The Omega-3 Fatty Acid Docosahexaenoic Acid Attenuates Organic Dust-Induced Airway Inflammation
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Swine Confinement Animal Feeding Operation Organic Dust Extract (ODE)
2.3. Cell Culture
2.4. Precision-Cut Mouse Lung Slice Cultures
2.5. Animal Care
2.6. Animal Model of ODE Exposure
2.7. Cytokine/Mediator Analyses
2.8. Flow Cytometry
2.9. Statistical Analyses
3. Results
3.1. DHA Pretreatment Reduces Organic Dust-Induced Inflammatory Cytokine/Chemokine Production from Bronchial Epithelial Cells, Monocytes, and Fibroblasts
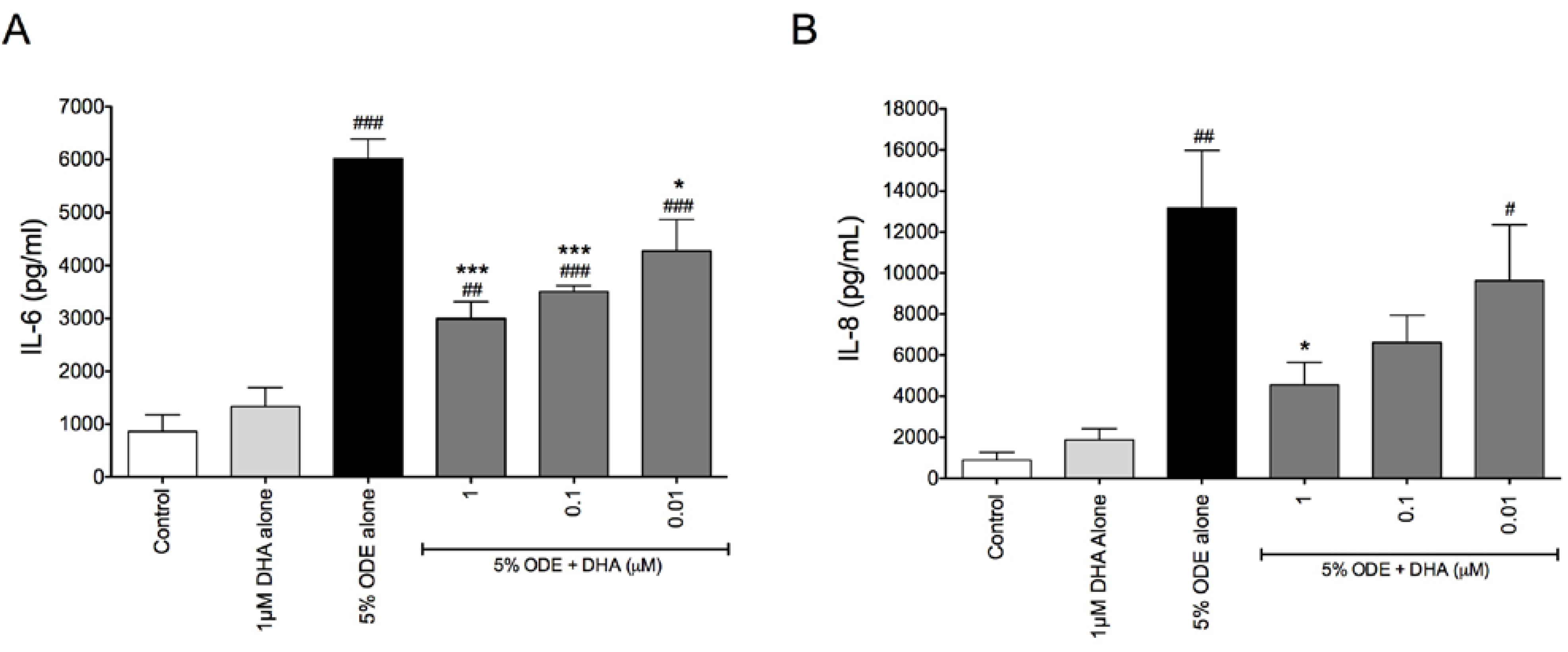
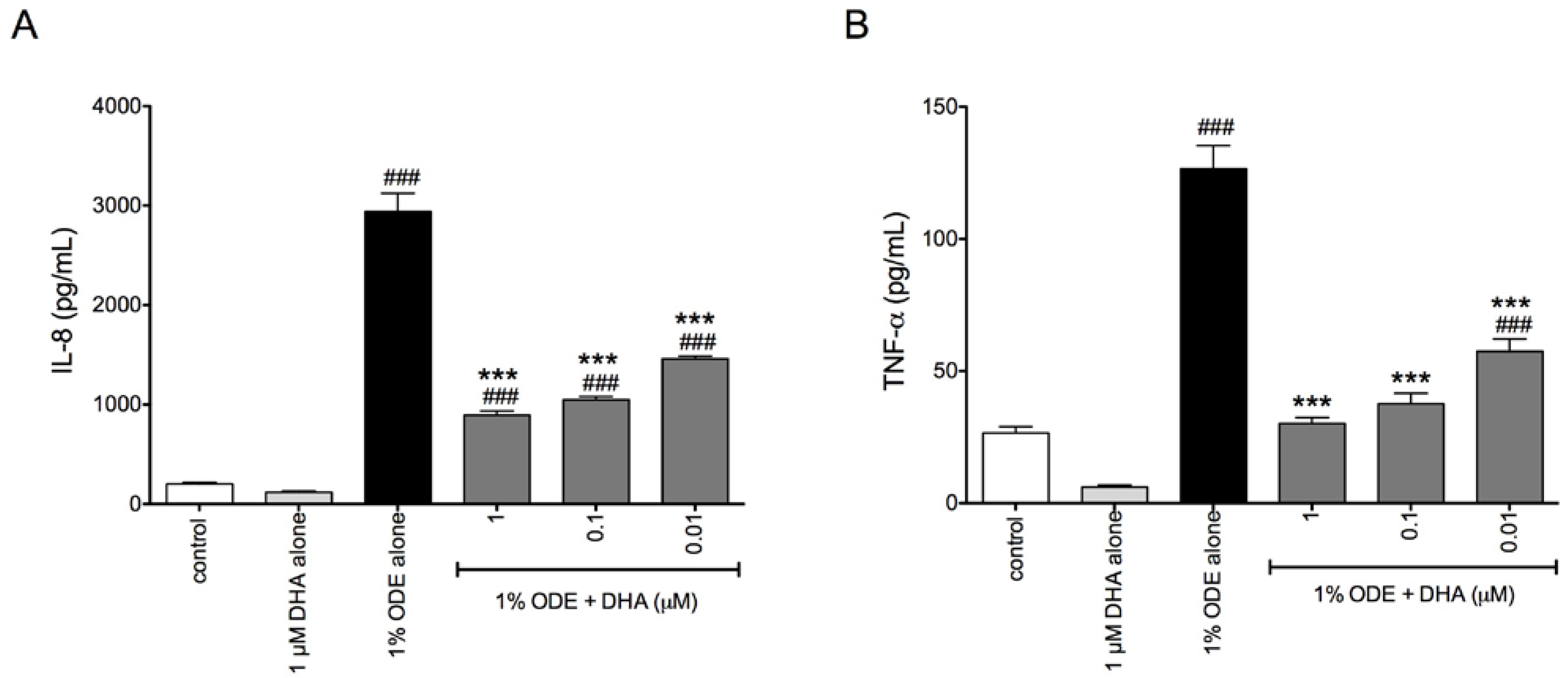
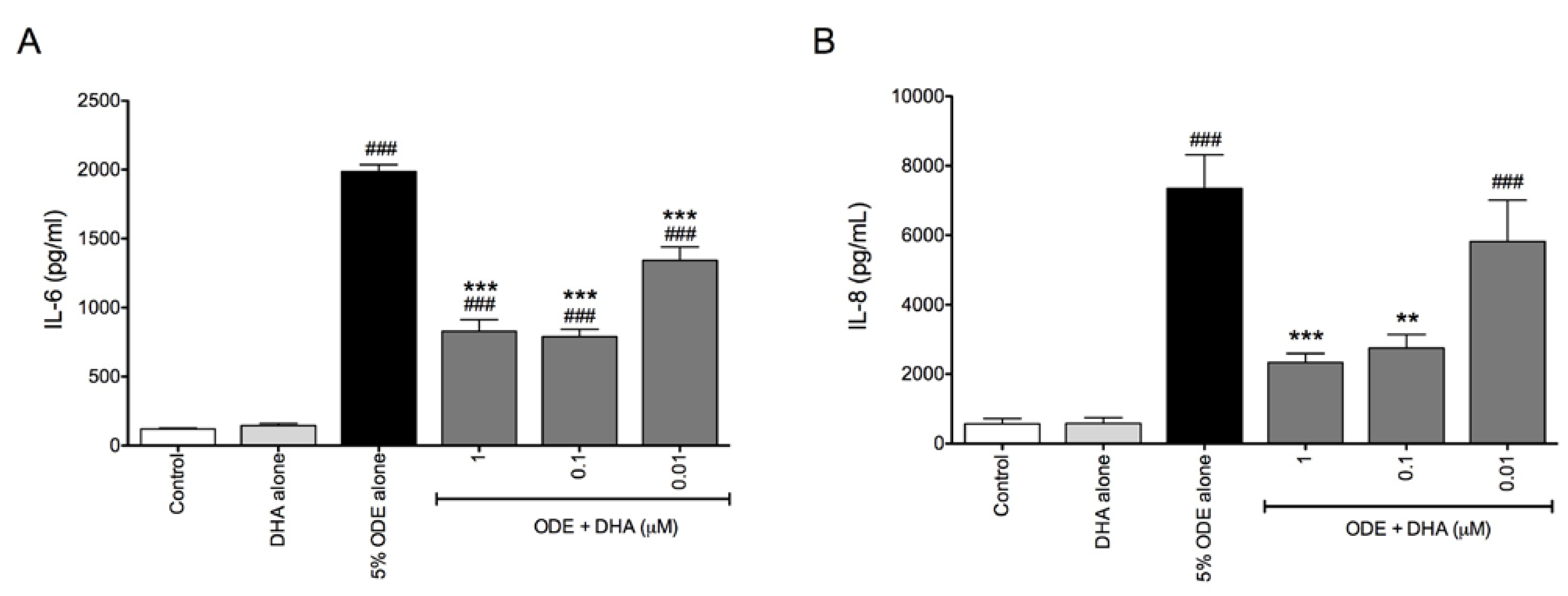
3.2. DHA Pretreatment Reduces ODE-Induced Inflammatory Cytokine Production in Ex Vivo Precision-Cut Mouse Lung Slice Cultures
3.3. DHA Pretreatment Decreases ODE-Induced ICAM-1 Up-Regulation on Bronchial Epithelial Cells
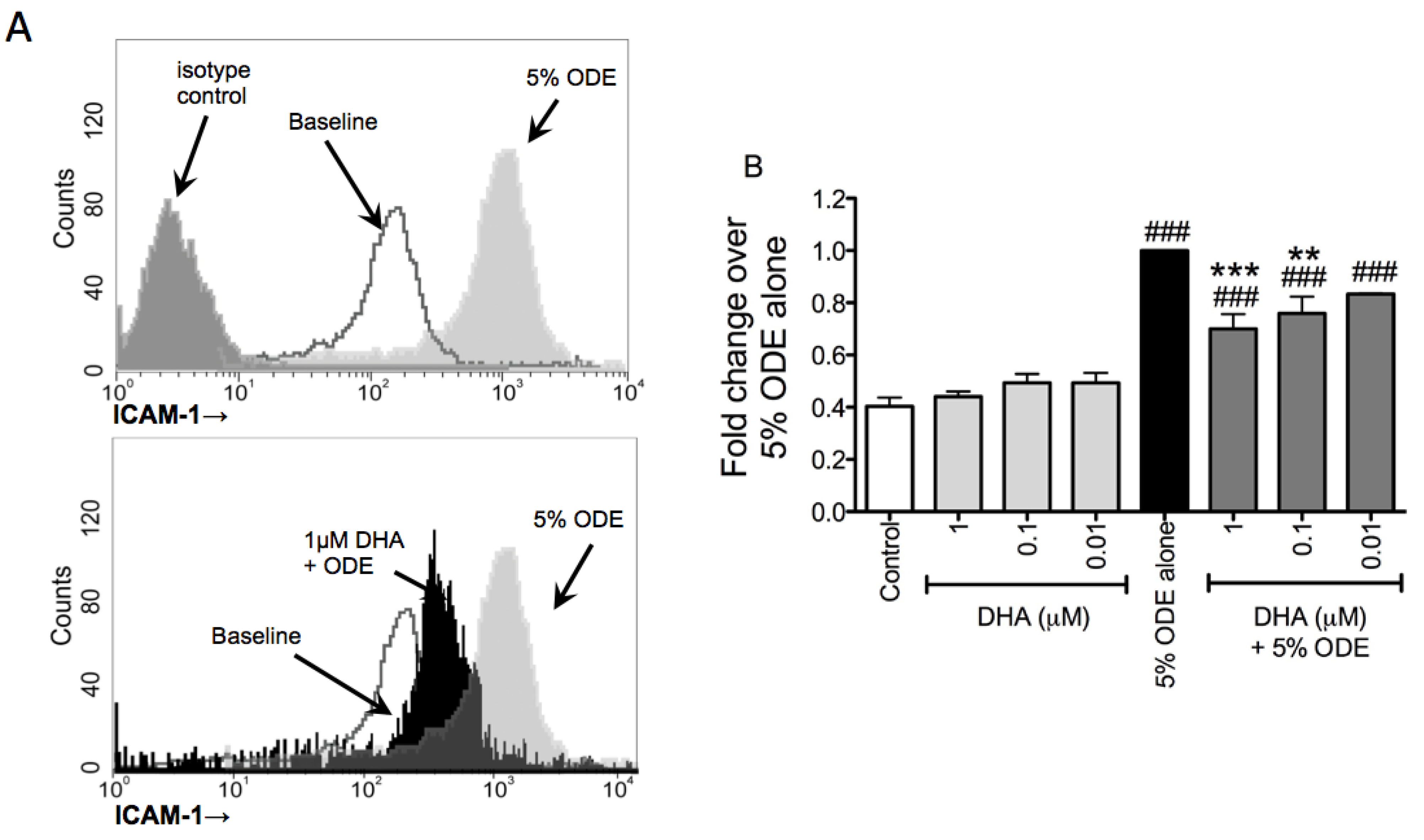
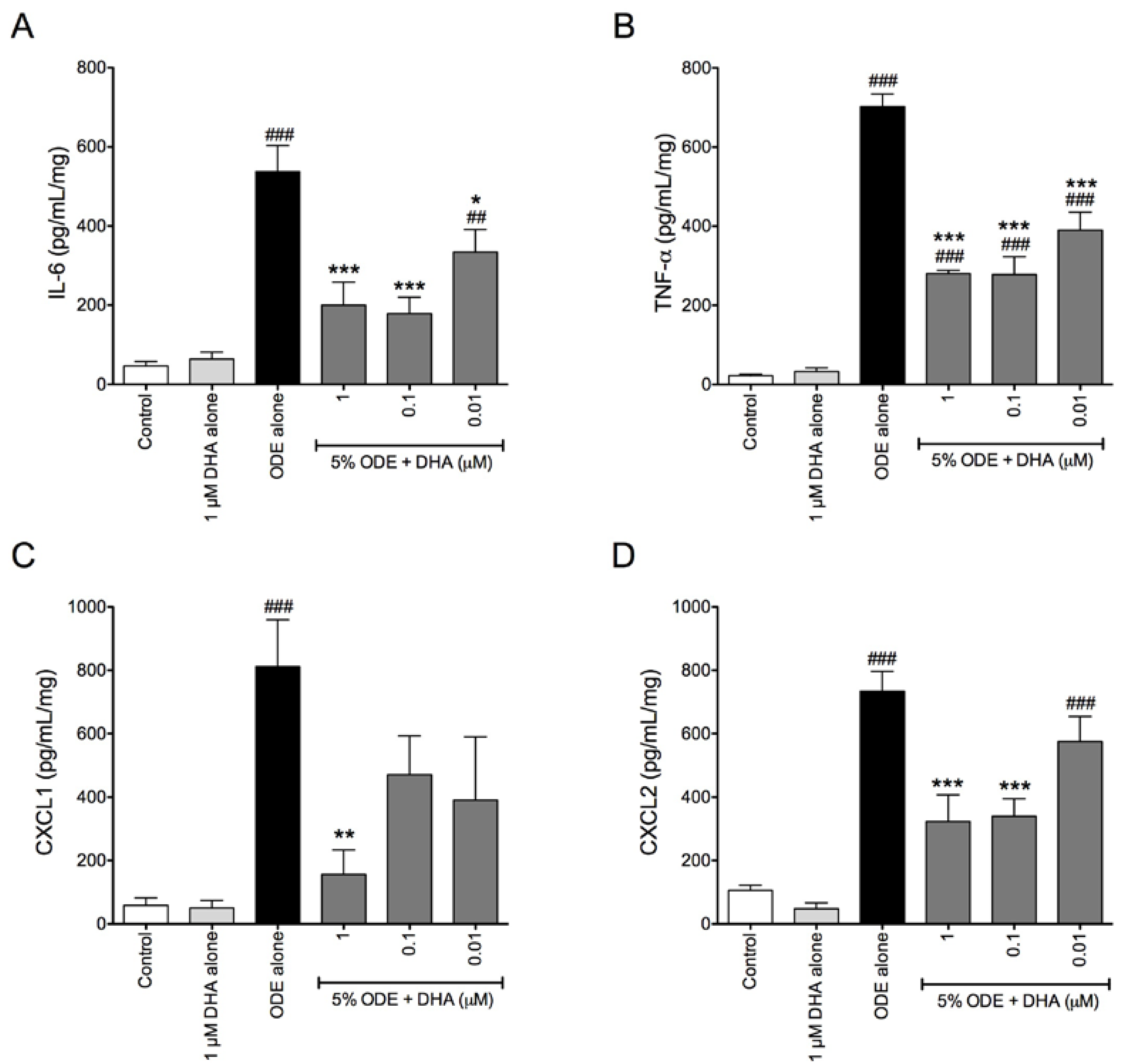
3.4. One-Week Supplementation with DHA Reduces the Airway Inflammatory Response of Mice to ODE Inhalation

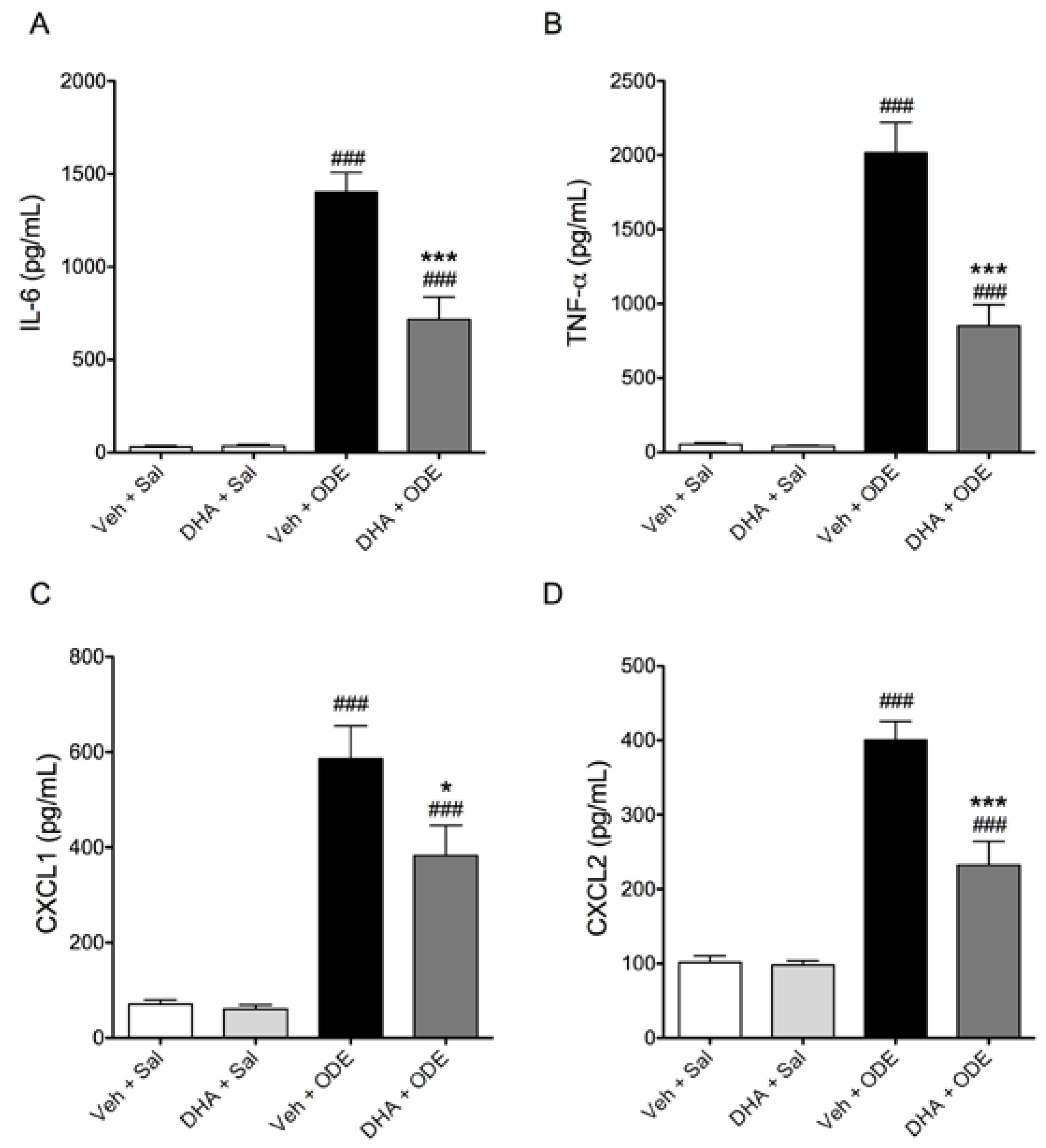
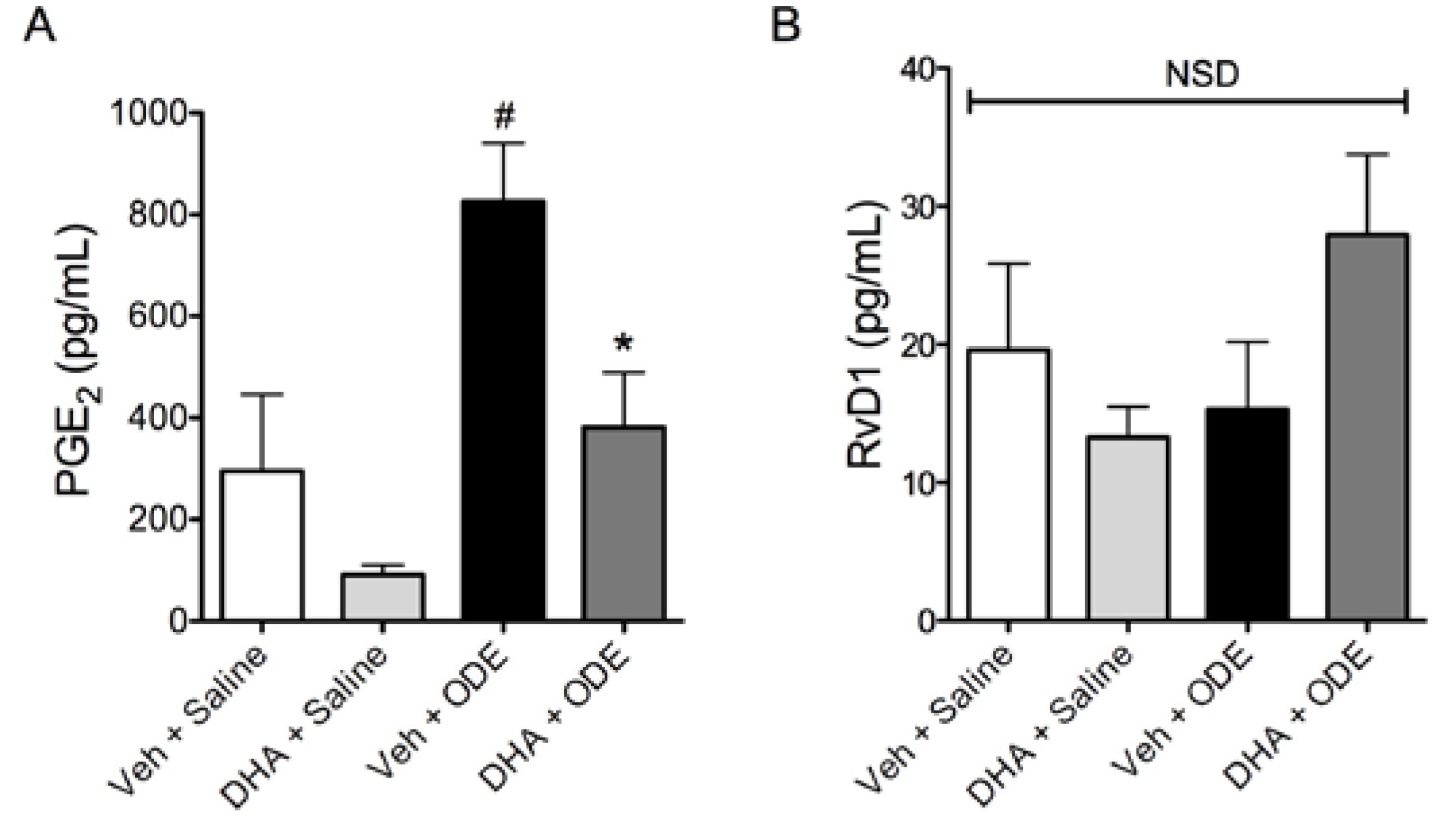
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kirkhorn, S.R.; Garry, V.F. Agricultural lung diseases. Environ. Health Perspect. 2000, 108, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.L. Consequences of respiratory exposures in the farm environment. N. C. Med. J. 2011, 72, 477–480. [Google Scholar] [PubMed]
- American Thoracic Society; National Institute for Occupational Safety and Health. Respiratory Health Hazards in Agriculture. Am. J. Respir. Crit. Care Med. 1998, 158, S1–S76. [Google Scholar]
