Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health
Abstract
:1. Introduction
2. Polyphenol Bioavailability and Bioactivity: A Complex Field of Research
2.1. Classification of Polyphenols and Their Dietary Sources
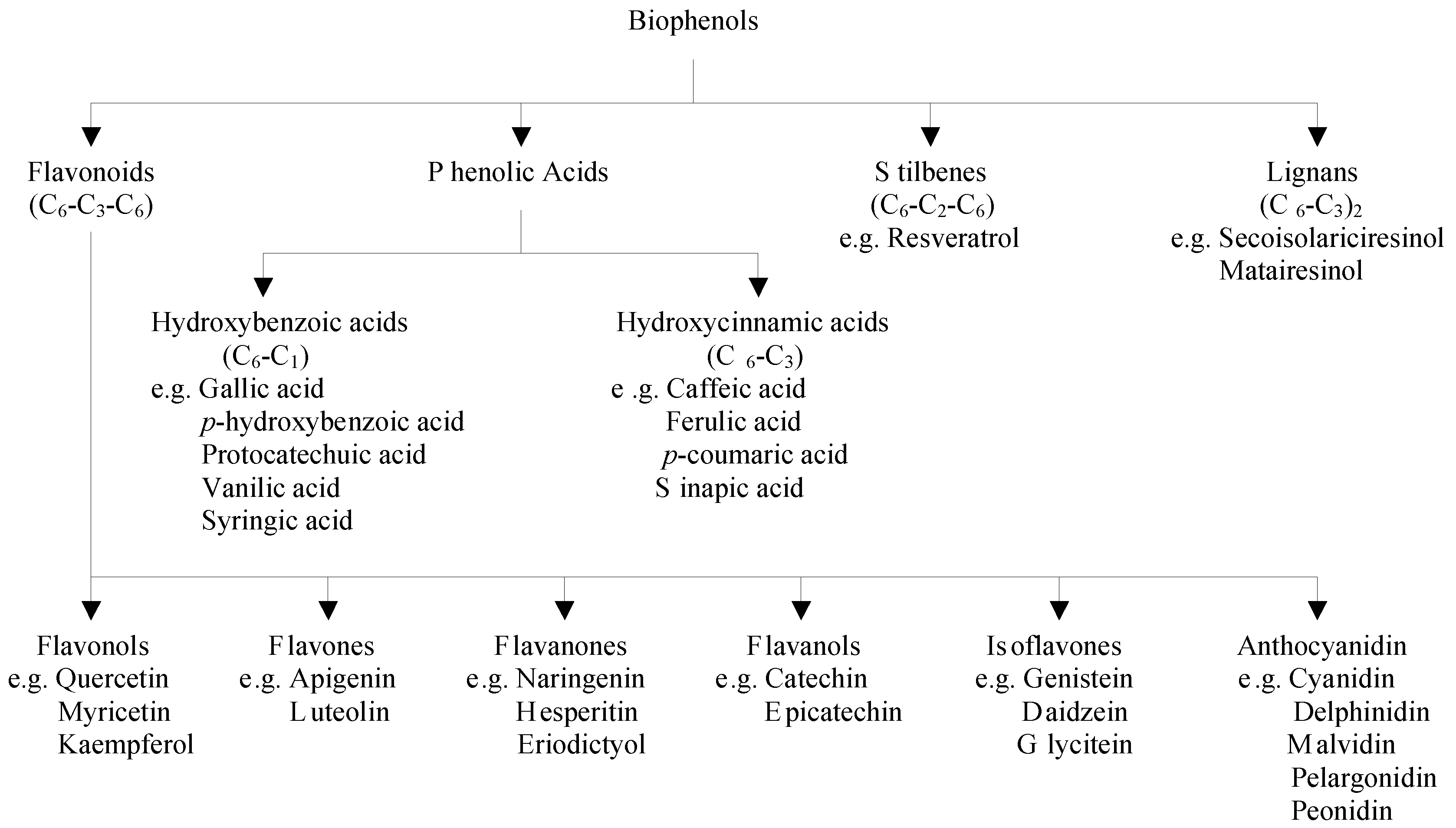
2.2. Factors Affecting the Bioavailability of Polyphenols
2.3. Protective Effects of Polyphenols Against Cardiovascular Diseases
2.4. Polyphenol Absorption and Biotransformation
3. Messenger Molecules in Cardiovascular Health Affected by Polyphenols
3.1. General Biomarkers of Cardiovascular Health
3.2. Signal Molecules Involved in the Regulation of Cardiovascular Health
| Biomarkers | Polyphenols | Cell Types | Ref. | |
|---|---|---|---|---|
| INTESTINE | ||||
| Transport | GLUT4, C36, FATP4 | Epigallocatechin | Rat intestinal tissue | [33] |
| Inflammatory markers | NF-kB, TNF-α, IL-1β, IL-6 | Apple peel polyphenols, Black tea polyphenols, Chrysin, Cinnamon polyphenols, Epicatechins, Epigallocatechin-3-gallate, Genistein, Grape seed polyphenols, Green tea polyphenols, Oak polyphenols, Pomegranate polyphenols, Resveratrol, Sugar cane polyphenols, Theaflavin | Caco-2/15, Caco-2, SW480, IEC6, isolated rat cells, HT-29 | [34,35,36,37,38,39,40,41,42] |
| Cholesterol | Cholesterol uptake | Grape seed polyphenols, Red wine polyphenol, chokeberry polyphenol | Caco-2, HT29, HuTu80 | [43,44] |
| ApoA-1, HDL | Isoquercetin, Quercetin | Caco-2 | [45] | |
| ADIPOSE TISSUE | ||||
| Energy storage | Lipid staining | Blueberry polyphenols, Chlorogenic acid, Cocoa polyphenols, Ellagic acid, Epigallocatechin-3-gallate, Episesamin, Fisetin, Hydroxytyrosol, Luteolin, Maysin, Oleuropein, Resveratrol, Rutin | 3T3-L1, 3T3-F442A, SGBS, hASC (human adipogenic stem cells) | [46,47,48,49,50,51,52,53,54,55,56,57] |
| GLUT-4, FASN4, HSL, FAS | Daidzein, Ellagic acid, Fisetin, Hydroxytyrosol, Naringenin, Oleuropein, Pycnogenol, Resveratrol, Sakuranetin | 3T3-L1, isolated human adipocytes, hASC | [46,48,57,58,59,60,61,62] | |
| PPAR-γ, LPL, aP2 | Apple polyphenols, Catechin, Chlorogenic acid, Cocoa polyphenols, Curcumin, Cyanidin-3-O-glucoside, Ellagic acid, Episesamin, Fisetin, Genistein, Hydroxytyrosol, Luteolin, Maysin, Oleuropein, Protocatechuic acid, Quercetin, Resveratrol, Rutin, Sakuranetin | 3T3-L1, primary human adipocytes, mesenchymal stem cells, hASC | [46,48,50,52,53,54,57,58,61,63,64,65,66,67,68,69,70,71] | |
| HSL, ATGL | Ellagic acid | hASC | [57] | |
| Proliferation | MAPK, p38, Erk, JNK | Cocoa polyphenols, Curcumin, Epigallocatechin-3-gallate, Episesamin, Green tea polyphenols, Oligonol, Pycnogenol | 3T3-L1, isoalted rat adipocytes, primary rat adipocytes | [52,53,62,66,72,73,74,75] |
| Apoptosis | caspases, PARP | Epigallocatechin-3-gallate, Episesamin | 3T3-L1 | [52,56] |
| Differentiation | Blueberry polyphenols, Curcumin, Cyanidine-3-O-glucoside, Delphinidin-3-O-glucoside, Episesamin, Genistein, Naringenin, Oleuropein, Petunidin-3-O-glucoside | 3T3-L1, 3T3-F442A, mesenchymal stem cells | [52,55,58,68,70] | |
| Satiety hormones | leptin, resistin, adiponectin | Apple polyphenols, Catechin, Chlorogenic acid, Cyanidin-3-O-glucoside, Gallic acid, Protocatechuic acid, Resveratrol, Rutin | 3T3-L1, isolated human and mice adipocytes, SGBS; mesenchymal stem cells | [51,54,58,59,63,65,66,76,77,78,79,80] |
| Inflammatory markers | TNF-α, IL-6, IL-1β | Chlorogenic acid, Naringenin, Oligonol, Quercetin, Resveratrol, Rutin | 3T3-L1, 3T3-L1/RAW263 coculture, isolated human and rat adipocytes, human primary adipocytes | [52,54,67,74,81,82,83] |
| MCP-1 | Naringenin, Quercetin, Resveratrol | primary human adipocytes, 3T3-L1/RAW263 coculture | [67,81] | |
| Hypoxia | VEGF | Cinnamon polyphenols, Episesamin, Resveratrol, | 3T3-L1, isolated adipose tissue | [52,82,83] |
| C/EBPα | Ellagic acid | hASC | [57] | |
| ENDOTHELIUM | ||||
| Transport | GLUT-4, Akt | Silibinin, Xanthohumol | HUVEC | [84] |
| Vasorelaxation | NO, eNOS | Red wine polyphenols, Resveratrol, Sinapic acid | EaHy.926, HUVEC | [85,86,87] |
| ACE | Billberry anthocyanidins, Butein, Kaempferol Oak polyphenols, Tannins, Tea polyphenols | ACE-test, HUVEC | [88,89,90,91,92,93] | |
| ET-1 | Quercetin | Isolated human umbilial chord veins | [94] | |
| Proliferation | MAPK, p38, Erk, JNK | Apigenin, Catechins, Cocoa procyanidins, Genistein, Quercetin, | EC, VSMC, HMEC, HUVEC | [95,96,97] |
| Migration | MMPs | Cyanidin, Delphinidin, Epigallocatechin-3-gallate, Green tea polyphenols, Hydroxytyrosol, Isoxanthohumol, Malvidin, Oleuropein, Pelargonidin, Peonidin, Petunidin, Quercetin, Resveratrol, Xanthohumol | HUVEC, HMEC-1 | [84,95,98,99] |
| Tubulus formation | Hydroxytyrosol, Oleuropein, Quercetin, Resveratrol Xanthohumol, | HUVEC and HMEC-1 | [95,98,100] | |
| Inflammatory markers | NF-κB, TNF-α | Catechins, Isoxanthohumol, Silibinin | HUVEC, VSMC | [84,95,96] |
| COX-2 | Hydroxytyrosol, Oleuropein, Quercetin, Resveratrol | EC | [98,101] | |
| LIVER | ||||
| Energy metabolism | Ser9 and Ser641 glycogen synthase | Epigallocatechin | HepG2, isolated rat hepatocytes | [102,103] |
| fat storage | 3-caffeoyl,4-dihydrocaffeoylquinic acid, Blueberry anthocyanins, Curcumin, Cyanidin-3- glucoside, Ellagic acid, Ginko bilonba polyphenols, Quercetin, Resveratrol, Sechium edule shoots polyphenols | HepG2, H4IIEC3, Huh7, isolated rat hepatocytes | [57,104,105,106,107,108,109,110,111,112,113,114,115] | |
| CPT-1, ACC | Cyanidin-3-O-β-glucoside, Ginko biloba polyphenols, Resveratrol, Sechium edule shoots polyphenols | isolated rat hepatocytes, HepG2 | [107,110,111,116,117,118] | |
| AMPK, LXR, FAS, PPAR-α, SREBP1c | 3-caffeoyl,4-dihydrocaffeoylquinic acid, Blackberry polyphenols, Cocoa polyphenols, Curcumin, Cyanidin-3-O-β-glucoside, Cyanidin chloride, Ellagic acid, Epicatechin, Epigallocatechin-3-gallate, Ginko biloba polyphenols, Mulberry anthocyanins, Resveratrol, Sechium edule shoots polyphenols, Sweet potato anthocyanins | HepG2, isolated rat hepatocytes, Huh7 | [57,103,104,107,109,110,111,112,113,116,117,118,119,120,121,122,123,124,125] | |
| Akt/PI3K | Epicatechin, Quercetin | HepG2 | [126,127] | |
| GPAT1 | Cyanidin-3-O-glucoside | HepG2 | [115,117] | |
| Cholesterol metabolism | Cholesterol storage | Grape seed polyphenols, Red wine polyphenols | HepG2 | [43] |
| ApoA1, ApoB100, HDL, HMGCoR | Epigallocatechin, Epigallocatechin gallate, Gallic acid, Quercetin, Red wine polyphenols, Resveratrol, Sechium edule shoots polyphenols | HepG2 | [45,107,128,129] | |
| Apoptosis | others (DNA fragmentation, PI staining) | Cyanidin-3-ol | HepG2 | [130] |
| Caspases | Black tea polyphenols, Epigallocatechin-3-gallate, Quercetin, Resveratrol, Solanum nigrum polyphenols | HepG2, HLE | [106,126,131,132,133] | |
| IMMUNE CELLS | ||||
| Inflammatory markers | MCP-1, NF-κB, COX-2; TNF-α; IκBα; IL-1α; IL-1β; IL-6; IL-8; IL-10 | Cacao polyphenols, Caffeic acid, Caffeoylquinic acids, Curcumin, Cyanidin-3-O-β-glucoside, Epicatechin, Gallic acid, Grape seed proanthocyanidins, Hydroxytyrosol, Naringenin chalcone, Oleuropein, Olive oil polyphenols, Quince peel polyphenols, Resveratrol, Rosmarinic acid | THP-1, RAW 264.7, HMC-1, NR8383, U-937 | [81,134,135,136,137,138,139,140,141,142,143,144,145,146,147] |
