Supporting Neurologic Health with Mushroom Nutrition
Abstract
1. Introduction
2. Mental Health
3. Gut Microbiota Involvement in Neurological Diseases
4. Improvement of Brain Function
5. Diet and Nutrition Impact on Mental Health
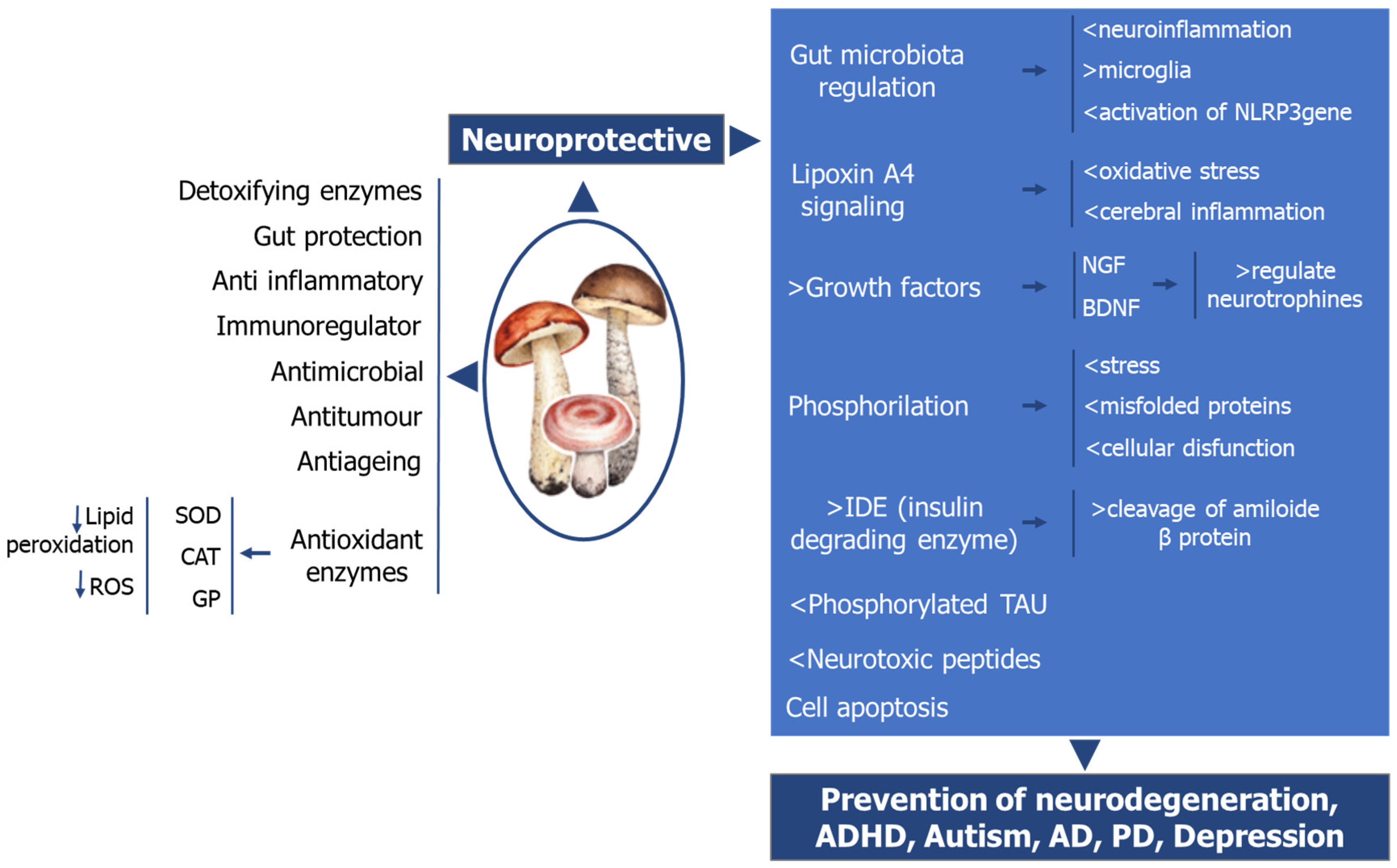
6. The Role of Mushrooms on Neurologic Health
6.1. Lentinula Edodes (Berk.) Pegler (“Shiitake”)
6.2. Grifola Frondosa (Dicks.) S.F.Gray (“Maitake”)
6.3. Trametes Versicolor (L.) C.G. Lloyd) Coriolus Versicolor (“Turkey Tail”)
6.4. Inonotus Obliquus (Fr.) Pilát. “Chaga”
6.5. Hericium Erinaceus (Bull.:Fr.) Pers. (“Lion’s Mane”)
6.6. Ophiocordyceps Sinensis (Berk.). Cordyceps (“Caterpillar”)
6.7. Ganoderma. Lucidum (Curtis) P. Karst (“Reishi”)
6.8. Pleurotus Eryngii (DC. ex Fr.) Quel. (“King Oyster”)
6.9. Mixture Hericium Erinaceus and Coriolus Versicolor
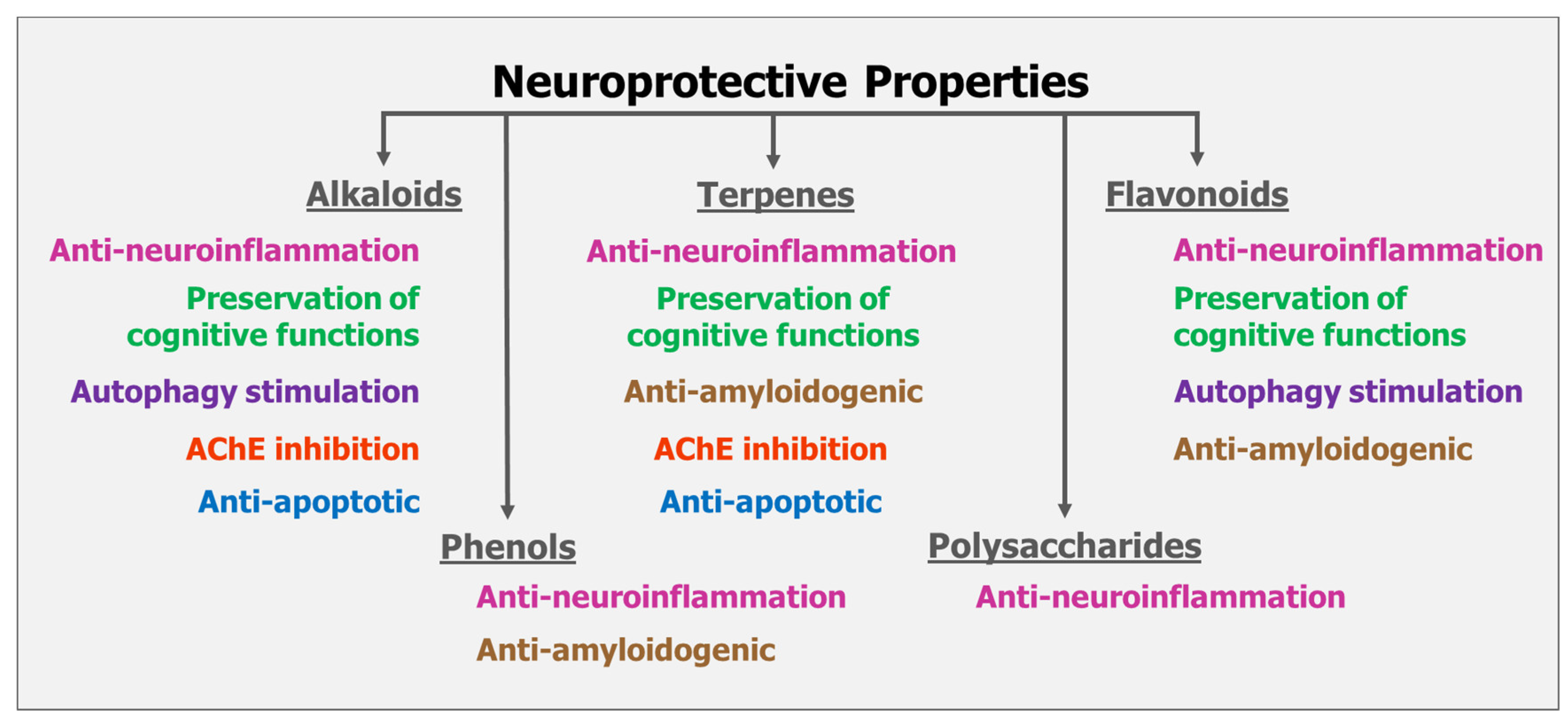
7. Summary on the Mode of Actions of Mushrooms on Neuroprotection
