Are We Meeting the Needs? A Systematic Review of Nutritional Gaps and Growth Outcomes in Children with Multiple Food Allergies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
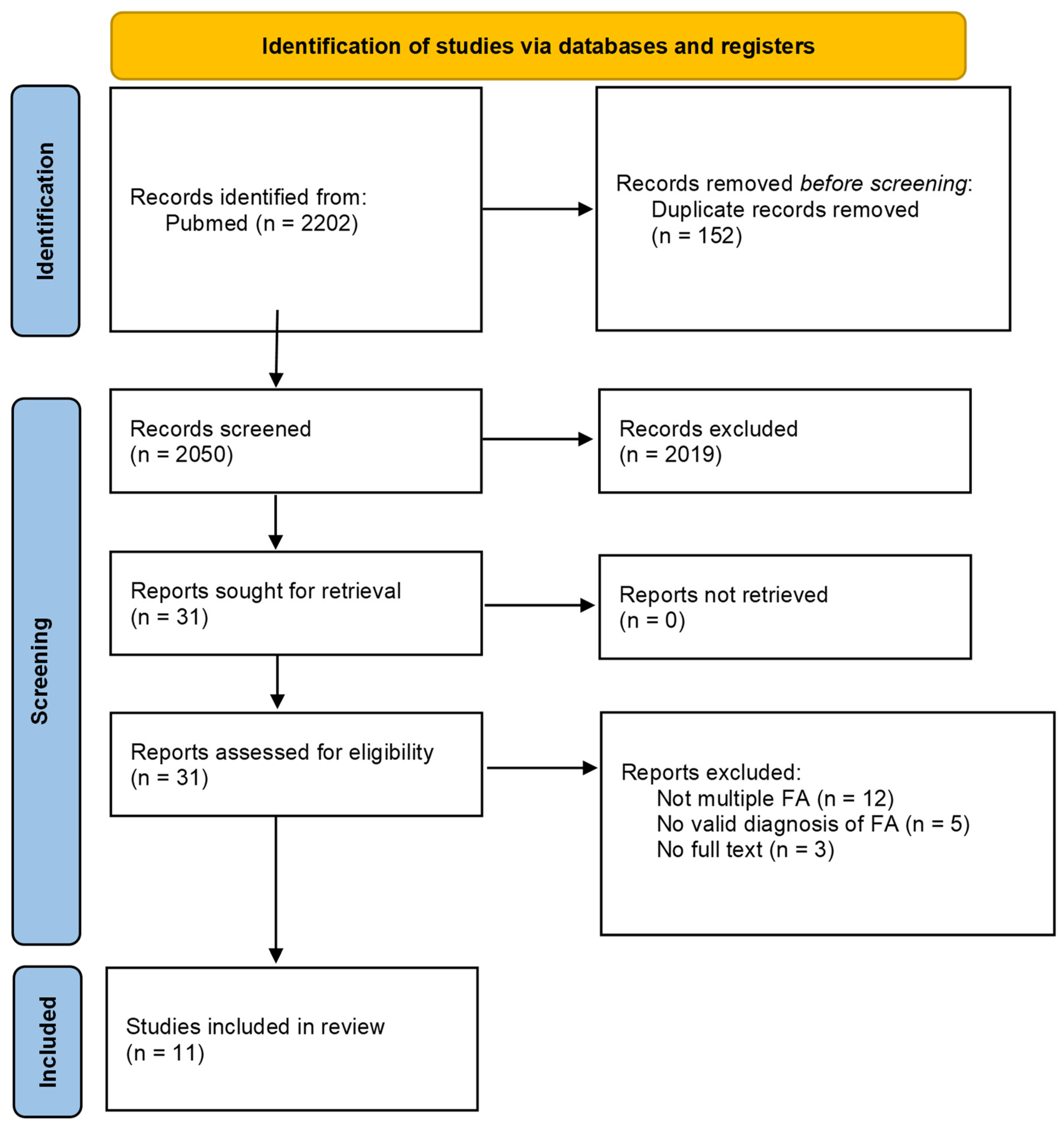
2.2.3. Selection Process
3. Results
3.1. Impact of Multiple Pediatric FAs in Nutritional Intake and Nutrient Deficiencies
| Study, Design, Class/Rating | Subjects | Comparison Group | Relationship of Multiple FAs to Growth (Standard Used) | Relationship of Multiple FAs to Nutrient Intake (Nutrition Assessment Method/Standard Used) | Limitations and Comments |
|---|---|---|---|---|---|
| [8] Christie et al. (2002), cross-sectional | consecutive patients with ≥1 FA * (n = 98) age 3.7 ± 2.3 years * FA: peanut, egg, and cow’s milk | age-matched controls without FA (n = 99) age 4.1 ± 2.4 years | (CDC growth charts) ≥2 FAs more likely to be <25th percentile height-for-age than 1 FA (35% and 16%, respectively) 1 FA more likely to be >75th percentile height-for-age than ≥2 FAs c (34% and 13%, respectively) | (3-day food record/10th edition DRIs) ≥1 FA more likely to consume >100% DRI for vitamin E than controls (78% and 92%, respectively) milk allergy more likely to consume <100% DRI for calcium than other allergies (58% and 31%, respectively) other allergies more likely to consume <100% DRI vitamin E than milk allergy (90% and 62%, respectively) FA without nutrition counseling more likely to consume< 67% DRI for calcium and vitamin D than those with counseling (calcium: 39% and 15%, respectively; vitamin D: 55% and 29%, respectively) | used 25th percentile height–forage as indicator of potential undernutrition did not report weight data unknown time avoiding allergens |
| [9] Flammarion et al. (2011), cross-sectional | children with ≥1 FA * (n = 96) age 4.6 ± 2.58 years * FA: peanut, cow’s milk, soy, and fish | age- and sex- matched controls (n = 95) age 4.7 ± 2.7 years | (Sempé reference values) ≥3 FAs more likely to have weight–for-age and height-for-age z-score < −2 than children with 1–2 FAs (weight: 14.5% and 1.8%, respectively; height: 12.1% and 3.6%, respectively) FA more likely than controls to have weight-for-age and height-for-age z-scores < −2 (weight: 9.3% and 0%, respectively; height: 7.2% and 2.7%, respectively) | (3-day food record/RDI for healthy children in France) FA consumed more vitamins A and E than controls (vitamin A: 95% RDA and 65% RDA, respectively; vitamin E: 92% RDA and 53% RDA, respectively) | 88% of children with food allergies received nutrition counseling by a dietitian avoiding allergens for ≥4 months prior to study |
| [10] Cho et al. (2011), case series | children with atopic dermatitis and ≥1 FA * (n = 112) classified according to sensitized FA * age 16.00 ± 10.93 months * FA: egg white, cow’s milk, soy, peanut, wheat, and fish | children with atopic dermatitis and no FA (n = 53) | (Korean growth charts) weight-for-age z-score decreased as sensitized FA increased (−0.21 ± 1.08, 0.02 ± 0.87, −0.14 ± 0.96, and −0.57 ± 1.90 for 0, 1, 2, and ≥3 sensitized FA, respectively) ≥3 FAs significantly shorter than 1 FA (−0.50 ± 1.01 and 1.10 ± 0.90, respectively) | unknown time avoiding allergens did not state if they were actually avoiding the sensitized FA | |
| [11] Aldámiz-Echevarría et al (2008), cross-sectional | children with ≥2 FAs * (n = 25) age 3.8 ± 1.6 years * FA: egg, cow’s milk, fish, legumes, meat, cereals, fruits, vegetables, molluscs, and crustaceans | healthy children (n = 61) age 5.1 ± 2.6 years | WNL | (no nutrition assessment measure used/no nutrient intake standard used) EFA index [(ω3 + ω6)/(ω7 + ω9)] was lower in FA than controls (1.5 and 1.9, respectively) DHA:DPA ratio was lower in FA than controls (4.0 and 11.7, respectively) lower percentage of α-linoleic acid in FA than controls (27.4 and 30.7, respectively) | unknown time avoiding allergens diets of the control patients were not described |