| Proliferation | MAPK, p38, ERK1/2 | Quince peel polyphenols, Resveratrol | THP-1, HMC-1 | [135,138,147] |
| Vasorelaxation | eNOS, NO | Cacao polyphenols, Epicatechin, Hydroxytyrosol, Naringenin chalcone, Resveratrol | THP-1, RAW 264.7 | [81,134,135,140] |
| Apoptosis | PI3K, Akt | Quince peel polyphenols, Resveratrol | THP-1 | [135,147] |
| Migration | MMPs | Olive oil polyphenols | THP-1 | [141] |
| Energy metabolism | PPAR-γ; LXR-α | Cyanidin-3-O-β-glucoside | THP-1 | [136] |
3.2.1. The Gastro-Intestinal Tract
3.2.2. The Adipose Tissue
3.2.3. The Endothelium
3.2.4. The Liver
3.2.5. The Immune System
3.2.6. Overall Effect
4. Current Cell Culture Research: Trends and Potential Application for Polyphenol Research
| Intestinal Cell Lines | Co-Cultured Cell (Line) | Experimental Setup | Application | Ref. | |
|---|---|---|---|---|---|
| Intestine | Caco-2, Caco-2BBE | HT-29, HT-29-MTX, M-cells | Direct contact | Iron bioavailbaility, breast milk effects, nanoparticle uptake, curcumin bioavailability | [184,189,190,191,192,193] |
| Liver | Caco-2; Caco-2-TC7 | HepG2, HepaRG, murine 3A | Transwell and continuous perfused fluidic system | Benzo-a-pyrene toxicity, b-carotene and retinoid transport | [194,195,196] |
| Neuronal | Caco-2, HT-29 | PC12, glial cells, primary enteric neurocytes | Collagen-embedded system, Transwell system | Co-culture characteristics, LPS stimulation, pathogen invasion | [197,198,199,200] |
| Fibroblast | Caco-2, IEC-6, IPI-21, CRL-2102 | Primary human and rat fibroblasts, Rat-2 | Collagen-embedded, long term 3D | Co-culture characteristics | [201,202,203,204] |
| Immune cells | Caco-2; HT-29, m-ICcl2 | Whole blood cells, dendritic cells from isolated blood monocytes and bone marrow, lymphoblastoic TK6 cells, macrophage-like THP-1 and RAW264.7, murine lymphocytes of Peyers patches, Jurkat cells, RBL-2H3 (rat basophils), mast cells | Transwell system, floating filter system and direct contact, indirect micropattern surface | Co-culture characteristics, bioactivity of drugs, LPS, probiotica, benzo-a-pyrene, aflatoxin, fucoidan, immunoreactivity of ovalbumin | [205,206,207,208,209,210,211,212,213,214,215,216] |
| 3 or more cell types | Caco-2+HT29-MTX | Raji B, fibroblast + immunocytes, blood derived macrophages + dendritic cells | Transwell system, direct contact, collagen-embedded Transwell system, | (Peptide) drug transport and permeability | [217,218,219] |
| Adipocyte | Caco-2, HT29-19A | PAZ-6 | Transwell system | Co-culture characteristics | [185] |
| Endothelium | Caco-2, HT29-6B, LS180EB3 | Primary HMEC, immortalized isolated HMEC from lymph node, appendix, lung, skin and intestine microvessels, HUVEC, EA.hy926 cells | Transwell system, 3D dynamic model with decellularized jejunum segments, indirect contact | Co-culture characteristics, migration and adhesion of tumor cells, effect of anthocyanins of grape | [220,221] |
| Adipocyte Cells | Co-cultured Cell (Line) | Experimental Setup | Application | Ref. | |
| Immune cells | Mouse preadipocytes, 3T3-L1 | RAW264 | Direct contact | Cross-talk grape, Maqui, calafate, blueberry polyphenol extracts, naringenin chalcone | [81,222,223] |
5. General Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Spencer, J.P.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Rizvi, S. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann.Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Carpene, C.; Gomez-Zorita, S.; Deleruyelle, S.; Carpene, M.A. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr. Med. Chem. 2015, 22, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.; Riso, P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Kishimoto, Y.; Tani, M.; Kondo, K. Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur. J. Clin. Nutr. 2013, 67, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Higgins, S.; Duthie, G.G.; Duthie, S.J.; Howie, M.; Mullen, W.; Lean, M.E.J.; Crozier, A. The influence of moderate red wine consumption on antioxidant status and indices of oxidative stress associated with chd in healthy volunteers. Br. J. Nutr. 2005, 93, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Giongo, L.; Bozza, E.; Caciagli, P.; Valente, E.; Pasquazzo, M.T.; Pedrolli, C.; Iorio, E.L.; Costa, A. Short-term blueberry intake enhances biological antioxidant potential and modulates inflammation markers in overweight and obese children. J. Berry Res. 2011, 1, 147–158. [Google Scholar]
- Estruch, R.; Sacanella, E.; Badia, E.; Antunez, E.; Nicolas, J.M.; Fernandez-Sola, J.; Rotilio, D.; de Gaetano, G.; Rubin, E.; Urbano-Marquez, A. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: Aprospective randomized crossover trial. Effects of wine on inflammatory markers. Atherosclerosis 2004, 175, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.; Lexis, L.; Lewandowski, P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr. J. 2007, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Napoli, R.; Cozzolino, D.; Guardasole, V.; Angelini, V.; Zarra, E.; Matarazzo, M.; Cittadini, A.; Sacca, L.; Torella, R. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metabolism 2005, 54, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Ntarladimas, I.; Antoniades, C.; Vasiliadou, C.; Tentolouris, C.; Papageorgiou, N.; Latsios, G.; Stefanadis, C. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin. Nutr. 2008, 27, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Teufel, K.; Wu, N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis 2001, 156, 67–72. [Google Scholar] [CrossRef]
- Waddington, E.; Puddey, I.B.; Croft, K.D. Red wine polyphenolic compounds inhibit atherosclerosis in apolipoprotein E-deficient mice independently of effects on lipid peroxidation. Am. J. Clin. Nutr. 2004, 79, 54–61. [Google Scholar] [PubMed]
- Mineharu, Y.; Koizumi, A.; Wada, Y.; Iso, H.; Watanabe, Y.; Date, C.; Yamamoto, A.; Kikuchi, S.; Inaba, Y.; Toyoshima, H.; et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in japanese men and women. J. Epidemiol. Commun. Health 2011, 65, 230–240. [Google Scholar] [CrossRef] [PubMed]
- de Koning Gans, J.M.; Uiterwaal, C.S.; van der Schouw, Y.T.; Boer, J.M.; Grobbee, D.E.; Verschuren, W.M.; Beulens, J.W. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Teufel, K.; Wu, N. Green and black teas inhibit atherosclerosis by lipid, antioxidant, and fibrinolytic mechanisms. J. Agric. Food Chem. 2004, 52, 3661–3665. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.M.; Baum, J.A.; Teng, H.Y.; Stillman, R.J.; Shay, N.F.; Erdman, J.W. Soy protein and isoflavones: Their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr. 1998, 68, 1375s–1379s. [Google Scholar] [PubMed]
