The Potential Role of Iron Homeostasis and Ferroptosis in Exercise Nutrition and Health
Abstract
1. Introduction
2. Iron Homeostasis and Importance in Maintaining Muscle Health
2.1. Iron Homeostasis and Function
2.2. The Effects of Iron Overload on Muscle Health
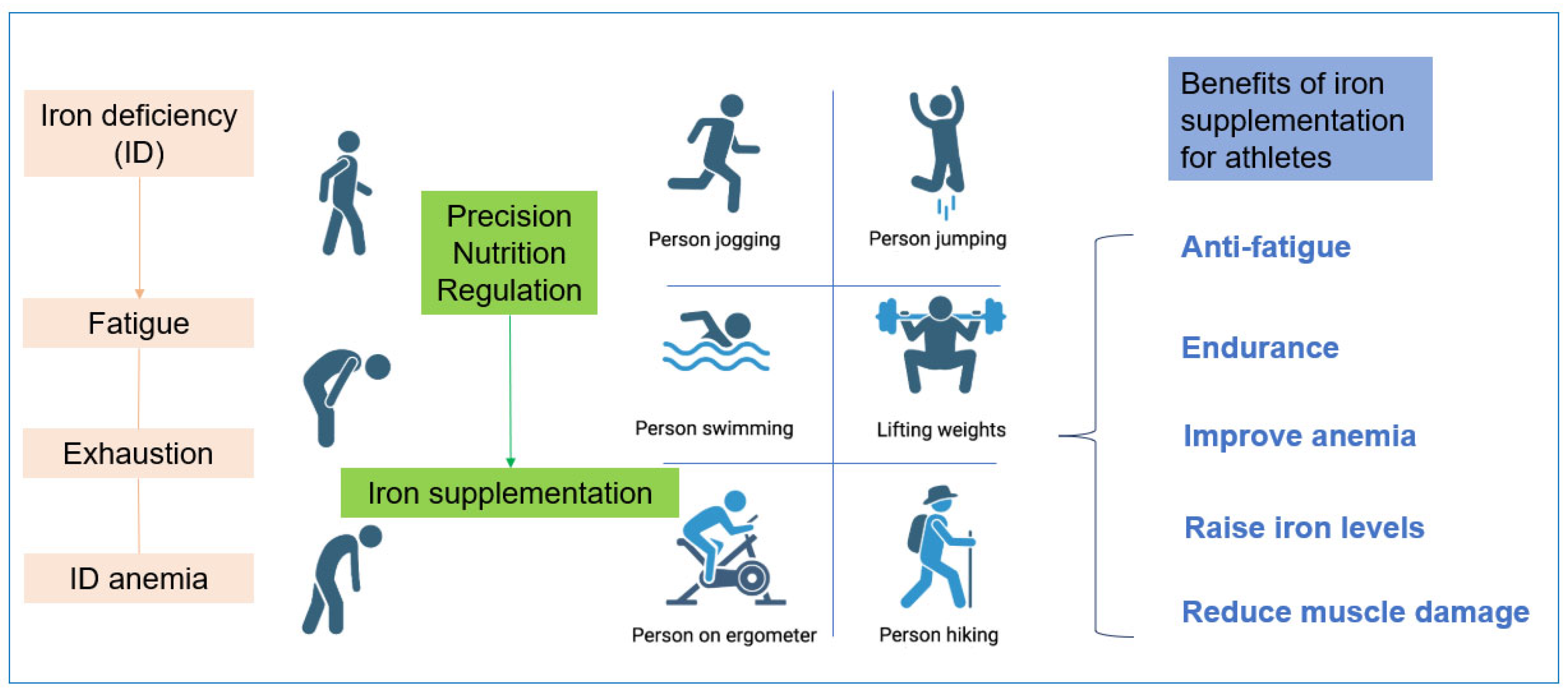
2.3. The Impact of Iron Deficiency on Muscle Health
3. Ferroptosis and Targeted Therapy
3.1. Ferroptosis Overview
3.2. The Role of Ferroptosis in Muscle Diseases
3.3. Research Progress on Targeted Therapy for Ferroptosis
4. The Connection Between Trace Element Iron and Athletes
4.1. Effects of Iron on Athletes’ Physiological Functions
4.2. Effects of Fe on Athletes’ Performance
5. The Development Prospects of Iron in Sports Nutrition Products
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cannas, D.; Loi, E.; Serra, M.; Firinu, D.; Valera, P.; Zavattari, P. Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients 2020, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, Y.; Zhang, Y.; Zhang, Y. Ferritin: Significance in viral infections. Rev. Med. Virol. 2024, 34, e2531. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Skalny, A.V.; Suliburska, J.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J. Trace Elem. Med. Biol. 2017, 41, 41–53. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, E.; Marley, A.; Samaan, M.A.; Brookes, M.J. Iron deficiency anaemia: Pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef]
- Auerbach, M.; DeLoughery, T.G.; Tirnauer, J.S. Iron Deficiency in Adults: A Review. JAMA 2025, 333, 1813–1823. [Google Scholar] [CrossRef]
- Siquier-Coll, J.; Bartolomé, I.; Perez-Quintero, M.; Grijota, F.J.; Arroyo, J.; Muñoz, D.; Maynar-Mariño, M. Serum, erythrocyte and urinary concentrations of iron, copper, selenium and zinc do not change during an incremental test to exhaustion in either normothermic or hyperthermic conditions. J. Therm. Biol. 2019, 86, 102425. [Google Scholar] [CrossRef]
- Maynar, M.; Muñoz, D.; Alves, J.; Barrientos, G.; Grijota, F.J.; Robles, M.C.; Llerena, F. Influence of an Acute Exercise Until Exhaustion on Serum and Urinary Concentrations of Molybdenum, Selenium, and Zinc in Athletes. Biol. Trace Elem. Res. 2018, 186, 361–369. [Google Scholar] [CrossRef]
- Solberg, A.; Reikvam, H. Iron. Status and Physical Performance in Athletes. Life 2023, 13, 2007. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, L.; Fei, F.; Yang, J. Effects of different exercise levels on serum trace element concentrations. Sci. Rep. 2025, 15, 37497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Zhang, J.; Luo, J.; Chen, T.; Zeng, X.; Nie, H. The association between iron deficiency and muscle mass/strength in patients undergoing maintenance hemodialysis. Front. Nutr. 2025, 12, 1628038. [Google Scholar] [CrossRef] [PubMed]
- De Biase, N.; Del Punta, L.; L’Hoyes, W.; Ellicori, P.; Cleland, J.G.F.; Masini, G.; Gargani, L.; Moura-Ferreira, S.; Hoedemakers, S.; Di Fiore, V.; et al. Associations of iron deficiency with cardiac function, congestion, exercise capacity and prognosis in heart failure. Eur. J. Heart Fail. 2025, 27, 889–900. [Google Scholar] [CrossRef]