| [12] Sicherer et al. (2001), non-controlled trial | children with ≥1 FA * (n = 29) age 6–210 months at enrollment * FA: soy, egg, pea, potato, banana, wheat, barley, beef, peanut, oat, rice, corn, pork, chicken | no FA (n = 2) | (NCHS) weight-for-age, height-for-age, and weight-for-height z-scores WNL at entry, 1 month, 4 months (−0.8 ± 0.2, −0.3 ± 0.2, and −0.6 ± 0.3, respectively, at 4 months) no statistically significant differences in z-scores between 0, 1, and 4 months | (diet record/RDA) consumed 152 ± 12% RDA for protein from formula at 4 months | unknown time avoiding allergens unclear how many had 18 multiple FAs vs. only milk allergy funded by formula manufacturer primary purpose to test efficacy of formula many with allergic eosinophilic gastroenteritis |
| [13] Zeiger et al. (1999), non-controlled trial | children with ≥1 FA * (n = 93) children with allergy to milk and soy (n = 13) 11 had additional allergies age 23.4 ± 2.5 months at enrollment * FA: egg, peanut, meat, and wheat | children with milk allergy but no soy allergy (n = 80) age 18.4 ± 0.9 months | (NCHS) children with milk and soy allergy had lower height-for-age z-scores than children with milk allergy (−0.73 ± 0.26 vs. −0.09 ± 0.12) | unknown time avoiding allergens purpose of study was to assess prevalence of soy allergy in children with milk allergy weight and height for milk and soy allergic children measured only one time | |
| [14] Papachristou et al. (2024), cross-sectional | children with diagnosed IgE- mediated FA * (n = 100) age 8.5 ± 3.7 children with multiple FAs constitute 50% (n = 50) * FA: nut, cow’s milk, egg, fish, legumes, fruits, sesame, wheat, shellfish, soy | children with respiratory allergy (n = 60) age 10.2 ± 3.6 | BMI (International Obesity Task Force). Children with FAs had lower mean BMI compared with controls (17.3 ± 3.2 vs. 19.4 ± 3.9; p < 0.001). The FA group presented higher frequency of underweight (19.6% vs. 5.1%), and lower frequencies of overweight (14.4% vs. 25.4%) and obesity (6.2% vs. 10.2%). | dietary intake was assessed by registered dietitians through 4 24 h recalls. Children with FAs consumed fewer servings of fermented dairy products (0.9; 95% CI, 0.7, 1.1 vs. 1.2; 95% CI, 0.9, 1.4 servings/day) but more servings of plant-based dairy alternatives (0.1; 95% CI, 0.05, 0.2 vs. 0.0; 95% CI, 0.0, 0.07 servings/day) and meat (1.7, 95% CI, 1.6, 1.9 vs. 1.5, 95% CI, 1.3, 1.7 servings/day) than controls. No difference was observed in the diet diversity between the two groups (11–12 different foods/day). Within the FA group, children with allergy to milk proteins had lower energy intake from protein, lower intake of calcium, lower consumption of commercially prepared sweets, and higher consumption of eggs, compared with children with nut or egg allergy, but no difference in diet diversity was observed. | study that does not allow for the assessment of growth trajectories time since diagnosis was not considered no biomarker was measured the higher percentage of underweight in children with FAs suggests the need for targeted nutrition intervention after FA diagnosis |
