Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays
Abstract
:1. Introduction

2. Results and Discussion
2.1. Total Antioxidant Power and Phenolics Content
| Assay | Methanol extract | Water extract |
|---|---|---|
| ORAC-PYR (mM TE/g) | 0.91 ± 0.04 | 1.18 ± 0.06 |
| DPPH (mM TE/g) | 0.52 ± 0.01 | 0.50 ± 0.2 |
| DPPH (IC50 μg/mL) | 54.20 ± 2.1 | 51.6 ± 3.2 |
| TEAC (mM TE/g) | 0.89 ± 0.05 | 0.99 ± 0.11 |
| TEAC (IC50 mg/mL) | 0.91 ± 0.08 | 0.89 ± 0.04 |
| FRAP (mM TE/g) | 0.58 ± 0.02 | 0.50 ± 0.01 |
| FRAP (mmol FeII/g) | 1.35 ± 0.04 | 1.10 ± 0.03 |
| Total phenolics (mM GAE/g) | 1.02 ± 0.03 | 0.64 ± 0.02 |
| Total flavonoids (mM CE/g) | 0.139 ± 0.002 | 0.128 ± 0.003 |
| Extract | Gallic acid | Cyanidin 3-O-glucoside | Other anthocyans # |
|---|---|---|---|
| SFE CO2, whole plant | 1.184 ± 0.079 | 0.009 ± 0.002 | nd |
| Solvent, whole plant | 4.573 ± 0.226 | 3.134 ± 0.071 | 0.042 ± 0.004 |
| Solvent, external layer | 3.413 ± 0.135 | 11.892 ± 0.676 | 0.507 ± 0.049 |
| Solvent, peeled plant | 2.894 ± 0.031 | 0.028 ± 0.002 | tr |
2.2. Antioxidant Power in Models of Lipid Oxidation

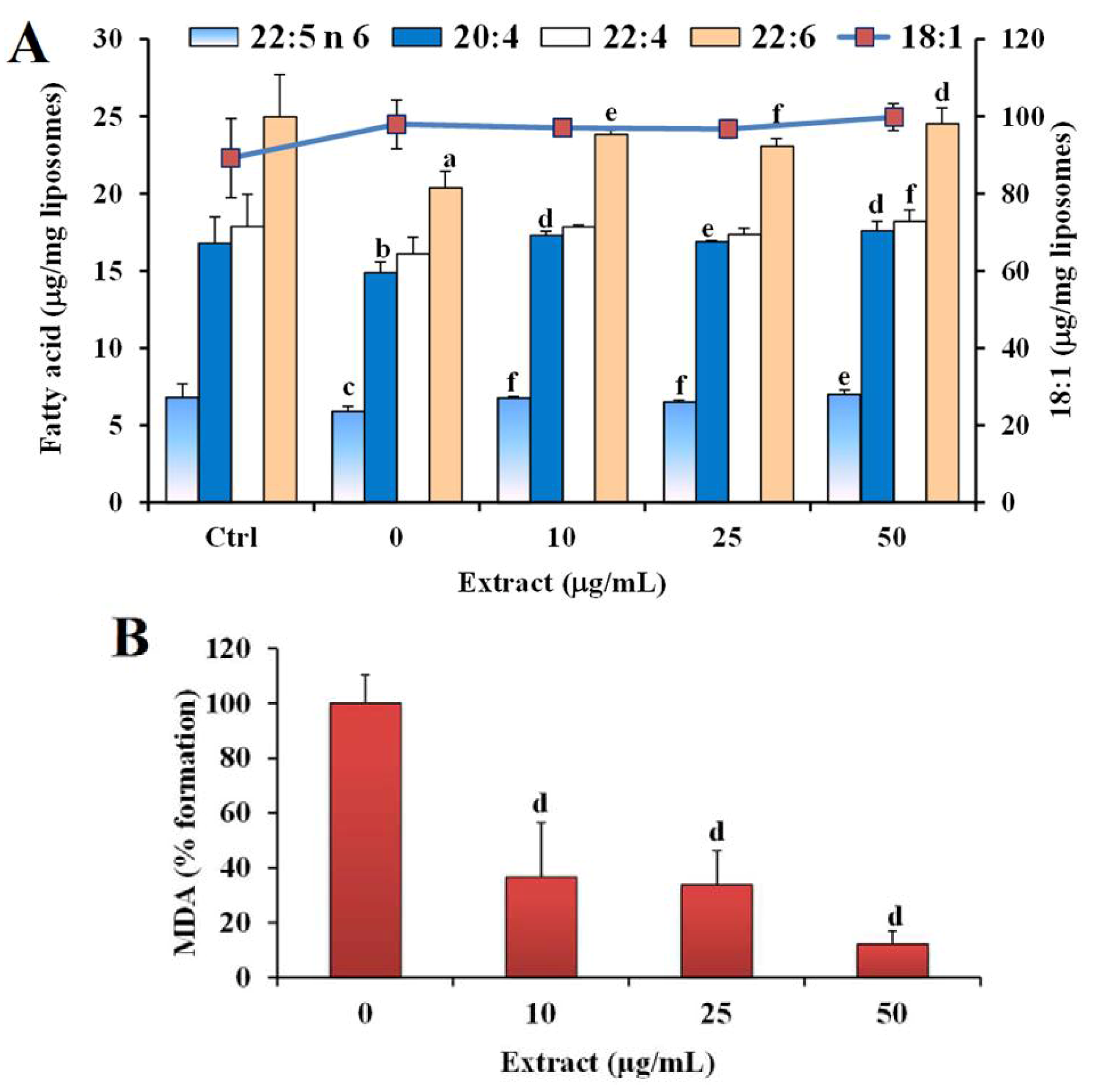
3. Experimental Section
3.1. Chemicals
3.2. Plant Materials
3.3. Preparation of Extracts
3.4. Total Phenolics Determination
3.5. Total Flavonoids Determination
3.6. HPLC-DAD Analysis
3.7. DPPH (1,1-Diphenyl-2-picrylhydrazyl Radical) Scavenging Assay
3.8. Ferric Reducing Antioxidant Power (FRAP)
3.9. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.10. ORAC-PYR (Oxygen Radical Absorbance Capacity-Pyrogallol Red) Determination
3.11. Cholesterol Oxidation Assay
3.12. Liposomes Preparation and Oxidation Assays
3.13. Statistical Analysis
4. Conclusions
Conflict of Interest
References
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant activity of mulberry fruit extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar]
- Hsu, F.L.; Huang, W.J.; Wu, T.H.; Lee, M.H.; Chen, L.C.; Lu, H.J.; Hou, W.C.; Lin, M.H. Evaluation of antioxidant and free radical scavenging capacities of polyphenolics from pods of Caesalpinia pulcherrima. Int. J. Mol. Sci. 2012, 13, 6073–6088. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Oyedemi, B.O.; Arowosegbe, S.; Afolayan, A.J. Phytochemicals analysis and medicinal potentials of hydroalcoholic extract from Curtisia dentata (Burm.f) C.A. Sm stem bark. Int. J. Mol. Sci. 2012, 13, 6189–6203. [Google Scholar] [CrossRef]
- Dharmananda, S. Cynomorium—Parasitic Plant Widely Used in Traditional Medicine. Available online: http://www.itmonline.org/arts/cynomorium.htm (accessed on 8 January 2013).
