The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris. Bioorg. Med. Chem. Lett. 2009, 19, 3607–3610. [Google Scholar]
- Nguyen, A.T.; Malonne, H.; Duez, P.; Vanhaelen-Fastre, R.; Vanhaelen, M.; Fontaine, J. Cytotoxic constituents from Plumbago zeylanica. Fitoterapia 2004, 75, 500–504. [Google Scholar]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009, 80, 123–126. [Google Scholar]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar]
- Omoloye, A.A.; Vidal, S. Abundance of 24-methylenecholesterol in traditional African rice as an indicator of resistance to the African rice gall midge, Orseolia oryzivora Harris & Gagné. Entomol. Sci. 2007, 10, 249–257. [Google Scholar]
- Przybylski, R.; Klensporf-Pawlik, D.; Anwar, F.; Rudzinska, M. Lipid components of North American wild rice (Zizania palustris). J. Am. Oil Chem. Soc. 2009, 86, 553–559. [Google Scholar]
- Itoh, T.; Sakurai, S.; Tamura, T.; Matsumoto, T. Occurrence of 24(E)-ethylidene sterols in two solanaceae seed oils and rice bran oil. Lipids 1980, 15, 22–25. [Google Scholar]
- Lei, M. Rice bran oil extraction by supercritical carbon dioxide. Shipin Kexue (Beijing) 1993, 159, 43–45. [Google Scholar]
- Sadish, K.S.; Kumar, Y.; Khan, M.S.Y.; Anbu, J.; De, C.E. Antihistaminic, anticholinergic and antiviral activities of fucosterol from Turbinaria conoides (J.Agardh) kutzing. Pharmacologyonline 2009, 1, 1104–1112. [Google Scholar]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar]
- Kongkathip, N.; Dhumma-upakorn, P.; Kongkathip, B.; Chawananoraset, K.; Sangchomkaeo, P.; Hatthakitpanichakul, S. Study on cardiac contractility of cycloeucalenol and cycloeucalenone isolated from Tinospora crispa. J. Ethnopharmacol. 2002, 83, 95–99. [Google Scholar]
- Na, M.; Kim, B.Y.; Osada, H.; Ahn, J.S. Inhibition of protein tyrosine phosphatase 1B by lupeol and lupenone isolated from Sorbus commixta. J. Enzyme Inhib. Med. Chem. 2009, 24, 1056–1059. [Google Scholar]
- Wal, P.; Wal, A.; Sharma, G.; Rai, A.K. Biological activities of lupeol. Syst. Rev. Pharm. 2011, 2, 96–103. [Google Scholar]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Jenkins, A.L.; Connelly, P.W.; Jones, P.J.H.; Vuksam, V. The Garden of Eden-plant based diets, the genetic drive to conserve cholesterol and its implications for heart disease in the 21st century. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2003, 136, 141–151. [Google Scholar]
- Ostlund, R.E., Jr. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar]
- Cui, X.; Dai, Q.; Tseng, M.; Shu, X.O.; Gao, Y.T.; Zheng, W. Dietary patterns and breast cancer risk in the shanghai breast cancer study. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1443–1448. [Google Scholar]
- Heinemann, T.; Axtmann, G.; Vonbergmann, K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Invest. 1993, 23, 827–831. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice. Nutrients 2015, 7, 1672-1687. https://doi.org/10.3390/nu7031672
Suttiarporn P, Chumpolsri W, Mahatheeranont S, Luangkamin S, Teepsawang S, Leardkamolkarn V. Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice. Nutrients. 2015; 7(3):1672-1687. https://doi.org/10.3390/nu7031672
Chicago/Turabian StyleSuttiarporn, Panawan, Watcharapong Chumpolsri, Sugunya Mahatheeranont, Suwaporn Luangkamin, Somsuda Teepsawang, and Vijittra Leardkamolkarn. 2015. "Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice" Nutrients 7, no. 3: 1672-1687. https://doi.org/10.3390/nu7031672
APA StyleSuttiarporn, P., Chumpolsri, W., Mahatheeranont, S., Luangkamin, S., Teepsawang, S., & Leardkamolkarn, V. (2015). Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice. Nutrients, 7(3), 1672-1687. https://doi.org/10.3390/nu7031672