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynolds, C.F.; Jeste, D.V.; Sachdev, P.S.; Blazer, D.G. Mental Health Care for Older Adults: Recent Advances and New Directions in Clinical Practice and Research. World Psychiatry Off. J. World Psychiatr. Assoc. 2022, 21, 336–363. [Google Scholar] [CrossRef] [PubMed]
- WHO Mental Health of Adolescents. Available online: https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health (accessed on 13 March 2025).
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Lee, J.E.; Lee, C.; Kim, Y.T. Natural Products and Their Neuroprotective Effects in Degenerative Brain Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 11223. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef]
- Singh, O. Comprehensive Mental Health Action Plan 2013–2030: We Must Rise to the Challenge. Indian J. Psychiatry 2021, 63, 415–417. [Google Scholar] [CrossRef]
- Dumurgier, J.; Tzourio, C. Epidemiology of Neurological Diseases in Older Adults. Rev. Neurol. 2020, 176, 642–648. [Google Scholar] [CrossRef]
- Stein, D.J.; Palk, A.C.; Kendler, K.S. What Is a Mental Disorder? An Exemplar-Focused Approach. Psychol. Med. 2021, 51, 894–901. [Google Scholar] [CrossRef]
- Healthdirect Australia Mental Illness—Types, Causes and Diagnosis of Mental Health Issues. Available online: https://www.healthdirect.gov.au/mental-illness (accessed on 1 March 2025).
- Burback, L.; Brémault-Phillips, S.; Nijdam, M.J.; McFarlane, A.; Vermetten, E. Treatment of Posttraumatic Stress Disorder: A State-of-the-Art Review. Curr. Neuropharmacol. 2023, 22, 557–635. [Google Scholar] [CrossRef]
- WHO ICD-11 2022 Release. Available online: https://www.who.int/news/item/11-02-2022-icd-11-2022-release (accessed on 1 March 2025).
- Amand-Eeckhout, L. World Mental Health Day 2024: Mental Health at Work. 2024. Available online: https://policycommons.net/artifacts/17118250/world-mental-health-day-2024/18006911/ (accessed on 1 March 2025).
- Institute of Health Metrics and Evaluation Global Health Data Exchange. Available online: https://ghdx.healthdata.org/ (accessed on 1 March 2025).