- Von Essen, S.; Romberger, D. The respiratory inflammatory response to the swine confinement building environment: The adaptation to respiratory exposures in the chronically exposed worker. J. Agric. Saf. Health 2003, 9, 185–196. [Google Scholar]
- Wang, Z.; Larsson, K.; Palmberg, L.; Malmberg, P.; Larsson, P.; Larsson, L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur. Respir. J. 1997, 10, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Szczyrek, M.; Krawczyk, P.; Milanowski, J.; Jastrzebska, I.; Zwolak, A.; Daniluk, J. Chronic obstructive pulmonary disease in farmers and agricultural workers—An overview. Ann. Agric. Environ. Med. 2011, 18, 310–313. [Google Scholar] [PubMed]
- De Lorgeril, M.; Salen, P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Torrejon, C. The omega-3 fatty acid nutritional landscape: Health benefits and sources. J. Nutr. 2012, 142, 587S–591S. [Google Scholar] [CrossRef] [PubMed]
- Giudetti, A.M.; Cagnazzo, R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012, 99, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Strichartz, G.; Serhan, C.N. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011, 34, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Serhan, C.N. Lipoxins and new lipid mediators in the resolution of inflammation. Curr. Opin. Pharmacol. 2006, 6, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Controlling the resolution of acute inflammation: A new genus of dual anti-inflammatory and proresolving mediators. J. Periodontol. 2008, 79, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.K.; Xu, Z.Z.; Ji, R.R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [PubMed]