- Scherbakov, N.; Sandek, A.; Valentova, M.; Mayer, A.; von Haehling, S.; Jankowska, E.; Anker, S.D.; Doehner, W. Iron Deficiency and Reduced Muscle Strength in Patients with Acute and Chronic Ischemic Stroke. J. Clin. Med. 2022, 11, 595. [Google Scholar] [CrossRef]
- Zeng, W.; Hu, M.; Ma, L.; Huang, F.; Jiang, Z. Copper and iron as unique trace elements linked to fibromyalgia risk. Sci. Rep. 2025, 15, 4019. [Google Scholar] [CrossRef]
- Vinke, J.S.J.; Gorter, A.R.; Eisenga, M.F.; Dam, W.A.; van der Meer, P.; van den Born, J.; Bakker, S.J.L.; Hoes, M.F.; de Borst, M.H. Iron deficiency is related to lower muscle mass in community-dwelling individuals and impairs myoblast proliferation. J. Cachexia Sarcopenia Muscle 2023, 14, 1865–1879. [Google Scholar] [CrossRef]
- Dziegala, M.; Josiak, K.; Kasztura, M.; Kobak, K.; von Haehling, S.; Banasiak, W.; Anker, S.D.; Ponikowski, P.; Jankowska, E. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J. Cachexia Sarcopenia Muscle 2018, 9, 802–815. [Google Scholar] [CrossRef]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef]
- Zhang, D.L.; Ghosh, M.C.; Rouault, T.A. The physiological functions of iron regulatory proteins in iron homeostasis—An update. Front. Pharmacol. 2014, 5, 124. [Google Scholar] [CrossRef]
- Mu, Q.; Chen, L.; Gao, X.; Shen, S.; Sheng, W.; Min, J.; Wang, F. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci. Bull. 2021, 66, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron metabolism: Interactions with normal and disordered erythropoiesis. Cold Spring Harb. Perspect. Med. 2012, 2, a011668. [Google Scholar] [CrossRef] [PubMed]
- Korolnek, T.; Hamza, I. Macrophages and iron trafficking at the birth and death of red cells. Blood 2015, 125, 2893–2897. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Systemic iron homeostasis and erythropoiesis. IUBMB Life 2017, 69, 399–413. [Google Scholar] [CrossRef]
- Steffen, D.; Kjaer, M.; Yeung, C.C. Exercise entrainment of musculoskeletal connective tissue clocks. Am. J. Physiol. Cell Physiol. 2024, 327, C270–C277. [Google Scholar] [CrossRef]
- McLeod, M.; Breen, L.; Hamilton, D.L.; Philp, A. Live strong and prosper: The importance of skeletal muscle strength for healthy ageing. Biogerontology 2016, 17, 497–510. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef]
- Qiu, Z.; Hou, C.; Xue, X.; Zhang, Y.; Zhang, Y.; Lin, J.; Li, J.; Zhang, H.; Liu, Y.; Hou, Q. The causal relationships between iron status and sarcopenia in Europeans: A bidirectional two-sample Mendelian randomization study. Eur. J. Clin. Nutr. 2025, 79, 207–213. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, D.; Wu, C.; Wu, Z.; Lin, J.; Liu, W. ATF3 as a molecular nexus linking ferroptosis regulation to sarcopenia pathogenesis via PI3K/Akt pathway activation. Exp. Gerontol. 2025, 209, 112830. [Google Scholar] [CrossRef]