- Moitra, M.; Santomauro, D.; Collins, P.Y.; Vos, T.; Whiteford, H.; Saxena, S.; Ferrari, A.J. The Global Gap in Treatment Coverage for Major Depressive Disorder in 84 Countries from 2000–2019: A Systematic Review and Bayesian Meta-Regression Analysis. PLoS Med. 2022, 19, e1003901. [Google Scholar] [CrossRef]
- Colizzi, M.; Lasalvia, A.; Ruggeri, M. Prevention and Early Intervention in Youth Mental Health: Is It Time for a Multidisciplinary and Trans-Diagnostic Model for Care? Int. J. Ment. Health Syst. 2020, 14, 23. [Google Scholar] [CrossRef]
- Carbone, S. Evidence Review: The Primary Prevention of Mental Health Conditions; Victorian Health Promotion Foundation: Melboune, VIC, Australia, 2020. [Google Scholar]
- Fusar-Poli, P.; Correll, C.U.; Arango, C.; Berk, M.; Patel, V.; Ioannidis, J.P.A. Preventive Psychiatry: A Blueprint for Improving the Mental Health of Young People. World Psychiatry 2021, 20, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Prevention of Mental Disorders. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research; Mrazek, P.J., Haggerty, R.J., Eds.; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Wu, Y.; Wang, L.; Tao, M.; Cao, H.; Yuan, H.; Ye, M.; Chen, X.; Wang, K.; Zhu, C. Changing Trends in the Global Burden of Mental Disorders from 1990 to 2019 and Predicted Levels in 25 Years. Epidemiol. Psychiatr. Sci. 2023, 32, e63. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Fernandes, T.H. Mushrooms as Functional Foods for Ménière’s Disease. Appl. Sci. 2023, 13, 12348. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Osakabe, N.; Anfuso, C.D.; Jacob, U.M.; Sidenkova, A.; Fritsch, T.; Abdelhameed, A.S.; Rashan, L.; Wenzel, U.; Calabrese, E.J.; Calabrese, V. Phytochemicals and Vitagenes for a Healthy Brain. In Brain and Mental Health in Ageing; Kaur, G., Rattan, S.I.S., Eds.; Springer: Cham, Switzerland, 2024; pp. 215–253. ISBN 978-3-031-68513-2. [Google Scholar]
- Ciancarelli, I.; Morone, G.; Iosa, M.; Cerasa, A.; Calabrò, R.S.; Tozzi Ciancarelli, M.G. Neuronutrition and Its Impact on Post-Stroke Neurorehabilitation: Modulating Plasticity Through Diet. Nutrients 2024, 16, 3705. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Parisi, A.; Seminara, F.; Vernuccio, L.; Catanese, G.; Barbagallo, M. Mediterranean Diet and Lifestyle in Persons with Mild to Moderate Alzheimer’s Disease. Nutrients 2024, 16, 3421. [Google Scholar] [CrossRef]
- Wren-Lewis, S.; Alexandrova, A. Mental Health Without Well-Being. J. Med. Philos. 2021, 46, 684–703. [Google Scholar] [CrossRef]
- Kirkbride, J.B.; Anglin, D.M.; Colman, I.; Dykxhoorn, J.; Jones, P.B.; Patalay, P.; Pitman, A.; Soneson, E.; Steare, T.; Wright, T.; et al. The Social Determinants of Mental Health and Disorder: Evidence, Prevention and Recommendations. World Psychiatry Off. J. World Psychiatr. Assoc. 2024, 23, 58–90. [Google Scholar] [CrossRef]
- Selloni, A. Social Determinants of Psychosis: An Examination of Loneliness, Stress, Discrimination, and Neighborhood Cohesion in Psychotic Disorders; City University of New York (CUNY): New York, NY, USA, 2024. [Google Scholar]
- WHO Mental Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-health-strengthening-our-response (accessed on 27 February 2025).