- Adams, M.R.; Golden, D.L.; Register, T.C.; Anthony, M.S.; Hodgin, J.B.; Maeda, N.; Williams, J.K. The atheroprotective effect of dietary soy isoflavones in apolipoprotein E−/− mice requires the presence of estrogen receptor-α. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1859–1864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol. 2003, 41, 631–636. [Google Scholar] [CrossRef]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Petzke, K.J.; Raederstorff, D.; Wolfram, S.; Klaus, S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int. J. Obes. 2012, 36, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.L.; Dawson, H.D.; Schoene, N.W.; Polansky, M.M.; Anderson, R.A. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes. Nutrition 2012, 28, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.C.; Furtos, A.; Dudonne, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple peel polyphenols and their beneficial actions on oxidative stress and inflammation. PLoS ONE 2013, 8, e53725. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cárdeno, A.; Alarcón de la Lastra, C. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Angel-Morales, G.; Noratto, G.; Mertens-Talcott, S. Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18CO myofibroblast cells: Potential role of microRNA-126. Food Funct. 2012, 3, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Siebers, M.; Keller, J.; Kloster, J.; Wen, G.; Eder, K. Inhibition of the pro-inflammatory NF-κB pathway by a grape seed and grape marc meal extract in intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.A.; Park, Y.L.; Yoon, S.H.; Kim, K.Y.; Cho, S.B.; Lee, W.S.; Chung, I.J.; Joo, Y.E. Black tea polyphenol theaflavin suppresses LPS-induced ICAM-1 and VCAM-1 expression via blockage of NF-kB and JNK activation in intestinal epithelial cells. Inflamm. Res. 2011, 60, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Romier-Crouzet, B.; Van De Walle, J.; During, A.; Joly, A.; Rousseau, C.; Henry, O.; Larondelle, Y.; Schneider, Y.-J. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem. Toxicol. 2009, 47, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Haller, D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-κB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J. Nutr. 2006, 136, 664–671. [Google Scholar] [PubMed]
- Yang, F.; Oz, H.S.; Barve, S.; de Villiers, W.J.S.; McClain, C.J.; Varilek, G.W. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-κB activation by inhibiting IκB kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001, 60, 528–533. [Google Scholar] [PubMed]
- Leifert, W.R.; Abeywardena, M.Y. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr. Res. 2008, 28, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.J.; Onstead-Haas, L.M.; Szafran-Swietlik, A.; Kojanian, H.; Davis, T.; Armstrong, P.; Wong, N.C.W.; Mooradian, A.D. Induction of hepatic apolipoprotein A-I gene expression by the isoflavones quercetin and isoquercetrin. Life Sci. 2014, 110, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3T3-L1 cells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Warnke, I.; Goralczyk, R.; Fuhrer, E.; Schwager, J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr. Metab. 2011, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bae, E.J. Inhibition of mitotic clonal expansion mediates fisetin-exerted prevention of adipocyte differentiation in 3T3-L1 cells. Arch. Pharmacal Res. 2013, 36, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zorita, S.; Treguer, K.; Mercader, J.; Carpene, C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J. Physiol. Biochem. 2013, 69, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Chung, B.Y.; Lee, M.K.; Song, Y.; Lee, S.S.; Chu, G.M.; Kang, S.N.; Song, Y.M.; Kim, G.S.; Cho, J.H. Centipede grass exerts anti-adipogenic activity through inhibition of C/EBPβ, C/EBPα, and PPARγ expression and the Akt signaling pathway in 3T3-L1 adipocytes. BMC Complement. Altern. Med. 2012, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, A.; Noben, J.-P.; Jocken, J.; Kallendrusch, S.; Fischer-Posovszky, P.; Mariman, E.C.M.; Renes, J. Resveratrol-induced changes of the human adipocyte secretion profile. J. Proteome Res. 2012, 11, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Trowitzsch-Kienast, W.; Erben, U.; Seehofer, D.; Kim, K.Y.; Zeitz, M.; Ruehl, M.; Somasundaram, R. (+)-episesamin inhibits adipogenesis and exerts anti-inflammatory effects in 3T3-L1 (pre)adipocytes by sustained WNT signaling, down-regulation of PPARγ and induction of iNOS. J. Nutr. Biochem. 2013, 24, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yang, H.; Seo, S.G.; Shin, S.H.; Chung, M.Y.; Kim, J.; Lee, S.J.; Lee, H.J.; Lee, K.W. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. Int. J. Obes. 2013, 37, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, G.; Barlette, A.G.; Dhamer, T.; Arcari, D.P.; Santos, J.C.; de Camargo, E.R.; Acedo, S.; Gambero, A.; Gnoatto, S.C.; Ribeiro, M.L. Phenolic compounds from mate (ilex paraguariensis) inhibit adipogenesis in 3T3-L1 preadipocytes. Plant Foods Hum. Nutr. 2012, 67, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Moghe, S.S.; Juma, S.; Imrhan, V.; Vijayagopal, P. Effect of blueberry polyphenols on 3T3-F442a preadipocyte differentiation. J. Med. Food 2012, 15, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Okla, M.; Kang, I.; Kim, D.M.; Gourineni, V.; Shay, N.; Gu, L.; Chung, S. Ellagic acid modulates lipid accumulation in primary human adipocytes and human hepatoma Huh7 cells via discrete mechanisms. J. Nutr. Biochem. 2015, 26, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.S.; Snook, L.A.; Arkell, A.M.; Simpson, J.A.; Holloway, G.P.; Wright, D.C. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R542–R551. [Google Scholar] [CrossRef] [PubMed]
- Claussnitzer, M.; Skurk, T.; Hauner, H.; Daniel, H.; Rist, M.J. Effect of flavonoids on basal and insulin-stimulated 2-deoxyglucose uptake in adipocytes. Mol. Nutr. Food Res. 2011, 55, S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Abe, D.; Sekiya, K. Sakuranetin induces adipogenesis of 3T3-L1 cells through enhanced expression of PPARγ2. Biochem. Biophys. Res. Commun. 2008, 372, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Kim, K.-J.; Lee, O.-H.; Lee, B.-Y. Effect of pycnogenol® on glucose transport in mature 3T3-L1 adipocytes. Phytother. Res. 2010, 24, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Hasan, S.T.; Meydani, M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors 2013, 39, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Boque, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Banuelos, O.; Soria, A.C.; Rodriguez-Sanchez, S.; Martinez, J.A.; Campion, J. Prevention of diet-induced obesity by apple polyphenols in wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Ye, X.; Zhang, R.; Long, J.; Ren, W.; Ding, S.; Liao, D.; Jin, X.; Wu, H.; Xu, S.; et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via Erk1/2-PPARγ-adiponectin pathway. PLoS ONE 2013, 8, e53796. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; Bumrungpert, A.; Kennedy, A.; Overman, A.; West, T.; Dawson, B.; McIntosh, M.K. Grape powder extract attenuates tumor necrosis factor α-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. Biochem. 2011, 22, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Mora, R.; Casado-Diaz, A.; De Castro, M.D.; Quesada-Gomez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Kim, S.N.; Lee, S.M.; Lee, W.; Song, M.J.; Park, S.M.; Lee, T.R.; Baik, J.-H.; Kim, H.K.; Hong, J.-H.; et al. (−)-Catechin promotes adipocyte differentiation in human bone marrow mesenchymal stem cells through PPARγ transactivation. Biochem. Pharmacol. 2009, 77, 125–133. [Google Scholar] [CrossRef]