- Alves, F.M.; Ayton, S.; Bush, A.I.; Lynch, G.S.; Koopman, R. Age-Related Changes in Skeletal Muscle Iron Homeostasis. J. Gerontol. Ser. A 2023, 78, 16–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Yu, R.; Zhang, D.; Wang, Y.; Wang, J.; Chen, X. A Cross-sectional Study on Age-Related Changes in Muscle Iron Deposition and Fat Infiltration: Associations with Grip Strength in a Healthy Adult Cohort. Biol. Trace Elem. Res. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tounaoua, M.R.; Chen, H.; Shaibu, Z.; Guo-Yang, Z. Correlation between iron accumulation and sarcopenia in middle-aged and elderly populations. BMC Musculoskelet. Disord. 2025, 26, 638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G. Is Iron Accumulation a Possible Risk Factor for Sarcopenia? Biol. Trace Elem. Res. 2018, 186, 379–383. [Google Scholar] [CrossRef] [PubMed]
- DeRuisseau, K.C.; Park, Y.M.; DeRuisseau, L.R.; Cowley, P.M.; Fazen, C.H.; Doyle, R.P. Aging-related changes in the iron status of skeletal muscle. Exp. Gerontol. 2013, 48, 1294–1302. [Google Scholar] [CrossRef]
- Koppenol, W.H. The Haber-Weiss cycle—70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Dong, D.; Shang, P. Effects of Iron Overload and Oxidative Damage on the Musculoskeletal System in the Space Environment: Data from Spaceflights and Ground-Based Simulation Models. Int. J. Mol. Sci. 2018, 19, 2608. [Google Scholar] [CrossRef]
- Zwart, S.R.; Morgan, J.L.; Smith, S.M. Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the International Space Station. Am. J. Clin. Nutr. 2013, 98, 217–223. [Google Scholar] [CrossRef]
- Smith, S.M. Red blood cell and iron metabolism during space flight. Nutrition 2002, 18, 864–866. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Y.; Mamtawla, G.; Wan, S.; Gao, X.; Zhang, L.; Li, G.; Wang, X. Iron overload is related to muscle wasting in patients with cachexia of gastric cancer: Using quantitative proteome analysis. Med. Oncol. 2020, 37, 113. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Huang, L.; Huhulea, E.N.; Enwere, C.; Aifuwa, E.; Frishman, W.H.; Aronow, W.S. Cardiac Manifestations of Nutritional Deficiencies. Cardiol. Rev. 2025, 10. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pinho, C.P.S.; da Silveira, A.C. Nutritional aspects in heart failure. J. Nutr. Health Sci. 2014, 1, 305. [Google Scholar] [CrossRef]
- Asperti, M.; Cantamessa, L.; Gryzik, M.; Bugatti, M.; Codenotti, S.; Denardo, A.; Vermi, W.; Fanzani, A.; Poli, M. The modulation of iron metabolism affects the Rhabdomyosarcoma tumor growth in vitro and in vivo. Clin. Exp. Med. 2023, 23, 2487–2502. [Google Scholar] [CrossRef] [PubMed]
- Lo Martire, V.; Alvente, S.; Bastianini, S.; Berteotti, C.; Valli, A.; Manconi, M.; Zoccoli, G.; Silvani, A. Sleep and Tibialis Anterior Muscle Activity in Mice with Mild Hypoxia and Iron Deficiency: Implications for the Restless Legs Syndrome. Front. Physiol. 2018, 9, 1818. [Google Scholar] [CrossRef]
- Barrientos, T.; Laothamatas, I.; Koves, T.R.; Soderblom, E.J.; Bryan, M.; Moseley, M.A.; Muoio, D.M.; Andrews, N.C. Metabolic Catastrophe in Mice Lacking Transferrin Receptor in Muscle. eBioMedicine 2015, 2, 1705–1717. [Google Scholar] [CrossRef]