- Hasturk, H.; Kantarci, A.; Goguet-Surmenian, E.; Blackwood, A.; Andry, C.; Serhan, C.N.; van Dyke, T.E. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007, 179, 7021–7029. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.M.; Sapinoro, R.E.; Thatcher, T.H.; Croasdell, A.; Levy, E.P.; Fulton, R.A.; Olsen, K.C.; Pollock, S.J.; Serhan, C.N.; Phipps, R.P.; et al. A novel anti-inflammatory and pro-resolving role for resolvin d1 in acute cigarette smoke-induced lung inflammation. PLoS One 2013. [Google Scholar] [CrossRef]
- Seki, H.; Fukunaga, K.; Arita, M.; Arai, H.; Nakanishi, H.; Taguchi, R.; Miyasho, T.; Takamiya, R.; Asano, K.; Ishizaka, A.; et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 2010, 184, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Kawata, T.; Shimizu, Y.; Okajima, F.; Dobashi, K.; Mori, M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008, 367, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, T.M.; Heires, A.J.; Wyatt, T.A.; Poole, J.A.; LeVan, T.D.; Cerutis, D.R.; Romberger, D.J. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir. Res. 2013. [Google Scholar] [CrossRef]
- Poole, J.A.; Wyatt, T.A.; Oldenburg, P.J.; Elliott, M.K.; West, W.W.; Sisson, J.H.; Von Essen, S.G.; Romberger, D.J. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L1085–L1095. [Google Scholar] [CrossRef] [PubMed]
- Romberger, D.J.; Bodlak, V.; von Essen, S.G.; Mathisen, T.; Wyatt, T.A. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J. Appl. Physiol. 2002, 93, 289–296. [Google Scholar] [PubMed]