- Harmon, A.W.; Harp, J.B. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, C807–C813. [Google Scholar] [PubMed]
- Hu, P.; Zhao, L.; Chen, J.G. Physiologically achievable doses of resveratrol enhance 3T3-L1 adipocyte differentiation. Eur. J. Nutr. 2015, 54, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of WNT/β-catenin signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, J.; Kitadate, K.; Nishioka, H.; Fujii, H.; Sakurai, T.; Kizaki, T.; Izawa, T.; Ishida, H.; Ohno, H. Comparison of the effect of oligonol, a new lychee fruit-derived low molecular form of polyphenol, and epigallocatechin-3-gallate on lipolysis in rat primary adipocytes. Phytother. Res. 2011, 25, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, J.; Kitadate, K.; Nishioka, H.; Fujii, H.; Sakurai, T.; Kizaki, T.; Izawa, T.; Ishida, H.; Tanno, M.; Ohno, H. Oligonol, an oligomerized lychee fruit-derived polyphenol, activates the Ras/Raf-1/MEK1/2 cascade independent of the IL-6 signaling pathway in rat primary adipocytes. Biochem. Biophys. Res. Commun. 2010, 402, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Zhou, N.M.; Zhang, Z.L.; Li, W.X.; Zhu, W. Resveratrol induces cell apoptosis in adipocytes via AMPK activation. Biochem. Biophys. Res. Commun. 2015, 457, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Nishii, S.; Zaima, N.; Moriyama, T.; Kawamura, Y. Ellagic acid improves hepatic steatosis and serum lipid composition through reduction of serum resistin levels and transcriptional activation of hepatic PPARα in obese, diabetic KK-Ay mice. Biochem. Biophys. Res. Commun. 2013, 434, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.; Palou, A.; Bonet, M.L. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and retinol-binding protein 4 expression in white adipocytes. J. Nutr. Biochem. 2011, 22, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Yanez-Gascon, M.J.; Garcia-Almagro, F.J.; Ruiz-Ros, J.A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, M.; Liu, X.; Dong, L.Q.; Glickman, R.D.; Slaga, T.J.; Zhou, Z.; Liu, F. Up-regulation of adiponectin by resveratrol: The essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and Dsba-L. J. Biol. Chem. 2011, 286, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Misra, C.S.; Arumugam, S.; Roy, S.; Shah, V.; Davis, J.A.; Shirumalla, R.K.; Ray, A. Antidiabetic activity of resveratrol, a known SIRT1 activator in a genetic model for type-2 diabetes. Phytother. Res. 2011, 25, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Kim, Y., II; Goto, T.; Kang, M.-S.; Yoshimura, M.; Obata, A.; Yu, R.; Kawada, T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007, 81, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Cullberg, K.B.; Olholm, J.; Paulsen, S.K.; Foldager, C.B.; Lind, M.; Richelsen, B.; Pedersen, S.B. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro. Eur. J. Pharm. Sci. 2013, 49, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Anderson, R.A. Cinnamon polyphenol extract regulates tristetraprolin and related gene expression in mouse adipocytes. J. Agric. Food Chem. 2011, 59, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Duluc, L.; Soleti, R.; Clere, N.; Andriantsitohaina, R.; Simard, G. Mitochondria as potential targets of flavonoids: Focus on adipocytes and endothelial cells. Curr. Med. Chem. 2012, 19, 4462–4474. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, T.; Manivannan, J.; Priya, M.K.; Suganya, N.; Chatterjee, S.; Raja, B. Sinapic acid prevents hypertension and cardiovascular remodeling in pharmacological model of nitric oxide inhibited rats. PLoS ONE 2014, 9, e115682. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, X.; Liang, Y.; Head, R.; Bennett, L. Inhibition of angiotensin converting enzyme (ACE) activity by polyphenols from tea (Camellia sinensis) and links to processing method. Food Funct. 2011, 2, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Arreola, M.J.; Rocha-Guzman, N.E.; Gallegos-Infante, J.A.; Gonzalez-Laredo, R.F.; Rosales-Castro, M.; Bacon, J.R.; Cao, R.; Proulx, A.; Intriago-Ortega, P. Antioxidant activity of oak (Quercus) leaves infusions against free radicals and their cardioprotective potential. Pakistan J. Biol. Sci. 2010, 13, 537–545. [Google Scholar] [CrossRef]
- Persson, I.A.; Persson, K.; Andersson, R.G. Effect of vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J. Agric. Food Chem. 2009, 57, 4626–4629. [Google Scholar] [CrossRef] [PubMed]
- Olszanecki, R.; Bujak-Gizycka, B.; Madej, J.; Suski, M.; Wolkow, P.P.; Jawien, J.; Korbut, R. Kaempferol, but not resveratrol inhibits angiotensin converting enzyme. J. Physiol. Pharmacol. 2008, 59, 387–392. [Google Scholar] [PubMed]
- Kang, D.G.; Kim, Y.C.; Sohn, E.J.; Lee, Y.M.; Lee, A.S.; Yin, M.H.; Lee, H.S. Hypotensive effect of butein via the inhibition of angiotensin converting enzyme. Biol. Pharm. Bull. 2003, 26, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-C.; Hsu, F.-L.; Tsai, J.-C.; Chan, P.; Liu, J.Y.-H.; Thomas, G.N.; Tomlinson, B.; Lo, M.-Y.; Lin, J.-Y. Antihypertensive effects of tannins isolated from traditional chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003, 73, 1543–1555. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, Z.; Attele, A.S.; Yuan, C.-S. Effects of quercetin on the release of endothelin, prostacyclin and tissue plasminogen activator from human endothelial cells in culture. J. Ethnopharmacol. 1999, 67, 279–285. [Google Scholar] [CrossRef]
- Negrão, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Azevedo, I.; Soares, R. Different effects of catechin on angiogenesis and inflammation depending on VEGF levels. J. Nutr. Biochem. 2013, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Negrao, R.; Duarte, D.; Costa, R.; Soares, R. Isoxanthohumol modulates angiogenesis and inflammation via vascular endothelial growth factor receptor, tumor necrosis factor α and nuclear factor κB pathways. Biofactors 2013, 39, 608–622. [Google Scholar] [CrossRef]
- Kenny, T.P.; Keen, C.L.; Jones, P.; Kung, H.J.; Schmitz, H.H.; Gershwin, M.E. Cocoa procyanidins inhibit proliferation and angiogenic signals in human dermal microvascular endothelial cells following stimulation by low-level H2O2. Exp. Biol. Med. 2004, 229, 765–771. [Google Scholar]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Matsukawa, M.; Yamakawa, S.; Asai, T.; Yahara, S.; Hashimoto, F.; Akizawa, T. Inhibitory effect of green tea polyphenols on membrane-type 1 matrix metalloproteinase, MT1-MMP. Biol. Pharm. Bull. 2003, 26, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Elgass, S.; Cooper, A.; Chopra, M. Lycopene inhibits angiogenesis in human umbilical vein endothelial cells and rat aortic rings. Br. J. Nutr. 2012, 108, 431–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, M.; Tang, S.-N.; Marsh, J.L.; Shankar, S.; Srivastava, R.K. Ellagic acid inhibits human pancreatic cancer growth in BALB c nude mice. Cancer Lett. 2013, 337, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Tan, Y.; Xiao, L.; Sun, Y.L.; Qu, X. Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytes. Biomed Res. Int. 2013, 2013, 920128. [Google Scholar] [CrossRef]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef]