- Wiecha, S.; Jarocka, M.; Wiśniowski, P.; Cieśliński, M.; Price, S.; Makaruk, B.; Kotowska, J.; Drabarek, D.; Cieśliński, I.; Sacewicz, T. The efficacy of intermittent pneumatic compression and negative pressure therapy on muscle function, soreness and serum indices of muscle damage: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2021, 13, 144. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Xie, W.; Ding, Y.; Chen, L.; Xu, G.; Wu, Y.; Wang, F. Fighting age-related orthopedic diseases: Focusing on ferroptosis. Bone Res. 2023, 11, 12. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Le, Y.; Zhang, Z.; Wang, C.; Lu, D. Ferroptotic Cell Death: New Regulatory Mechanisms for Metabolic Diseases. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 785–800. [Google Scholar] [CrossRef]
- Minotti, G.; Aust, S.D. The role of iron in oxygen radical mediated lipid peroxidation. Chem. Biol. Interact. 1989, 71, 1–19. [Google Scholar] [CrossRef]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kodumuru, V.; Sviridov, S.; Liu, S.; Chafeev, M.; Chowdhury, S.; Chakka, N.; Sun, J.; Gauthier, S.J.; Mattice, M.; et al. Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5108–5113. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Chen, Z.; Putt, D.A.; Lash, L.H. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: Further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch. Biochem. Biophys. 2000, 373, 193–202. [Google Scholar] [CrossRef]
- Da Dalt, L.; Cabodevilla, A.G.; Goldberg, I.J.; Norata, G.D. Cardiac lipid metabolism, mitochondrial function, and heart failure. Cardiovasc. Res. 2023, 119, 1905–1914. [Google Scholar] [CrossRef]
- Nichtová, Z.; Fernandez-Sanz, C.; De La Fuente, S.; Yuan, Y.; Hurst, S.; Lanvermann, S.; Tsai, H.Y.; Weaver, D.; Baggett, A.; Thompson, C.; et al. Enhanced Mitochondria-SR Tethering Triggers Adaptive Cardiac Muscle Remodeling. Circ. Res. 2023, 132, e171–e187. [Google Scholar] [CrossRef]
- Reardon, T.F.; Allen, D.G. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp. Physiol. 2009, 94, 720–730. [Google Scholar] [CrossRef]
- Altun, M.; Edström, E.; Spooner, E.; Flores-Moralez, A.; Bergman, E.; Tollet-Egnell, P.; Norstedt, G.; Kessler, B.M.; Ulfhake, B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve 2007, 36, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Bucchini, D.; Martin, M.E.; Levi, S.; Arosio, P.; Grandchamp, B.; Beaumont, C. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 2000, 275, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, W.; Leng, Y.; Xiong, Y.; Xia, Z. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol. 2020, 39, 210–225. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Jie, H.; Li, S.; Li, H.; Su, Y.; Li, L.; Kang, L.; Dong, B.; Zhang, Y. Downregulation of the (pro)renin receptor alleviates ferroptosis-associated cardiac pathological changes via the NCOA 4-mediated ferritinophagy pathway in diabetic cardiomyopathy. Int. Immunopharmacol. 2024, 138, 112605. [Google Scholar] [CrossRef]