- Belfiore, C.I.; Galofaro, V.; Cotroneo, D.; Lopis, A.; Tringali, I.; Denaro, V.; Casu, M. A Multi-Level Analysis of Biological, Social, and Psychological Determinants of Substance Use Disorder and Co-Occurring Mental Health Outcomes. Psychoactives 2024, 3, 194–214. [Google Scholar] [CrossRef]
- Reed, G.M. What’s in a Name? Mental Disorders, Mental Health Conditions and Psychosocial Disability. World Psychiatry Off. J. World Psychiatr. Assoc. 2024, 23, 209–210. [Google Scholar] [CrossRef]
- Moukham, H.; Lambiase, A.; Barone, G.D.; Tripodi, F.; Coccetti, P. Exploiting Natural Niches with Neuroprotective Properties: A Comprehensive Review. Nutrients 2024, 16, 1298. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Charoensup, R.; Kalieva, K.; Habibi, E.; Guo, M.; Wang, D.; Kvasnica, M.; Onder, A.; Sarker, S.D. Natural Products in Neurodegenerative Diseases: Recent Advances and Future Outlook. Front. Pharmacol. 2025, 16, 1529194. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.B.; Bell, V.; Ferrão, J.; Calabrese, V.; Fernandes, T.H. Mushroom Biomass: Some Clinical Implications of β-Glucans and Enzymes. Curr. Res. Nutr. Food Sci. 2016, 4, 37–47. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kołat, D.; Yang, K.; Hu, J.K. Role of the Gut Microbiota in Anticancer Therapy: From Molecular Mechanisms to Clinical Applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota Revolution: How Gut Microbes Regulate Our Lives. World J. Gastroenterol. 2023, 29, 4368. [Google Scholar] [CrossRef]
- Młynarska, E.; Jakubowska, P.; Frąk, W.; Gajewska, A.; Sornowska, J.; Skwira, S.; Wasiak, J.; Rysz, J.; Franczyk, B. Associations of Microbiota and Nutrition with Cognitive Impairment in Diseases. Nutrients 2024, 16, 3570. [Google Scholar] [CrossRef]
- Ghaffar, T.; Ubaldi, F.; Volpini, V.; Valeriani, F.; Romano Spica, V. The Role of Gut Microbiota in Different Types of Physical Activity and Their Intensity: Systematic Review and Meta-Analysis. Sports 2024, 12, 221. [Google Scholar] [CrossRef]
- Vitale, M.; Costabile, G.; Testa, R.; D’Abbronzo, G.; Nettore, I.C.; Macchia, P.E.; Giacco, R. Ultra-Processed Foods and Human Health: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2024, 15, 100121. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Speranza, B.; Racioppo, A.; Santillo, A.; Albenzio, M.; Derossi, A.; Caporizzi, R.; Francavilla, M.; Racca, D.; Flagella, Z.; et al. Ultra-Processed Food and Gut Microbiota: Do Additives Affect Eubiosis? A Narrative Review. Nutrients 2024, 17, 2. [Google Scholar] [CrossRef]
- Ratan, Y.; Rajput, A.; Pareek, A.; Pareek, A.; Jain, V.; Sonia, S.; Farooqui, Z.; Kaur, R.; Singh, G. Advancements in Genetic and Biochemical Insights: Unraveling the Etiopathogenesis of Neurodegeneration in Parkinson’s Disease. Biomolecules 2024, 14, 73. [Google Scholar] [CrossRef]
- Junyi, L.; Yueyang, W.; Bin, L.; Xiaohong, D.; Wenhui, C.; Ning, Z.; Hong, Z. Gut Microbiota Mediates Neuroinflammation in Alzheimer’s Disease: Unraveling Key Factors and Mechanistic Insights. Mol. Neurobiol. 2025, 62, 3746–3763. [Google Scholar] [CrossRef] [PubMed]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of Gut Microbiota and Brain via Immune and Neuroendocrine Signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the Microbiota-Gut-Brain Axis: Diet, Microbiome, and Neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Major, D.; Fazekas-Pongor, V.; Csípő, T.; Tarantini, S.; Csizmadia, Z.; Varga, J.T. Exploring the Influence of Gut-Brain Axis Modulation on Cognitive Health: A Comprehensive Review of Prebiotics, Probiotics, and Symbiotics. Nutrients 2024, 16, 789. [Google Scholar] [CrossRef]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2017, 16, 1059–1085. [Google Scholar] [CrossRef]