| [15] Dziechciarz P. et al. (2024), cross-sectional, multi-center study | children with confirmed CMA (n = 144, 57% multiple FAs *) and suspected CMA (n = 88, 78% multiple FAs *) 51% of all participants with multiple FAs aged 6–24 months mean duration of CMP-free diet was 6.5 months * FA: cow’s milk | (weight for length and BMI for age were converted into SD scores (z-scores) based on WHO Standards using the WHO Anthro Software) according to weight for length z-score, 36% of all included children (n = 232) had any nutritional impairment (weight for length z-score above 1 or below −1): moderate malnutrition (10%), mild malnutrition (10.3%), possible risk of overweight (11.2%), no significant changes was found between nutritional status in children with confirmed and suspected CMA according to BMI for age z-score, 31.5% of all included children (n = 232) had any nutritional impairment (BMI for age z-score above 1 or below −1): mild malnutrition (9%), possible risk of overweight (9.9%) among patients with confirmed CMA, 30.5% had any nutritional impairment: mild malnutrition (9%), possible risk of overweight (9.7%) | the absence of a control group prevents comparison with a healthy population nutritional status was assessed at only one time point the cross-sectional nature of the survey precludes any causal inferences malnutrition and overweight are a burden in Polish children up to 2 years of age, with suspected and confirmed by oral food challenge CMA on a CMP-free diet | ||
| [7] Roberto Berni Canani et al. (2014), non-randomized, prospective, multicenter intervention study | children with FAs * aged 6 to 36 months who were following an elimination diet without dietary counseling for at least 60 days (n = 91) age 18.9 months (95% CI, 16.5 to 21.3) * FA: cow’s milk, hen’s egg, soy, fish, tomato, wheat, legumes, and rice multiple food allergy n = 42 (46.1%) | healthy children without FAs (aged 6 to 36 months) (n = 66) mean age 20.3 months (95% CI 17.7 to 22.8) | anthropometric indexes (z score for weight, length/height, and head circumference) were determined using the Euro-Growth References. A weight-to-length ratio <2 standard deviations was more frequent in children with FAs than in children without FAs (21% vs. 3%; p < 0.001). | at enrollment, energy and protein intakes were lower in children with FAs (91 kcal/kg/day, interquartile range [IQR] 15.1, minimum [MIN] 55.2, maximum [MAX] 130.6; and 2.2 g/kg/day, IQR 0.5, MIN 1.5, MAX 2.7, respectively) than in children without FAs (96 kcal/kg/day, IQR 6.1, MIN 83.6, MAX 118.0; and 4.6 g/kg/day, IQR 1.2, MIN 2.0, MAX 6.1, respectively; p < 0.001). At 6 months following dietary counseling, the total energy intake of children with FAs was similar to the baseline values of control children | the lack of follow-up data on children without FA and the lack of a control group of children with FA not receiving dietary counseling since suboptimal nutritional status is a frequent problem for children with FAs; dietary counseling, provided by a dietitian, is an effective strategy to promote rapid nutritional recovery in these children |
| [16] Mehta H. et al. (2014), retrospective chart review | FA * children (n = 439), median age 49 months (range, 0–238 months; t-test, p < 0.001) commercial insurance (n = 79) vs. state insurance (n = 360) * FA: peanut, tree nut, egg, fish, shell fish, wheat, and cow’s milk allergy | non food allergic children (n = 9499), median age 68 months (range, 0–240 months) commercial insurance (n = 1560) vs. state insurance (n = 7939) | (height and weight z-scores were calculated using the CDC and Prevention 2000 growth curves for children aged 2 and older (using the CDC and Prevention growth chart algorithm for SAS—SAS Version 9.3; SAS Inc., Cary, NC, USA) and