- Kawada, S.; Nagasawa, Y.; Kawabe, M.; Ohyama, H.; Kida, A.; Kato-Kogoe, N.; Nanami, M.; Hasuike, Y.; Kuragano, T.; Kishimoto, H.; et al. Iron-induced calcification in human aortic vascular smooth muscle cells through interleukin-24 (IL-24), with/without TNF-alpha. Sci. Rep. 2018, 8, 658. [Google Scholar] [CrossRef]
- Drüeke, T.B.; Massy, Z.A. Vascular calcification in chronic kidney disease: Contribution of ferroptosis? Kidney Int. 2022, 102, 209–1211. [Google Scholar] [CrossRef]
- Jacobs, W.; Lammens, M.; Kerckhofs, A.; Voets, E.; Van San, E.; Van Coillie, S.; Peleman, C.; Mergeay, M.; Sirimsi, S.; Matheeussen, V.; et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): Autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020, 7, 3772–3781. [Google Scholar] [CrossRef]
- Ashekyan, O.; Rudnicki, M.A. Inflammation sends old muscle stem cells into a rusty meltdown. Nat. Aging 2025, 5, 1380–1382. [Google Scholar] [CrossRef]
- Wu, K.; Vaughan, A.J.; Bossowski, J.P.; Hao, Y.; Ziogou, A.; Kim, S.M.; Kim, T.H.; Nakamura, M.N.; Pillai, R.; Mancini, M.; et al. Targeting FSP1 triggers ferroptosis in lung cancer. Nature 2025, ahead of print. [Google Scholar] [CrossRef]
- Palma, M.; Chaufan, M.; Breuer, C.B.; Müller, S.; Sabatier, M.; Fraser, C.S.; Szylo, K.J.; Yavari, M.; Carmona, A.; Kaur, M.; et al. Lymph node environment drives FSP1 targetability in metastasizing melanoma. Nature 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Li, Y.; Zhang, X.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in muscle diseases and disorders: Mechanisms and therapeutic prospects. Bone Res. 2025, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Xu, H.; Ma, H.; Abedi, S.A.A.; Wang, S.; Zhang, X.; Liu, X.; Xu, H.; Wang, W.; Lou, K. A PET-based fluorescent probe for monitoring labile Fe (II) pools in macrophage activations and ferroptosis. Chem. Commun. 2022, 58, 2979–2982. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Graham, E.T.; Naowarojna, N.; Shi, Z.; Wang, Y.; Xie, G.; Zhou, L.; Salmon, W.; Jia, J.M.; Wang, X.; et al. PALP: A rapid imaging technique for stratifying ferroptosis sensitivity in normal and tumor tissues in situ. Cell Chem. Biol. 2022, 29, 157–170.e6. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, X.; Shui, S.; Wang, B.; Cui, Y.; Dong, S.; Yuwen, T.; Liu, G. PDTAC: Targeted Photodegradation of GPX4 Triggers Ferroptosis and Potent Antitumor Immunity. J. Med. Chem. 2022, 65, 12176–12187. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Zhan, M.; Yang, L.; Wang, H. Targeting ferroptosis and cuproptosis in gastrointestinal cancers: Molecular mechanisms, metabolic vulnerabilities, and therapeutic interventions. Mol. Biomed. 2025, 6, 101. [Google Scholar] [CrossRef]
- Lal, A.; Porter, J.; Sweeters, N.; Ng, V.; Evans, P.; Neumayr, L.; Kurio, G.; Harmatz, P.; Vichinsky, E. Combined chelation therapy with deferasirox and deferoxamine in thalassemia. Blood Cells Mol. Dis. 2013, 50, 99–104. [Google Scholar] [CrossRef]