- Damiani, F.; Cornuti, S.; Tognini, P. The Gut-Brain Connection: Exploring the Influence of the Gut Microbiota on Neuroplasticity and Neurodevelopmental Disorders. Neuropharmacology 2023, 231, 109491. [Google Scholar] [CrossRef]
- Daliry, A.; Pereira, E.N.G.d.S. Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders. Nutrients 2021, 13, 3533. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, F.; Chen, B.; Shin, W.S.; Chen, W.; He, Y.; Leung, K.T.; Tse, G.M.K.; Yu, J.; To, K.F.; et al. The Nerve Cells in Gastrointestinal Cancers: From Molecular Mechanisms to Clinical Intervention. Oncogene 2024, 43, 77–91. [Google Scholar] [CrossRef]
- Nakhal, M.M.; Yassin, L.K.; Alyaqoubi, R.; Saeed, S.; Alderei, A.; Alhammadi, A.; Alshehhi, M.; Almehairbi, A.; Al Houqani, S.; BaniYas, S.; et al. The Microbiota-Gut-Brain Axis and Neurological Disorders: A Comprehensive Review. Life 2024, 14, 1234. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Mihailovich, M.; Soković Bajić, S.; Dinić, M.; Đokić, J.; Živković, M.; Radojević, D.; Golić, N. Cutting-Edge IPSC-Based Approaches in Studying Host-Microbe Interactions in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 156. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells 2023, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Kuźniar, J.; Kozubek, P.; Czaja, M.; Leszek, J. Correlation between Alzheimer’s Disease and Gastrointestinal Tract Disorders. Nutrients 2024, 16, 2366. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, Y.; Zhong, H.; Liu, Z.; Geng, J.; Wang, H.; Wang, W. Gut Microbes in Central Nervous System Development and Related Disorders. Front. Immunol. 2024, 14, 1288256. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the Gut-Brain-Axis: Implications for New Therapeutic Design in the CNS. eBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.P.S.S.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The Impact of Microbiota on the Gut-Brain Axis: Examining the Complex Interplay and Implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef]
- Naveed, M.; Zhou, Q.G.; Xu, C.; Taleb, A.; Meng, F.; Ahmed, B.; Zhang, Y.; Fukunaga, K.; Han, F. Gut-Brain Axis: A Matter of Concern in Neuropsychiatric Disorders…! Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110051. [Google Scholar] [CrossRef]
- Settanni, C.R.; Ianiro, G.; Bibbò, S.; Cammarota, G.; Gasbarrini, A. Gut Microbiota Alteration and Modulation in Psychiatric Disorders: Current Evidence on Fecal Microbiota Transplantation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef]
- Ahmed, G.K.; Ramadan, H.K.A.; Elbeh, K.; Haridy, N.A. Bridging the Gap: Associations between Gut Microbiota and Psychiatric Disorders. Middle East Curr. Psychiatry 2024, 31, 2. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Sorond, F.A. What Is Brain Health? Cereb. Circ.—Cogn. Behav. 2024, 6, 100190. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain Foods: The Effects of Nutrients on Brain Function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhao, L.; Long, J.; Feng, Z.; Su, J.; Gao, F.; Liu, J. Mitochondria as a Sensor, a Central Hub and a Biological Clock in Psychological Stress-Accelerated Aging. Ageing Res. Rev. 2024, 93, 102145. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional Psychiatry: The Present State of the Evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R.; Al Nishan, A.; Habiba, S.U.; Moon, I.S. Brain Modulation by the Gut Microbiota: From Disease to Therapy. J. Adv. Res. 2023, 53, 153–173. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Osetrova, M.; Tkachev, A.; Mair, W.; Guijarro Larraz, P.; Efimova, O.; Kurochkin, I.; Stekolshchikova, E.; Anikanov, N.; Foo, J.C.; Cazenave-Gassiot, A.; et al. Lipidome Atlas of the Adult Human Brain. Nat. Commun. 2024, 15, 4455. [Google Scholar] [CrossRef]