WHO growth curves for ages younger than 2 years of age). Of those with commercial insurance, children with food allergies were significantly shorter (mean height z-score = 0.06; p = 0.01) and weighed less (mean weight z-score −0.1; p = 0.006) than children without food allergies (mean height z-score = 0.42; mean weight z-score = 0.07) | children with food allergies may have easier access to processed foods that may be poor substitutions for the allergen(s) that they are avoiding in regard to nutritional content but may contain a high caloric value there was a significant effect in children aged 2–5 and a trend toward significance in children 6–11 years of age, with children having a milk allergy being smaller as older children may not have an adequate substitution with alternative nutrient sources. Milk substitutes for this older age group are lower in fat and most are very low in protein or protein-free; thus, even when they are used, they provide a poor nutritional substitute. | limitations of this study include the retrospective design, reliance on physician diagnosis of food allergy, and lack of serum IgE, prick skin test, and oral food challenge data comorbidities, medication use, nutritional supplementation (e.g., vitamin D and calcium), visit to a dietitian, education regarding nutrition, and systemic symptoms indicating underlying inflammation were not obtained and can all impact growth |
| (identified by the International Classification of Diseases, 9th Revision [ICD-9] code for well child visit [v20.2]) | (identified by the International Classification of Diseases, 9th Revision [ICD-9] code for well child visit [v20.2]) | children with food allergies and state insurance were not smaller in height or weight compared with children without food allergies among White subjects, there was a significant effect of food allergies on height and weight (ANOVA for height p = 0.012, for weight p = 0.0036) that was not observed for Hispanic/Latino, Black, or Asian subjects children with allergies to milk weighed significantly less than children without milk allergies (p = 0.0006) | the combination of poor milk substitution and the elimination of a variety of foods because of milk content in this age group may be contributing to lower energy and protein intakes | results indicate the importance of closely monitoring the growth of children with food allergies, particularly those avoiding milk nutritional counseling should be provided to the families of these children to better educate caregivers on appropriate nutritionally dense substitutes | |
| [17] K. Maslin et al. (2018) Cross-sectional study | presence of a confirmed diagnosis of IgE or non-IgE mediated allergy to at least | healthy participants, who consumed an unrestricted diet | there was no difference in BMI according to food allergic status | record of everything eaten and drunk over four consecutive days, including one weekend day. Food allergic adolescents consumed a significantly lower percentage energy from fat but higher percentage energy from carbohydrate than control participants | there was a greater proportion of males in the food allergy adolescent group |