- Pennell, D.J.; Porter, J.B.; Piga, A.; Lai, Y.R.; El-Beshlawy, A.; Elalfy, M.; Yesilipek, A.; Kilinç, Y.; Habr, D.; Musallam, K.M.; et al. Sustained improvements in myocardial T2* over 2 years in severely iron-overloaded patients with beta thalassemia major treated with deferasirox or deferoxamine. Am. J. Hematol. 2015, 90, 91–96. [Google Scholar] [CrossRef]
- Aydinok, Y.; Kattamis, A.; Cappellini, M.D.; El-Beshlawy, A.; Origa, R.; Elalfy, M.; Kilinç, Y.; Perrotta, S.; Karakas, Z.; Viprakasit, V.; et al. Effects of deferasirox-deferoxamine on myocardial and liver iron in patients with severe transfusional iron overload. Blood 2015, 125, 3868–3877. [Google Scholar] [CrossRef]
- Michailidis, Y.; Karagounis, L.G.; Terzis, G.; Jamurtas, A.Z.; Spengos, K.; Tsoukas, D.; Chatzinikolaou, A.; Mandalidis, D.; Stefanetti, R.J.; Papassotiriou, I.; et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 2013, 98, 233–245. [Google Scholar] [CrossRef]
- Vidart, J.; Wajner, S.M.; Leite, R.S.; Manica, A.; Schaan, B.D.; Larsen, P.R.; Maia, A.L. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: A randomized clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, 4537–4545. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; Shen, Q.; Mahoney, D.E.; Rahman, F.; Krueger, K.J.; Diaz, F.J.; Clark, L.; Smith, C.; Vacek, J.; Hiebert, J.B. Effects of Ubiquinol and/or D-ribose in Patients with Heart Failure with Preserved Ejection Fraction. Am. J. Cardiol. 2022, 176, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Buyse, G.M.; Voit, T.; Schara, U.; Straathof, C.S.M.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.M.; Finkel, R.S.; Goemans, N.; McDonald, C.M.; et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomised placebo-controlled phase 3 trial. Lancet 2015, 385, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Auersperger, I.; Škof, B.; Leskošek, B.; Knap, B.; Jerin, A.; Lainscak, M. Exercise-induced changes in iron status and hepcidin response in female runners. PLoS ONE 2013, 8, e58090. [Google Scholar] [CrossRef]
- Domínguez, R.; Sánchez-Oliver, A.J.; Mata-Ordoñez, F.; Feria-Madueño, A.; Grimaldi-Puyana, M.; López-Samanes, Á.; Pérez-López, A. Effects of an Acute Exercise Bout on Serum Hepcidin Levels. Nutrients 2018, 10, 209. [Google Scholar] [CrossRef]
- Auersperger, I.; Knap, B.; Jerin, A.; Blagus, R.; Lainscak, M.; Skitek, M.; Skof, B. The effects of 8 weeks of endurance running on hepcidin concentrations, inflammatory parameters, and iron status in female runners. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 55–63. [Google Scholar] [CrossRef]
- Parks, R.B.; Hetzel, S.J.; Brooks, M.A. Iron Deficiency and Anemia among Collegiate Athletes: A Retrospective Chart Review. Med. Sci. Sports Exerc. 2017, 49, 1711–1715. [Google Scholar] [CrossRef]
- Connie, M.; Weaver, S.R. Exercise and Iron Status1. J. Nutr. 1992, 122, 782–787. [Google Scholar] [CrossRef]
- Bejder, J.; Graae, J.; Andersen, J.B.; Barbieri, R.A.; Campos, E.Z.; Bangsbo, J.; Nybo, L.; Nordsborg, N.B. Time-course of muscle fatigue development during intense exercise in hypoxia and normoxia. Sci. Rep. 2025, 15, 14065. [Google Scholar] [CrossRef]