- Poole, J.A.; Dooley, G.P.; Saito, R.; Burrell, A.M.; Bailey, K.L.; Romberger, D.J.; Mehaffy, J.; Reynolds, S.J. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J. Toxicol. Environ. Health 2010, 73, 684–700. [Google Scholar] [CrossRef]
- Wyatt, T.A.; Kharbanda, K.K.; McCaskill, M.L.; Tuma, D.J.; Yanov, D.; DeVasure, J.; Sisson, J.H. Malondialdehyde-acetaldehyde-adducted protein inhalation causes lung injury. Alcohol 2012, 46, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gurzell, E.A.; Wiesinger, J.A.; Morkam, C.; Hemmrich, S.; Harris, W.S.; Fenton, J.I. Is the omega-3 index a valid marker of intestinal membrane phospholipid EPA + DHA content? Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Mathisen, T.; von Essen, S.G.; Wyatt, T.A.; Romberger, D.J. Hog barn dust extract augments lymphocyte adhesion to human airway epithelial cells. J. Appl. Physiol. 2004, 96, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Wyatt, T.A.; von Essen, S.G.; Hervert, J.; Parks, C.; Mathisen, T.; Romberger, D.J. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J. Allergy Clin. Immunol. 2007, 120, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Laberge, S.; El Bassam, S. Cytokines, structural cells of the lungs and airway inflammation. Paediatr. Respir. Rev. 2004, 5, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Chow, C.W.; Downey, G.P. Role of innate immune cells and their products in lung immunopathology. Int. J. Biochem. Cell Biol. 2008, 40, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Tosi, M.F.; Stark, J.M.; Smith, C.W.; Hamedani, A.; Gruenert, D.C.; Infeld, M.D. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: Effects on neutrophil-epithelial cell adhesion. Am. J. Respir. Cell Mol. Biol. 1992, 7, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, P.G.; van den Tweel, M.C.; Henricks, P.A.; Engels, F.; Wagenaar, S.S.; Rutten, A.A.; Nijkamp, F.P. Expression and modulation of adhesion molecules on human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1993, 9, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H. Airway epithelial cells as regulators of airway inflammation (review). Int. J. Mol. Med. 1998, 1, 367–378. [Google Scholar] [PubMed]
- Message, S.D.; Johnston, S.L. Host defense function of the airway epithelium in health and disease: Clinical background. J. Leukoc. Biol. 2004, 75, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.A.; Slager, R.E.; Heires, A.J.; Devasure, J.M.; Vonessen, S.G.; Poole, J.A.; Romberger, D.J. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am. J. Respir. Cell. Mol. Biol. 2010, 42, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Gleason, A.M.; Bauer, C.; West, W.W.; Alexis, N.; van Rooijen, N.; Reynolds, S.J.; Romberger, D.J.; Kielian, T.L. CD11c+/CD11b+ cells are critical for organic dust—Elicited murine lung inflammation. Am. J. Respir. Cell. Mol. Biol. 2012, 47, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Romberger, D.J. Immunological and inflammatory responses to organic dust in agriculture. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 126–132. [Google Scholar] [PubMed]