| one of the following foods: egg, milk, peanuts, tree nuts, sesame, crustaceans, fish, or wheat adolescents with FAs * (n = 50) medium age 14.5 (SD 2.4) adults with FAs * (n = 23) medium age 39.4 (SD 13.7) * FA: peanut, tree nut, fruits, egg, milk, and sesame | adolescents without FAs (n = 33) medium age 14.5 (2.2) adults without FAs (n = 47) medium age 30.2 (SD 9.5) | adolescents with food allergy had higher intakes of niacin and selenium than adolescents without (p < 0.05). This difference persisted when dietary supplements were removed from the analysis across all participants, the intake of several micronutrients was suboptimal. There was no difference in protein or energy intake, according to food allergic status. | the food-allergic participants recruited were predominantly members of a support charity, while the majority of control participants were recruited through advertisements on university websites a substantial proportion of all participants did not meet RNIs for micronutrients, particularly minerals, which may indicate a poor choice of foods with low nutrient density |
3.2. Impact of Multiple Pediatric FAs on Growth Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FAs | Food allergies |
| EFSA | European Food Safety Authority |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| JBI | Joanna Briggs Institute |
| CMA | Cow’s milk allergy |
| CMP | Cow’s Milk Protein |
| CDC | Centers for Disease Control and Prevention |
| DHA | docosahexanoic acid |
| DPA | docosapentaenoic acid |
| DRI | dietary reference intake |
| EFA | essential fatty acid |
| NCHS | National Center for Health Statistics |
| RDA | recommended daily allowance |
| RDI | reference daily intake |
| WNL | within normal limits |
References
- Warren, C.M.; Sehgal, S.; Sicherer, S.H.; Gupta, R.S. Epidemiology and the Growing Epidemic of Food Allergy in Children and Adults Across the Globe. Curr. Allergy Asthma Rep. 2024, 24, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Food allergen detection by mass spectrometry: From common to novel protein ingredients. Proteomics 2023, 23, e2200427. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Risso, D.; DunnGalvin, A.; González Díaz, S.N.; Monaci, L.; Fierro, V.; Ansotegui, I.J. Food Labeling Issues for Severe Food Allergic Patients. World Allergy Organ. J. 2021, 14, 100598. [Google Scholar] [CrossRef] [PubMed]
- Spolidoro, G.C.I.; Amera, Y.T.; Ali, M.M.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Frequency of Food Allergy in Europe: An Updated Systematic Review and Meta-analysis. Allergy 2023, 78, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, R.; Mercuri, C.; Bosco, V.; Giordano, V.; Simeone, S.; Guillari, A.; Rea, T. Development and Management of Avoidant/Restrictive Food Intake Disorder and Food Neophobia in Pediatric Patients with Food Allergy: A Comprehensive Review. Nutrients 2024, 16, 3034. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Leone, L.; D’Auria, E.; Riva, E.; Nocerino, R.; Ruotolo, S.; Terrin, G.; Cosenza, L.; Di Costanzo, M.; Passariello, A.; et al. The Effects of Dietary Counseling on Children with Food Allergy: A Prospective, Multicenter Intervention Study. J. Acad. Nutr. Diet. 2014, 114, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Christie, L.; Hine, R.J.; Parker, J.G.; Burk, W. Food Allergies in Children Affect Nutrient Intake and Growth. J. Am. Diet. Assoc. 2002, 102, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Flammarion, S.; Santos, C.; Guimber, D.; Jouannic, L.; Thumerelle, C.; Gottrand, F.; Deschildre, A. Diet and Nutritional Status of Children with Food Allergies. Pediatr. Allergy Immunol. 2011, 22, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-N.; Hong, S.; Lee, S.-H.; Yum, H.-Y. Nutritional Status According to Sensitized Food Allergens in Children with Atopic Dermatitis. Allergy Asthma Immunol. Res. 2011, 3, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; Bilbao, A.; Andrade, F.; Elorz, J.; Prieto, J.A.; Rodríguez-Soriano, J. Fatty Acid Deficiency Profile in Children with Food Allergy Managed with Elimination Diets. Acta Paediatr. 