- Cui, J.; Shi, C.; Xia, P.; Ning, K.; Xiang, H.; Xie, Q. Fermented Deer Blood Ameliorates Intense Exercise-Induced Fatigue via Modulating Small Intestine Microbiota and Metabolites in Mice. Nutrients 2021, 13, 1543. [Google Scholar] [CrossRef]
- Koehler, K.; Braun, H.; Achtzehn, S.; Hildebrand, U.; Predel, H.G.; Mester, J.; Schänzer, W. Iron status in elite young athletes: Gender-dependent influences of diet and exercise. Eur. J. Appl. Physiol. 2012, 112, 513–523. [Google Scholar] [CrossRef]
- Yang, L. Investigating the effects of persistent physical training on iron metabolism. Volkswagen Stand. 2021, 12, 84–86. [Google Scholar]
- Pengelly, M.; Pumpa, K.L.; Pyne, D.B.; Etxebarria, N. Iron’s True Weight: Does the Amount of Iron in the Body Equate to the Amount of Iron on the Bar in Australian Football League Women’s Players? Nutrients 2025, 17, 1691. [Google Scholar] [CrossRef] [PubMed]
- Pengelly, M.; Pumpa, K.; Pyne, D.B.; Etxebarria, N. Iron deficiency, supplementation, and sports performance in female athletes: A systematic review. J. Sport Health Sci. 2024, 14, 101009. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S. Iron supplementation prevents a decline in iron stores and enhances strength performance in elite female volleyball players during the competitive season. Appl. Physiol. Nutr. Metab. 2015, 40, 615–622. [Google Scholar] [CrossRef]
- Hinton, P.S.; Sinclair, L.M. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur. J. Clin. Nutr. 2007, 61, 30–39. [Google Scholar] [CrossRef]
- Silva Neto, L.G.R.; Santos Neto, J.E.D.; Bueno, N.B.; de Oliveira, S.L.; Ataide, T.D.R. Effects of iron supplementation versus dietary iron on the nutritional iron status: Systematic review with meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2553–2561. [Google Scholar] [CrossRef]
- The Chinese Nutrition Society has released the 2023 edition of the Dietary Reference Intakes for Nutrients for Chinese Residents. Acta Nutr. Sin. 2023, 45, 414. [CrossRef]
- Penggalih, M.H.S.T.; Sutanto, Y.S.; Taslim, N.A.; Syahputra, R.A.; Hardinsyah, H.; Tjandrawinata, R.R.; Nurkolis, F. Precision nutrition in sports science: An opinion on omics-based personalization and athletic outcomes. Front. Nutr. 2025, 12, 1611440. [Google Scholar] [CrossRef]
- Zare, R.; Kimble, R.; Ali Redha, A.; Cerullo, G.; Clifford, T. How can chokeberry (Aronia) (poly)phenol-rich supplementation help athletes? A systematic review of human clinical trials. Food Funct. 2023, 14, 5478–5491. [Google Scholar] [CrossRef]
- Stankiewicz, B.; Cieślicka, M.; Mieszkowski, J.; Kochanowicz, A.; Niespodziński, B.; Szwarc, A.; Waldziński, T.; Reczkowicz, J.; Piskorska, E.; Petr, M.; et al. Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial. Nutrients 2023, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Welk, A.K.; Mehlhose, C.; Daum, D.; Enneking, U. Exploring customer segmentation for food products with additional health benefits: A case study on iron-biofortified vegetables, functional foods, and dietary supplements. Appetite 2025, 211, 108004. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Tyal, A.; Kaur, J.; Sadeghi, P.; Maitta, R.W. Molecular Mechanisms of Iron Metabolism and Overload. Biomedicines 2025, 13, 2067. [Google Scholar] [CrossRef]