- Duke, J.A.; Duke, P.-A.K.; duCellier, J.L. Duke’s Handbook of Medicinal Plants of the Bible, 1st ed; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Abdel-Magied, E.M.; Abdel-Rahman, H.A.; Harraz, F.M. The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature wistar rats. J. Ethnopharmacol. 2001, 75, 1–4. [Google Scholar] [CrossRef]
- Al-Qarawi, A.A.; Abdel-Rahman, H.A.; El-Badry, A.A.; Harraz, F.; Razig, N.A.; Abdel-Magied, E.M. The effect of extracts of Cynomorium coccineum and Withania somnifera on gonadotrophins and ovarian follicles of immature wistar rats. Phytother. Res. 2000, 14, 288–290. [Google Scholar] [CrossRef]
- Rosa, A.; Rescigno, A.; Piras, A.; Atzeri, A.; Scano, P.; Porcedda, S.; Zucca, P.; Assunta Dessì, M. Chemical composition and effect on intestinal Caco-2 cell viability and lipid profile of fixed oil from Cynomorium coccineum L. Food Chem. Toxicol. 2012, 50, 3799–3807. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. Combined use of multiple methodologies for the measurement of total antioxidant capacity in UK commercially available vegetable juices. Plant Foods Hum. Nutr. 2012, 67, 142–147. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E.; Trogu, E.; Sollai, F.; Rescigno, A. Evaluation of antioxidant capacity of antioxidant-declared beverages marketed in Italy. Ital. J. Food Sci. 2010, 22, 313–319. [Google Scholar]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Rached, W.; Benamar, H.; Bennaceur, M.; Marouf, A. Screening of the antioxidant potential of some Algerian indigenous plants. J. Biol. Sci. 2010, 10, 316–324. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Wang, X.; Zhang, G.; Wang, Y.; Di, D. Evaluation of the free radical scavenging activity of Cynomorium songaricum Rupr. by a novel DPPH-HPLC method. J. Food Sci. 2011, 76, C1245–C1249. [Google Scholar] [CrossRef]
- Wong, C.C.; Li, H.B.; Cheng, K.W.; Chen, F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Harborne, J.B.; Saito, N.; Detoni, C.H. Anthocyanins of Cephaelis, Cynomorium, Euterpe, Lavatera and Pinana. Biochem. Syst. Ecol. 1994, 22, 835–836. [Google Scholar] [CrossRef]
- Padula, M.C.; Lepore, L.; Milella, L.; Ovesna, J.; Malafronte, N.; Martelli, G.; de Tommasi, N. Cultivar based selection and genetic analysis of strawberry fruits with high levels of health promoting compounds. Food Chem. 2012, in press.. [Google Scholar]
- Mulabagal, V.; Keller, W.J.; Calderón, A.I. Quantitative analysis of anthocyanins in Euterpe oleracea (açaí) dietary supplement raw materials and capsules by Q-TOF liquid chromatography/mass spectrometry. Pharm. Biol. 2012, 50, 1289–1296. [Google Scholar] [CrossRef]
- Jung, S.; Choe, J.H.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef]