- Gong, J.; Wu, Z.Y.; Qi, H.; Chen, L.; Li, H.B.; Li, B.; Yao, C.Y.; Wang, Y.X.; Wu, J.; Yuan, S.Y.; et al. Maresin 1 mitigates lipopolysaccharide-induced acute lung injury in mice. Br. J. Pharmacol. 2014, 171, 3539–3550. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Dong, J.; Wu, W.; Yang, T.; Wang, T.; Guo, L.; Chen, L.; Xu, D.; Wen, F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir. Res. 2012, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Arruda, A.; Demichele, S.; Seth, A.; Singer, P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: A meta-analysis of outcome data. JPEN J. Parenter. Enter. Nutr. 2008, 32, 596–605. [Google Scholar] [CrossRef]
- Oliver, C.; Jahnke, N. Omega-3 fatty acids for cystic fibrosis. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Strasser, T.; Fischer, S.; Weber, P.C. Leukotriene B5 is formed in human neutrophils after dietary supplementation with icosapentaenoic acid. Proc. Natl. Acad. Sci. USA 1985, 82, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chan-Li, Y.; Collins, S.L.; Zhang, Y.; Hallowell, R.W.; Mitzner, W.; Horton, M.R. Pulmonary delivery of docosahexaenoic acid mitigates bleomycin-induced pulmonary fibrosis. BMC Pulm. Med. 2014, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.U.; Bratt, J.M.; Jiang, X.; Pedersen, T.L.; Grapov, D.; Adkins, Y.; Kelley, D.S.; Newman, J.W.; Kenyon, N.J.; Stephensen, C.B. Dietary long-chain omega-3 fatty acids do not diminish eosinophilic pulmonary inflammation in mice. Am. J. Respir. Cell. Mol. Biol. 2014, 50, 626–636. [Google Scholar] [PubMed]
- Im, D.S. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog. Lipid Res. 2012, 51, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Dennis, E.A. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 8517–8522. [Google Scholar] [CrossRef] [PubMed]