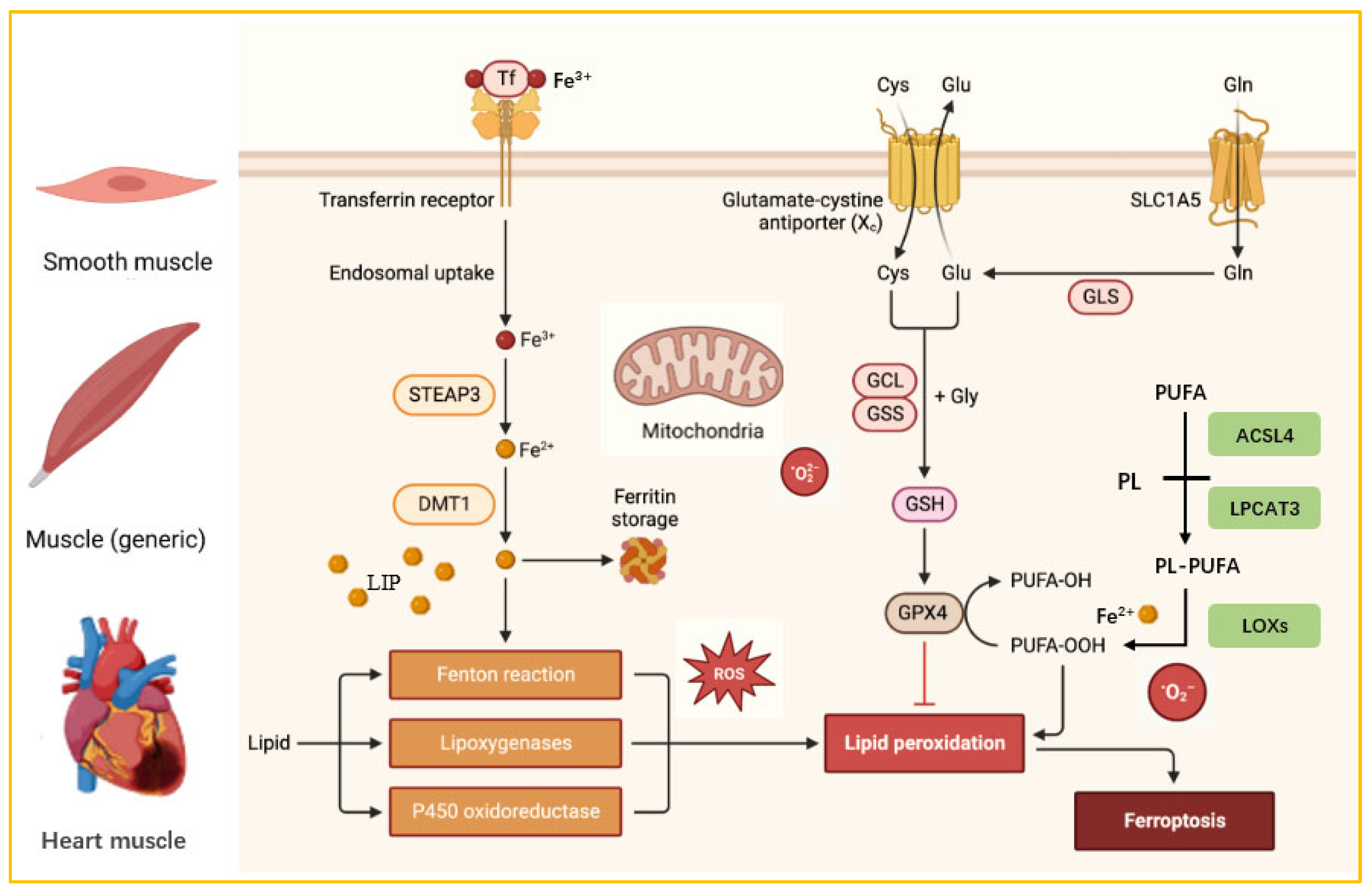
| Type of Disease | Targeted Drugs | Dosage | Treatment Cycle | Therapeutic Effect | References |
|---|---|---|---|---|---|
| Beta-thalassemia major | deferasirox (DFX) and deferoxamine (DFO) combination therapy | DFX 20–30 mg/kg daily; DFO 35–50 mg/kg on 3–7 days/week | 12 months | The concentrations of plasma ferritin, hepatic iron, myocardial iron and non-ferritin-binding iron in the plasma are reduced. | [78] |
| Iron overload beta-thalassemia major | DFX and DFO stand-alone treatment | DFX 40 mg/kg daily; DFO 50–60 mg/kg on 5–7 days/week | 24 months | The myocardial T2 signal intensity improved over a two-year period, while the hepatic iron concentration decreased. | [79] |
| Severe transfusional iron overload | DFX and DFO combination therapy | DFX 30.5 mg/kg per day; DFO 36.3 mg/kg per day | 24 months | The T2 value increased from 7.2 ms at baseline to 9.5 ms after 24 months, indicating a reduction in myocardial and hepatic iron concentrations. | [80] |
| Skeletal muscle injury | Thiol-based antioxidant N-acetylcysteine (NAC) | 20 mg NAC/kg in 3 daily dosages | 8 days | Reduce inflammation and exercise-induced muscle damage. Improve skeletal muscle performance and inhibit intracellular, redox-dependent signalling pathways. | [81] |
| Acute myocardial infarction | N-acetyl cysteine (NAC) | intravenous injection, total dose 6000 mg (12 mL) of NAC | 120 h | Prevention of Non-Thyroid Disease Syndrome. This is in patients with acute myocardial infarction. | [82] |
| Diastolic heart failure | Ubiquinol and D-ribose | 600 mg of ubiquinol and 15 g of d-ribose per day | 12 weeks. | Alleviate heart failure symptoms, reduce B-type natriuretic peptide and lactate levels, and increase ATP production. | [83] |
| Duchenne muscular dystrophy (DMD) | Idebenone | 300 mg three times a day | 52 weeks | It improves respiratory muscle function and reduces the loss of respiratory function. It is also safe and well tolerated. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, Q.; Gao, R.; Zhu, K.; Qiu, H.; Huang, J.; Zhang, X. The Potential Role of Iron Homeostasis and Ferroptosis in Exercise Nutrition and Health. Nutrients 2026, 18, 139. https://doi.org/10.3390/nu18010139
Wang Q, Gao R, Zhu K, Qiu H, Huang J, Zhang X. The Potential Role of Iron Homeostasis and Ferroptosis in Exercise Nutrition and Health. Nutrients. 2026; 18(1):139. https://doi.org/10.3390/nu18010139
Chicago/Turabian StyleWang, Qi, Ruiyang Gao, Kongdi Zhu, Huilong Qiu, Jiaqiang Huang, and Xia Zhang. 2026. "The Potential Role of Iron Homeostasis and Ferroptosis in Exercise Nutrition and Health" Nutrients 18, no. 1: 139. https://doi.org/10.3390/nu18010139
APA StyleWang, Q., Gao, R., Zhu, K., Qiu, H., Huang, J., & Zhang, X. (2026). The Potential Role of Iron Homeostasis and Ferroptosis in Exercise Nutrition and Health. Nutrients, 18(1), 139. https://doi.org/10.3390/nu18010139