- Hwang, P.Y.; Gyun Kim, H.; Choi, J.H.; Truong Do, M.; Tran, T.P.; Chun, H.K.; Chung, Y.C.; Jeong, T.C.; Jeong, H.G. 3-Caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia herbacea attenuates high glucose-induced hepatic lipogenesis in human HepG2 cells through activation of the liver kinase B1 and silent information regulator T1/APMK-dependent pathway. Mol. Nutr. Food Res. 2013, 57, 471–482. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, D.; Zhang, D.; Lv, Y.C.; Wei, Y.; Wu, W.; Zhou, F.; Tang, M.M.; Mao, T.; Li, M.M.; et al. Inhibitory effect of blueberry polyphenolic compounds on oleic acid-induced hepatic steatosis in vitro. J. Agric. Food Chem. 2011, 59, 12254–12263. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Chung, P.-J.; Wu, C.-H.; Lan, K.-P.; Yang, M.-Y.; Wang, C.-J. Solanum nigrum L. Polyphenolic extract inhibits hepatocarcinoma cell growth by inducing G2/M phase arrest and apoptosis. J. Sci. Food Agric. 2011, 91, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Ou, T.-T.; Chang, C.-H.; Chang, X.-Z.; Yang, M.-Y.; Wang, C.-J. The polyphenol extract from sechium edule shoots inhibits lipogenesis and stimulates lipolysis via activation of AMPK signals in HepG2 cells. J. Agric. Food Chem. 2013, 62, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Suh, H.R.; Yoon, Y.; Lee, K.J.; Kim, D.G.; Kim, S.; Lee, B.H. Protective effect of resveratrol derivatives on high-fat diet induced fatty liver by activating AMP-activated protein kinase. Arch. Pharmacal Res. 2014, 37, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Kim, S.B.; Seo, Y.S.; Joung, D.K.; Mun, S.H.; Choi, J.G.; Lee, Y.M.; Kang, D.G.; Lee, H.S.; Kwon, D.Y. Curcumin decreases oleic acid-induced lipid accumulation via AMPK phosphorylation in hepatocarcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2578–2586. [Google Scholar] [PubMed]
- Gnoni, G.V.; Paglialonga, G. Resveratrol inhibits fatty acid and triacylglycerol synthesis in rat hepatocytes. Eur. J. Clin. Investig. 2009, 39, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Chen, L.L.; Xiao, F.X.; Sun, H.; Ding, H.C.; Xiao, H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol. Sin. 2008, 29, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Fu, Y.C.; Xu, W.C.; Feng, Y.Q.; Fang, S.R.; Zhou, X.H. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem. Biophys. Res. Commun. 2009, 380, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Vidyashankar, S.; Sandeep Varma, R.; Patki, P.S. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol. Vitro 2013, 27, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, D.; Ling, W.; Feng, X.; Xia, M. Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ. J. Lipid Res. 2011, 52, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Small lipid-binding proteins in regulating endothelial and vascular functions: Focusing on adipocyte fatty acid binding protein and lipocalin-2. Br. J. Pharmacol. 2012, 165, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, G.; Zhong, R.; Wang, Y.; Wang, D.; Xia, M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012, 11, 10. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Han, E.H.; Kim, H.G.; Wee, J.H.; Jung, K.O.; Jung, K.H.; Kwon, K.I.; Jeong, T.C.; Chung, Y.C.; et al. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 2011, 31, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J.; Hsu, M.J.; Huang, H.P.; Chung, D.J.; Chang, Y.C.; Wang, C.J. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J. Agric. Food Chem. 2013, 61, 6069–6076. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Martin, M.A.; Goya, L.; Ramos, S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food Chem. Toxicol. 2014, 64, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol. Nutr. Food Res. 2012, 56, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Ryu, H.W.; Jin, C.H.; Choi, D.S.; Kang, S.Y.; Kim, D.S.; Byun, M.W.; Jeong, I.Y. Blackberry extract attenuates oxidative stress through up-regulation of Nrf2-dependent antioxidant enzymes in carbon tetrachloride-treated rats. J. Agric. Food Chem. 2011, 59, 11442–11448. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y.; Kim, J.Y.; Jun, H.J.; Kim, S.J.; Lee, J.H.; Hoang, M.H.; Kim, H.S.; Chang, H.I.; Hwang, K.Y.; Um, S.J.; et al. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-α reducing hepatic lipid. BBA-Mol. Cell Biol. Lett. 2013, 1831, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.A.; Roach, P.D. Modulation of cholesterol metabolism by the green tea polyphenol (−)-epigallocatechin gallate in cultured human liver (HepG2) cells. J. Agric. Food Chem. 2006, 54, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and Erk pathways in a human hepatoma cell line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [PubMed]
- Granado-Serrano, A.B.; Martin, M.A.; Izquierdo-Pulido, M.; Goya, L.; Bravo, L.; Ramos, S. Molecular mechanisms of (-)-epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line. J. Agric. Food Chem. 2007, 55, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ho, N.; Santos, C.; Dubois, P.; Mamo, J.; Croft, K.; Allister, E. Red wine polyphenolics increase ldl receptor expression and activity and suppress the secretion of ApoB100 from human HepG2 cells. J. Nutr. 2003, 133, 700–706. [Google Scholar] [PubMed]
- Nakagawa, S.; Kojima, Y.; Sekino, K.; Yamato, S. Effect of polyphenols on 3-hydroxy-3-methylglutaryl-coenzyme a lyase activity in human hepatoma HepG2 cell extracts. Biol. Pharm. Bull. 2013, 36, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Monga, J.; Pandit, S.; Chauhan, R.S.; Chauhan, C.S.; Chauhan, S.S.; Sharma, M. Growth inhibition and apoptosis induction by (+)-cyanidan-3-ol in hepatocellular carcinoma. PLoS ONE 2013, 8, e68710. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Chen, Y.; Cheng, X.; Zhang, X.; He, Q. Potentiation of resveratrol-induced apoptosis by matrine in human hepatoma HepG2 cells. Oncol. Rep. 2014, 32, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.S.; Priyadarsini, R.V.; Ramalingam, K.; Hara, Y.; Karunagaran, D.; Nagini, S. Intrinsic apoptosis and NF-κB signaling are potential molecular targets for chemoprevention by black tea polyphenols in HepG2 cells in vitro and in a rat hepatocarcinogenesis model in vivo. Food Chem. Toxicol. 2010, 48, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, E.; Franch, A.; Castellote, C.; Perez-Cano, F.; Permanyer, J.; Izquierdo-Pulido, M.; Castell, M. Flavonoids from theobroma cacao down-regulate inflammatory mediators. J. Agric. Food Chem. 2005, 53, 8506–8511. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.P.; Morrow, D.; Jin, Y.; von Offenberg Sweeney, N.; Sitzmann, J.V.; Cahill, P.A.; Redmond, E.M. Resveratrol inhibits expression and binding activity of the monocyte chemotactic protein-1 receptor, CCR2, on THP-1 monocytes. Atherosclerosis 2007, 195, e125–e133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xia, M.; Liu, C.; Guo, H.; Ye, Q.; Hu, Y.; Zhang, Y.; Hou, M.; Zhu, H.; Ma, J.; et al. Cyanidin-3-O-β-glucoside inhibits iNOS and COX-2 expression by inducing liver x receptor α activation in THP-1 macrophages. Life Sci. 2008, 83, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chacon, M.R.; Ceperuelo-Mallafre, V.; Maymo-Masip, E.; Mateo-Sanz, J.M.; Arola, L.; Guitierrez, C.; Fernandez-Real, J.M.; Ardevol, A.; Simon, I.; Vendrell, J. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine 2009, 47, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Jang, H.J.; Chae, H.S.; Oh, Y.C.; Choi, J.G.; Lee, Y.S.; Kim, J.H.; Kim, Y.C.; Sohn, D.H.; Park, H.; et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: Pivotal roles of NF-κ B and MAPK. Pharmacol. Res. 2009, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Hauck, C.; Yum, M.Y.; Rizshsky, L.; Widrlechner, M.P.; Mccoy, J.A.; Murphy, P.A.; Dixon, P.M.; Nikolau, B.J.; Birt, D.F. Rosmarinic acid in prunella vulgaris ethanol extract inhibits lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW 264.7 mouse macrophages. J. Agric. Food Chem. 2009, 57, 10579–10589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Cao, J.; Zhong, L.F. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn-Schmiedeberg’s Arch. Pharmacology 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Galli, G.V.; Maschi, O.; Gilardi, F.; Bellosta, S.; Crestani, M.; Bosisio, E.; De Fabiani, E.; Caruso, D. Olive oil phenols modulate the expression of metalloproteinase 9 in THP-1 cells by acting on nuclear factor-κB signaling. J. Agric. Food Chem. 2010, 58, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.D.; Chen, G.J.; Almeida, M.C.; Soares, D.M.; de Souza, G.E.P.; Lopes, N.P.; Lantz, R.C. Effects of caffeoylquinic acid derivatives and C-flavonoid from lychnophora ericoides on in vitro inflammatory mediator production. Nat. Prod. Commun. 2010, 5, 733–740. [Google Scholar] [PubMed]
- Kuppan, G.; Balasubramanyam, J.; Monickaraj, F.; Srinivasan, G.; Mohan, V.; Balasubramanyam, M. Transcriptional regulation of cytokines and oxidative stress by gallic acid in human THP-1 monocytes. Cytokine 2010, 49, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lian, F.; Zhu, Y.; Xia, M.; Wang, Q.; Ling, W.; Wang, X.D. Cyanidin-3-O-β-glucoside inhibits lps-induced expression of inflammatory mediators through decreasing IκBα phosphorylation in THP-1 cells. Inflamm. Res. 2010, 59, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Huang, H.W.; Lin, J.A.; Huang, S.M.; Yen, G.C. The proglycation effect of caffeic acid leads to the elevation of oxidative stress and inflammation in monocytes, macrophages and vascular endothelial cells. J. Nutr. Biochem. 2011, 22, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia oblonga miller) peel polyphenols modulates LPS-induced inflammation in human THP-1-derived macrophages through NF-kB, p38 MAPK and Akt inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assman, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [PubMed]
- Neutra, M.; Louvard, D. Differentiation of intestinal cells in vitro. Mod. Cell Biol. 1989, 8, 363–398. [Google Scholar]
- Mircheff, A.K.; Wright, E.M. Analytical isolation of plasma membranes of intestinal epithelial cells: Identification of Na, K-ATPase rich membranes and the distribution of the enzyme activities. J. Membr. Biol. 1976, 26, 309–333. [Google Scholar] [CrossRef]
- Zweibaum, A.; Pinto, M.; Chevalier, G.; Dussaulx, E.; Triadou, N.; Lacroix, B.; Haffen, K.; Brun, J.L.; Rousset, M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J. Cell Physiol. 1985, 122, 21–29. [Google Scholar] [PubMed]
- Gonzales, G.; Van Camp, J.; Zotti, M.; Kobayashi, V.; Grootaert, C.; Raes, K.; Smagghe, G. Two- and three-dimensional quantitative structure–permeability relationship of flavonoids in Caco-2 cells using stepwise multiple linear regression (SMLR), partial least squares regression (PLSR), and pharmacophore (GALAHAD)-based comparative molecular similarity index analysis (COMSIA). Med. Chem. Res. 2014, 24, 1696–1706. [Google Scholar]
- Hers, I.; Tavare, J.M. Mechanism of feedback regulation of insulin receptor substrate-1 phosphorylation in primary adipocytes. Biochem. J. 2005, 388, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Armoni, M.; Harel, C.; Karnieli, E. Transcriptional regulation of the GLUT4 gene: From PPAR-γ and FOXO1 to FFA and inflammation. Trends Endocrinol. Metab. 2007, 18, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Lam, K.S.; Vanhoutte, P.M.; Xu, A. Adiponectin and cardiovascular health: An update. Br. J. Pharmacol. 2012, 165, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.L.; Cheng, K.K.Y.; Lam, K.S.L.; Vanhoutte, P.M.; Xu, A. Cross-talk between adipose tissue and vasculature: Role of adiponectin. Acta Physiol. 2011, 203, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Rae, C.J.; Graham, A. Induction of angiogenesis by murine resistin: Putative role of PI3-kinase and no-dependent pathways. Regul. Pept. 2009, 152, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kunduzova, O.; Alet, N.; Delesque-Touchard, N.; Millet, L.; Castan-Laurell, I.; Muller, C.; Dray, C.; Schaeffer, P.; Herault, J.P.; Savi, P.; et al. Apelin/APJ signaling system: A potential link between adipose tissue and endothelial angiogenic processes. FASEB J. 2008, 22, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Kalea, A.Z.; Batlle, D. Apelin and ACE2 in cardiovascular disease. Curr. Opin. Investig. Drugs 2010, 11, 273–282. [Google Scholar] [PubMed]
- Heilbronn, L.K.; Campbell, L.V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des. 2008, 14, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Brenner, R.E.; Melzner, I.; Braun, M.; Moller, P.; Heinze, E.; Debatin, K.M.; Hauner, H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zilberfarb, V.; Siquier, K.; Strosberg, A.D.; Issad, T. Effect of dexamethasone on adipocyte differentiation markers and tumour necrosis factor-α expression in human PAZ6 cells. Diabetologia 2001, 44, 377–386. [Google Scholar] [PubMed]
- Forest, C.; Czerucka, D.; Negrel, R.; Ailhaud, G. Establishment of a human cell line after transformation by a plasmid containing the early region of the SV40 genome. Cell Biol. Int. Rep. 1983, 7, 73–81. [Google Scholar] [CrossRef]
- Tontonoz, P.; Singer, S.; Forman, B.M.; Sarraf, P.; Fletcher, J.A.; Fletcher, C.D.; Brun, R.P.; Mueller, E.; Altiok, S.; Oppenheim, H.; et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Hugo, E.R.; Brandebourg, T.D.; Comstock, C.E.S.; Gersin, K.S.; Sussman, J.J.; Ben-Jonathan, N. LS14: A novel human adipocyte cell line that produces prolactin. Endocrinology 2006, 147, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Targosz-Korecka, M.; Brzezinka, G.D.; Malek, K.E.; Stepien, E.; Szymonski, M. Stiffness memory of EA.Hy926 endothelial cells in response to chronic hyperglycemia. Cardiovasc. Diabetol. 2013, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, M.; Fujimura, T.; Hamada, Y.; Fujita, Y.; Hara, H.; Nishiyama, S.; Katsuoka, K.; Tamauchi, H.; Sakurai, Y. Establishment of a human hemangiosarcoma cell line (ISO-HAS). Int. J. Cancer 1999, 81, 305–308. [Google Scholar] [CrossRef]