2008, 97, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Noone, S.A.; Koerner, C.B.; Christie, L.; Burks, A.W.; Sampson, H.A. Hypoallergenicity and Efficacy of an Amino Acid–Based Formula in Children with Cow’s Milk and Multiple Food Hypersensitivities. J. Pediatr. 2001, 138, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, R.S.; Sampson, H.A.; Bock, S.A.; Burks, A.W.; Harden, K.; Noone, S.; Martin, D.; Leung, S.; Wilson, G. Soy Allergy in Infants and Children with IgE-Associated Cow’s Milk Allergy. J. Pediatr. 1999, 134, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, E.; Voutsina, M.; Vagianou, K.; Papadopoulos, N.; Xepapadaki, P.; Yannakoulia, M. Dietary Intake, Diet Diversity, and Weight Status of Children with Food Allergy. J. Acad. Nutr. Diet. 2024, 124, 1606–1613.e5. [Google Scholar] [CrossRef] [PubMed]
- Dziechciarz, P.; Stróżyk, A.; Horvath, A.; Cudowska, B.; Jedynak-Wąsowicz, U.; Mól, N.; Jarocka-Cyrta, E.; Zawadzka-Krajewska, A.; Krauze, A. Nutritional Status and Feeding Difficulties in Children up to 2 Years of Age with Cow’s Milk Allergy. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 131–139. [Google Scholar] [CrossRef]
- Mehta, H.; Ramesh, M.; Feuille, E.; Groetch, M.; Wang, J. Growth Comparison in Children with and without Food Allergies in 2 Different Demographic Populations. J. Pediatr. 2014, 165, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Maslin, K.; Venter, C.; MacKenzie, H.; Vlieg-Boerstra, B.; Dean, T.; Sommer, I. Comparison of Nutrient Intake in Adolescents and Adults with and without Food Allergies. J. Hum. Nutr. Diet. 2018, 31, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, B.; Binder, C.; Dupont, C.; Hadji, S.; Arvola, T.; Isolauri, E. Prospective, Controlled, Multi-center Study on the Effect of an Amino-acid-based Formula in Infants with Cow’s Milk Allergy/Intolerance and Atopic Dermatitis. Pediatr. Allergy Immunol. 2001, 12, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Sova, C.; Feuling, M.B.; Baumler, M.; Gleason, L.; Tam, J.S.; Zafra, H.; Goday, P.S. Systematic Review of Nutrient Intake and Growth in Children with Multiple IgE-Mediated Food Allergies. Nutr. Clin. Pract. 2013, 28, 669–675. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cesare, G.; Carciofi, A.; Borgiani, F.; Cappelletti, D.; Correani, A.; Monachesi, C.; Gatti, S.; Lionetti, M.E. Are We Meeting the Needs? A Systematic Review of Nutritional Gaps and Growth Outcomes in Children with Multiple Food Allergies. Nutrients 2025, 17, 1590. https://doi.org/10.3390/nu17091590
Di Cesare G, Carciofi A, Borgiani F, Cappelletti D, Correani A, Monachesi C, Gatti S, Lionetti ME. Are We Meeting the Needs? A Systematic Review of Nutritional Gaps and Growth Outcomes in Children with Multiple Food Allergies. Nutrients. 2025; 17(9):1590. https://doi.org/10.3390/nu17091590
Chicago/Turabian StyleDi Cesare, Gianluca, Annalisa Carciofi, Francesca Borgiani, Deborah Cappelletti, Alessio Correani, Chiara Monachesi, Simona Gatti, and Maria Elena Lionetti. 2025. "Are We Meeting the Needs? A Systematic Review of Nutritional Gaps and Growth Outcomes in Children with Multiple Food Allergies" Nutrients 17, no. 9: 1590. https://doi.org/10.3390/nu17091590
APA StyleDi Cesare, G., Carciofi, A., Borgiani, F., Cappelletti, D., Correani, A., Monachesi, C., Gatti, S., & Lionetti, M. E. (2025). Are We Meeting the Needs? A Systematic Review of Nutritional Gaps and Growth Outcomes in Children with Multiple Food Allergies. Nutrients, 17(9), 1590. https://doi.org/10.3390/nu17091590