- Hsu, C.L.; Yen, G.Y. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Rosa, A.; Melis, M.P.; Deiana, M.; Atzeri, A.; Appendino, G.; Corona, G.; Incani, A.; Loru, D.; Dessì, M.A. Protective effect of the oligomeric acylphloroglucinols from Myrtus communis on cholesterol and human low density lipoprotein oxidation. Chem. Phys. Lipids 2008, 155, 16–23. [Google Scholar] [CrossRef]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dess, M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef]
- Garenc, C.; Julien, P.; Levy, E. Oxysterols in biological systems: The gastrointestinal tract, liver, vascular wall and central nervous system. Free Radic. Res. 2010, 44, 47–73. [Google Scholar] [CrossRef]
- Mosca, M.; Ceglie, A.; Ambrosone, L. Effect of membrane composition on lipid oxidation in liposomes. Chem. Phys. Lipids 2011, 164, 158–165. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar]
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessì, M.A. Chemical composition and antioxidant activities of Myrtus communis L. Berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Alarcón, E.; Campos, A.M.; Edwards, A.M.; Lissi, E.; López-Alarcón, C. Antioxidant capacity of herbal infusions and tea extracts: A comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 2008, 107, 1114–1119. [Google Scholar] [CrossRef]
- Templar, J.; Kon, S.P.; Milligan, T.P.; Newman, D.J.; Raftery, M.J. Increased plasma malondialdehyde levels in glomerular disease as determined by a fully validated hplc method. Nephrol. Dial. Transplant. 1999, 14, 946–951. [Google Scholar] [CrossRef]
- Erithacus Software, GraFit Software, version 7, Erithacus Software: London, UK, 2010.
- The R Foundation for Statistical Computing, R Software, version 2.5.1, The R Foundation for Statistical Computing: Vienna, Austria, 2007.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zucca, P.; Rosa, A.; Tuberoso, C.I.G.; Piras, A.; Rinaldi, A.C.; Sanjust, E.; Dessì, M.A.; Rescigno, A. Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays. Nutrients 2013, 5, 149-161. https://doi.org/10.3390/nu5010149
Zucca P, Rosa A, Tuberoso CIG, Piras A, Rinaldi AC, Sanjust E, Dessì MA, Rescigno A. Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays. Nutrients. 2013; 5(1):149-161. https://doi.org/10.3390/nu5010149
Chicago/Turabian StyleZucca, Paolo, Antonella Rosa, Carlo I. G. Tuberoso, Alessandra Piras, Andrea C. Rinaldi, Enrico Sanjust, Maria A. Dessì, and Antonio Rescigno. 2013. "Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays" Nutrients 5, no. 1: 149-161. https://doi.org/10.3390/nu5010149
APA StyleZucca, P., Rosa, A., Tuberoso, C. I. G., Piras, A., Rinaldi, A. C., Sanjust, E., Dessì, M. A., & Rescigno, A. (2013). Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays. Nutrients, 5(1), 149-161. https://doi.org/10.3390/nu5010149