- Mimoun, M.; Coste, T.C.; Lebacq, J.; Lebecque, P.; Wallemacq, P.; Leal, T.; Armand, M. Increased tissue arachidonic acid and reduced linoleic acid in a mouse model of cystic fibrosis are reversed by supplemental glycerophospholipids enriched in docosahexaenoic acid. J. Nutr. 2009, 139, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Shek, L.P.; Chong, M.F.; Lim, J.Y.; Soh, S.E.; Chong, Y.S. Role of dietary long-chain polyunsaturated fatty acids in infant allergies and respiratory diseases. Clin. Dev. Immunol. 2012. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Poole, J.A.; Mathisen, T.L.; Wyatt, T.A.; von Essen, S.G.; Romberger, D.J. Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1049–L1054. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Wyatt, T.A.; Kielian, T.; Oldenburg, P.; Gleason, A.M.; Bauer, A.; Golden, G.; West, W.W.; Sisson, J.H.; Romberger, D.J. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.; Berube, J.; Rousseau, S. The protein kinase TPL2 is essential for ERK1/ERK2 activation and cytokine gene expression in airway epithelial cells exposed to pathogen-associated molecular patterns (PAMPs). PLoS One 2013. [Google Scholar] [CrossRef]
- Gardner, A.; Fisher, A.J.; Richter, C.; Johnson, G.E.; Moisey, E.J.; Brodlie, M.; Ward, C.; Krippner-Heidenreich, A.; Mann, D.A.; Borthwick, L.A. The critical role of TAK1 in accentuated epithelial to mesenchymal transition in obliterative bronchiolitis after lung transplantation. Am. J. Pathol. 2012, 180, 2293–2308. [Google Scholar] [PubMed]