- Ma, X.; Sickmann, A.; Pietsch, J.; Wildgruber, R.; Weber, G.; Infanger, M.; Bauer, J.; Grimm, D. Proteomic differences between microvascular endothelial cells and the EA.Hy926 cell line forming three-dimensional structures. Proteomics 2014, 14, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Portillo, M.P.; Hijona, E.; Bujanda, L. Effects of resveratrol and other polyphenols in hepatic steatosis. World J. Gastroenterol. 2014, 20, 7366–7380. [Google Scholar] [CrossRef] [PubMed]
- Walldius, G. Apolipoprotein B (apoB) more closely related to subclinical atherosclerosis than non-HDL cholesterol and LDL cholesterol. J. Internal Med. 2010, 268, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of primary human hepatocytes and hepatoma cell line HepG2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 2007, 211, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Mittal, N.; Sohi, K.K.; Pathak, C.M. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: A Bcl-2 independent mechanism. Biochim. Biophys. Acta 2006, 1760, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Agrewala, J.N. Dietary polyphenols in modulation of the immune system. Nova Sci. Publ. 2008. [Google Scholar] [CrossRef]
- Frostegard, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Auwerx, J. The human leukemia cell line, THP-1: A multifacetted model for the study of monocyte-macrophage differentiation. Experientia 1991, 47, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Gongora, M.C. Oxidative stress and hypertension. Med. Clin. N. Am. 2009, 93, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.L.; Hosseinkhani, H. Development of 3D in vitro technology for medical applications. Int. J. Mol. Sci. 2014, 15, 17938–17962. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.; Gulseren, I.; Corredig, M. Utilization of solid lipid nanoparticles for enhanced delivery of curcumin in cocultures of HT29-MTX and Caco-2 cells. Food Funct. 2013, 4, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Le Dréan, G.; Haure-Mirande, V.; Ferrier, L.; Bonnet, C.; Hulin, P.; de Coppet, P.; Segain, J.-P. Visceral adipose tissue and leptin increase colonic epithelial tight junction permeability via a RhoA-ROCK-dependent pathway. FASEB J. 2013, 28, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Schoeppner, S.; Kucharzik, T.; Kraft, M.; Schoenherr, E.; Domschke, W.; Luegering, N. Colonic epithelial cells induce endothelial cell expression of ICAM-1 and VCAM-1 by a NF-κB-dependent mechanism. Clin. Exp. Immunol. 2001, 124, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zgouras, D.; Wachtershauser, A.; Frings, D.; Stein, J. Butyrate impairs intestinal tumor cell-induced angiogenesis by inhibiting HIF-1α nuclear translocation. Biochem. Biophys. Res. Commun. 2003, 300, 832–838. [Google Scholar] [CrossRef]
- Kuntz, S.; Asseburg, H.; Dold, S.; Rompp, A.; Frohling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Woitiski, C.B.; Sarmento, B.; Carvalho, R.A.; Neufeld, R.J.; Veiga, F. Facilitated nanoscale delivery of insulin across intestinal membrane models. Int. J. Pharm. 2011, 412, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Nollevaux, G.; Deville, C.; El Moualij, B.; Zorzi, W.; Deloyer, P.; Schneider, Y.J.; Peulen, O.; Dandrifosse, G. Development of a serum-free co-culture of human intestinal epithelium cell-lines (Caco-2/HT29–5M21). BMC Cell Biol. 2006, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Glahn, R.P.; Miller, D.D. Different responses of fe transporters in Caco-2/HT29-MTX cocultures than in independent Caco-2 cell cultures. Cell Biol. Int. 2009, 33, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Friel, J.K.; Suh, M.; Diehl-Jones, W.L. Antioxidant properties of breast milk in a novel in vitro digestion/enterocyte model. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Poortman, J.; Peters, R.J.; Wijma, E.; Kramer, E.; Makama, S.; Puspitaninganindita, K.; Marvin, H.J.P.; Peijnenburg, A.A.C.M.; Hendriksen, P.J.M. Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium coculture model. ACS Nano 2011, 5, 4091–4103. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, D.A.; Choi, S.-H.; Sakai, Y.; Péry, A.R.R.; Brochot, C. Kinetic modelling of in vitro cell-based assays to characterize non-specific bindings and ADME processes in a static and a perfused fluidic system. Toxicol. Lett. 2011, 205, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Fukuda, O.; Choi, S.H.; Sakoda, A. Development of a biohybrid simulator for absorption and biotransformation processes in humans based on in vitro models of small intestine and liver tissues. J. Artif. Organs 2003, 6, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Guantario, B.; Ferruzza, S.; Guguen-Guillouzo, C.; Sambuy, Y.; Scarino, M.L.; Bellovino, D. Co-cultures of enterocytes and hepatocytes for retinoid transport and metabolism. Toxicol. Vitro 2012, 26, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Yokoyama, T.; Ogawa, N.; Fujiwara-Hatano, Y.; Shimizu, M. The changes in the neuronal PC12 and the intestinal epithelial Caco-2 cells during the coculture. The functional analysis using an in vitro coculture system. Cytotechnology 2001, 35, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.-D.; Chen, W.; Sun, L.-H.; Wang, W.-S.; Zhou, S.-W.; Yang, H. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol. Cell. Neurosci. 2011, 46, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Flamant, M.; Aubert, P.; Rolli-Derkinderen, M.; Bourreille, A.; Neunlist, M.R.; Mahé, M.M.; Meurette, G.; Marteyn, B.; Savidge, T.; Galmiche, J.P.; et al. Enteric glia protect against shigella flexneri invasion in intestinal epithelial cells: A role for S-nitrosoglutathione. Gut 2011, 60, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Holland-Cunz, S.; Bainczyk, S.; Hagl, C.; Wink, E.; Wedel, T.; Back, W.; Schafer, K.H. Three-dimensional co-culture model of enterocytes and primary enteric neuronal tissue. Pediatr. Surg. Int. 2004, 20, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Townley, A.K.; Schmidt, K.; Hodgson, L.; Stephens, D.J. Epithelial organization and cyst lumen expansion require efficient Sec13–Sec31-driven secretion. J. Cell Sci. 2012, 125, 673–684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lahar, N.; Lei, N.Y.; Wang, J.; Jabaji, Z.; Tung, S.C.; Joshi, V.; Lewis, M.; Stelzner, M.; Martin, M.G.; Dunn, J.C. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS ONE 2011, 6, e26898. [Google Scholar] [PubMed]