- Huang, F.; Kao, C.Y.; Wachi, S.; Thai, P.; Ryu, J.; Wu, R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-κB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007, 179, 6504–6513. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.L. Therapeutic applicability of anti-inflammatory and proresolving polyunsaturated fatty acid-derived lipid mediators. Sci. World J. 2010, 10, 676–712. [Google Scholar] [CrossRef]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014, 33, 1255–1264. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordgren, T.M.; Friemel, T.D.; Heires, A.J.; Poole, J.A.; Wyatt, T.A.; Romberger, D.J. The Omega-3 Fatty Acid Docosahexaenoic Acid Attenuates Organic Dust-Induced Airway Inflammation. Nutrients 2014, 6, 5434-5452. https://doi.org/10.3390/nu6125434
Nordgren TM, Friemel TD, Heires AJ, Poole JA, Wyatt TA, Romberger DJ. The Omega-3 Fatty Acid Docosahexaenoic Acid Attenuates Organic Dust-Induced Airway Inflammation. Nutrients. 2014; 6(12):5434-5452. https://doi.org/10.3390/nu6125434
Chicago/Turabian StyleNordgren, Tara M., Taylor D. Friemel, Art J. Heires, Jill A. Poole, Todd A. Wyatt, and Debra J. Romberger. 2014. "The Omega-3 Fatty Acid Docosahexaenoic Acid Attenuates Organic Dust-Induced Airway Inflammation" Nutrients 6, no. 12: 5434-5452. https://doi.org/10.3390/nu6125434
APA StyleNordgren, T. M., Friemel, T. D., Heires, A. J., Poole, J. A., Wyatt, T. A., & Romberger, D. J. (2014). The Omega-3 Fatty Acid Docosahexaenoic Acid Attenuates Organic Dust-Induced Airway Inflammation. Nutrients, 6(12), 5434-5452. https://doi.org/10.3390/nu6125434