- Yoshikawa, T.; Hamada, S.; Otsuji, E.; Tsujimoto, H.; Hagiwara, A. Endocrine differentiation of rat enterocytes in long-term three-dimensional co-culture with intestinal myofibroblasts. Vitro Cell. Dev. Biol. Anim. 2011, 47, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.E.; Bullock, A.J.; Day, M.J.; MacNeil, S. Co-culture of intestinal epithelial and stromal cells in 3D collagen-based environments. Regen. Med. 2009, 4, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, M.; Schneiderhan-Marra, N.; Baur, N.; Hefner, K.; Blum, M.; Stein, G.M.; Joos, T.O.; Schmolz, M. Characterization of immunologically active drugs in a novel organotypic co-culture model of the human gut and whole blood. Int. Immunopharmacol. 2012, 14, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Rubio, T.; Mujico, J.R.; Marcos, A.; Puertollano, E.; Nadal, I.; Sanz, Y.; Nova, E. Immunostimulatory effect of faecal Bifidobacterium species of breast-fed and formula-fed infants in a peripheral blood mononuclear cell/Caco-2 co-culture system. Br. J. Nutr. 2011, 106, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Tiscornia, I.; Sanchez-Martins, V.; Hernandez, A.; Bollati-Fogolin, M. Human monocyte-derived dendritic cells from leukoreduction system chambers after plateletpheresis are functional in an in vitro co-culture assay with intestinal epithelial cells. J. Immunol. Methods 2012, 384, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zoumpopoulou, G.; Tsakalidou, E.; Dewulf, J.; Pot, B.; Grangette, C. Differential crosstalk between epithelial cells, dendritic cells and bacteria in a co-culture model. Int. J. Food Microbiol. 2009, 131, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, M.; Chieppa, M.; Larghi, P.; Vulcano, M.; Allavena, P.; Rescigno, M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood 2005, 106, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Le Hegarat, L.; Huet, S.; Fessard, V. A co-culture system of human intestinal Caco-2 cells and lymphoblastoid TK6 cells for investigating the genotoxicity of oral compounds. Mutagenesis 2012, 27, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Satsu, H.; Totsuka, M.; Shimizu, M. Iex-1 suppresses apoptotic damage in human intestinal epithelial Caco-2 cells induced by co-culturing with macrophage-like THP-1 cells. Biosci. Rep. 2011, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Nishitani, Y.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. In vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264.7 cells. Biochem. Biophys. Res. Commun. 2008, 374, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ng, C.P.; Tsang, L.L.; Ho, L.S.; Xu, P.H.; Rowlands, D.K.; Gao, J.Y.; Chung, Y.W.; Li, T.Y.; Chan, H.C. Altered expression of inflammatory cytokine receptors in response to LPS challenge through interaction between intestinal epithelial cells and lymphocytes of Peyer’s patch. Cell Biol. Int. 2009, 33, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Stybayeva, G.; Zhu, H.; Ramanculov, E.; Dandekar, S.; George, M.; Revzin, A. Micropatterned co-cultures of T-lymphocytes and epithelial cells as a model of mucosal immune system. Biochem. Biophys. Res. Commun. 2009, 380, 575–580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thierry, A.C.; Bernasconi, E.; Mercenier, A.; Corthésy, B. Conditioned polarized Caco-2 cell monolayers allow to discriminate for the ability of gut-derived microorganisms to modulate permeability and antigen-induced basophil degranulation. Clin. Exp. Allergy 2009, 39, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Wilcz-Villega, E.M.; McClean, S.; O’Sullivan, M.A. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: Implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Andrade, F.; Araújo, F.; Ferreira, D.; Sarmento, B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur. J. Pharm. Biopharm. 2013, 83, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, D.; Sui, Z.; Qi, X.; Ji, L.; Wang, X.; Yang, L. Development of an improved three-dimensional in vitro intestinal mucosa model for drug absorption evaluation. Tissue Eng. 2013, 19, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Leonard, F.; Ali, H.; Collnot, E.M.; Crielaard, B.J.; Lammers, T.; Storm, G.; Lehr, C.M. Screening of budesonide nanoformulations for treatment of inflammatory bowel disease in an inflamed 3D cell-culture model. Altex 2012, 29, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Pusch, J.; Votteler, M.; Göhler, S.; Engl, J.; Hampel, M.; Walles, H.; Schenke-Layland, K. The physiological performance of a three-dimensional model that mimics the microenvironment of the small intestine. Biomaterials 2011, 32, 7469–7478. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, M.; Duś, D.; Mitterrand, M.; Lamerant-Fayel, N.; Kieda, C. Flow cytometric assay for quantitative and qualitative evaluation of adhesive interactions of tumor cells with endothelial cells. Microvasc. Res. 2008, 76, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Vasquez, K.; Ovalle-Marin, A.; Fuentes, F.; Parra, C.; Quitral, V.; Jimenez, P.; Garcia-Diaz, D.F. Chilean native fruit extracts inhibit inflammation linked to the pathogenic interaction between adipocytes and macrophages. J. Med. Food 2015, 18, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Kitadate, K.; Nishioka, H.; Fujii, H.; Kizaki, T.; Kondoh, Y.; Izawa, T.; Ishida, H.; Radak, Z.; Ohno, H. Oligomerized grape seed polyphenols attenuate inflammatory changes due to antioxidative properties in coculture of adipocytes and macrophages. J. Nutr. Biochem. 2010, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Ann. Rev. Med. 2015, 66, 343–359. [Google Scholar] [PubMed]
- Ginter, E.; Simko, V. Gut microorganisms and cardiovascular disease: Carnitine is the answer. Bratisl. Med. J. 2014, 115, 673–674. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Coconnier, M.H.; Bernet, M.F.; Kerneis, S.; Chauviere, G.; Fourniat, J.; Servin, A.L. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 1993, 110, 299–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morelli, L.; Garbagna, N.; Rizzello, F.; Zonenschain, D.; Grossi, E. In vivo association to human colon of Lactobacillus paracasei B21060: Map from biopsies. Dig. Liver Dis. 2006, 38, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.J.; Brassart, D.; Servin, A.L.; Rochat, F.; Donnet-Hughes, A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: Criteria for strain selection. Am. J. Clin. Nutr. 1997, 66, S515–S520. [Google Scholar]
- Elliott, S.N.; Buret, A.; McKnight, W.; Miller, M.J.; Wallace, J.L. Bacteria rapidly colonize and modulate healing of gastric ulcers in rats. Am. J. Physiol. 1998, 275, 425–432. [Google Scholar]
- Marzorati, M.; Vanhoecke, B.; De Ryck, T.; Sadaghian Sadabad, M.; Pinheiro, I.; Possemiers, S.; Van den Abbeele, P.; Derycke, L.; Bracke, M.; Pieters, J.; et al. The HMI module: A new tool to study the host-microbiota interaction in the human gastrointestinal tract in vitro. BMC Microbiol. 2014, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ingber, D.E. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grootaert, C.; Kamiloglu, S.; Capanoglu, E.; Van Camp, J. Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health. Nutrients 2015, 7, 9229-9255. https://doi.org/10.3390/nu7115462
Grootaert C, Kamiloglu S, Capanoglu E, Van Camp J. Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health. Nutrients. 2015; 7(11):9229-9255. https://doi.org/10.3390/nu7115462
Chicago/Turabian StyleGrootaert, Charlotte, Senem Kamiloglu, Esra Capanoglu, and John Van Camp. 2015. "Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health" Nutrients 7, no. 11: 9229-9255. https://doi.org/10.3390/nu7115462
APA StyleGrootaert, C., Kamiloglu, S., Capanoglu, E., & Van Camp, J. (2015). Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health. Nutrients, 7(11), 9229-9255. https://doi.org/10.3390/nu7115462