- Smolińska, K.; Szopa, A.; Sobczyński, J.; Serefko, A.; Dobrowolski, P. Nutritional Quality Implications: Exploring the Impact of a Fatty Acid-Rich Diet on Central Nervous System Development. Nutrients 2024, 16, 1093. [Google Scholar] [CrossRef]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of Polyunsaturated Fatty Acids in Human Brain Structure and Function across the Lifespan: An Update on Neuroimaging Findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- Raine, A.; Brodrick, L. Omega-3 Supplementation Reduces Aggressive Behavior: A Meta-Analytic Review of Randomized Controlled Trials. Aggress. Violent Behav. 2024, 78, 101956. [Google Scholar] [CrossRef]
- De Araújo, P.H.G.; Duarte, A.O.; Silva, M.C. da Influência Da Dieta Na Saúde Mental e Desempenho Cognitivo—Uma Revisão Da Literatura. Res. Soc. Dev. 2024, 13, e11013646103. [Google Scholar] [CrossRef]
- Swathi, M.; Manjusha, S.; Vadakkiniath, I.J.; Gururaj, A. Prevalence and Correlates of Stress, Anxiety, and Depression in Patients with Chronic Diseases: A Cross-Sectional Study. Middle East Curr. Psychiatry 2023, 30, 66. [Google Scholar] [CrossRef]
- Padamsey, Z.; Rochefort, N.L. Paying the Brain’s Energy Bill. Curr. Opin. Neurobiol. 2023, 78, 102668. [Google Scholar] [CrossRef] [PubMed]
- Komar-Fletcher, M.; Wojas, J.; Rutkowska, M.; Raczyńska, G.; Nowacka, A.; Jurek, J.M. Negative Environmental Influences on the Developing Brain Mediated by Epigenetic Modifications. Explor. Neurosci. 2023, 2, 193–211. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Hamazaki, K. Considering Mental Health from the Viewpoint of Diet: The Role and Possibilities of Nutritional Psychiatry. Seishin Shinkeigaku Zasshi 2016, 118, 880–894. [Google Scholar]
- Warren, M.; O’Connor, C.; Lee, J.E.; Burton, J.; Walton, D.; Keathley, J.; Wammes, M.; Osuch, E. Predispose, Precipitate, Perpetuate, and Protect: How Diet and the Gut Influence Mental Health in Emerging Adulthood. Front. Nutr. 2024, 11, 1339269. [Google Scholar] [CrossRef]
- Allendorf, D.H.; Brown, G.C. Neu1 Is Released from Activated Microglia, Stimulating Microglial Phagocytosis and Sensitizing Neurons to Glutamate. Front. Cell. Neurosci. 2022, 16, 917884. [Google Scholar] [CrossRef]
- Du, J.; Shui, H.; Chen, R.; Dong, Y.; Xiao, C.; Hu, Y.; Wong, N.K. Neuraminidase-1 (NEU1): Biological Roles and Therapeutic Relevance in Human Disease. Curr. Issues Mol. Biol. 2024, 46, 8031–8052. [Google Scholar] [CrossRef]
- Jiang, X.; Song, Y.; Lv, C.; Li, Y.; Feng, X.; Zhang, H.; Chen, Y.; Wang, Q. Mushroom-Derived Bioactive Components with Definite Structures in Alleviating the Pathogenesis of Alzheimer’s Disease. Front. Pharmacol. 2024, 15, 1373660. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.W.; Hwang, B.S.; Woo, E.E.; Lee, Y.J.; Jeong, K.W.; Lee, I.K.; Yun, B.S. Neuraminidase Inhibitors from the Fruiting Body of Phellinus Igniarius. Mycobiology 2016, 44, 117. [Google Scholar] [CrossRef]
- van Zonneveld, S.M.; van den Oever, E.J.; Haarman, B.C.M.; Grandjean, E.L.; Nuninga, J.O.; van de Rest, O.; Sommer, I.E.C. An Anti-Inflammatory Diet and Its Potential Benefit for Individuals with Mental Disorders and Neurodegenerative Diseases—A Narrative Review. Nutrients 2024, 16, 2646. [Google Scholar] [CrossRef] [PubMed]
- Grajek, M.; Krupa-Kotara, K.; Białek-Dratwa, A.; Sobczyk, K.; Grot, M.; Kowalski, O.; Staśkiewicz, W. Nutrition and Mental Health: A Review of Current Knowledge about the Impact of Diet on Mental Health. Front. Nutr. 2022, 9, 943998. [Google Scholar] [CrossRef] [PubMed]
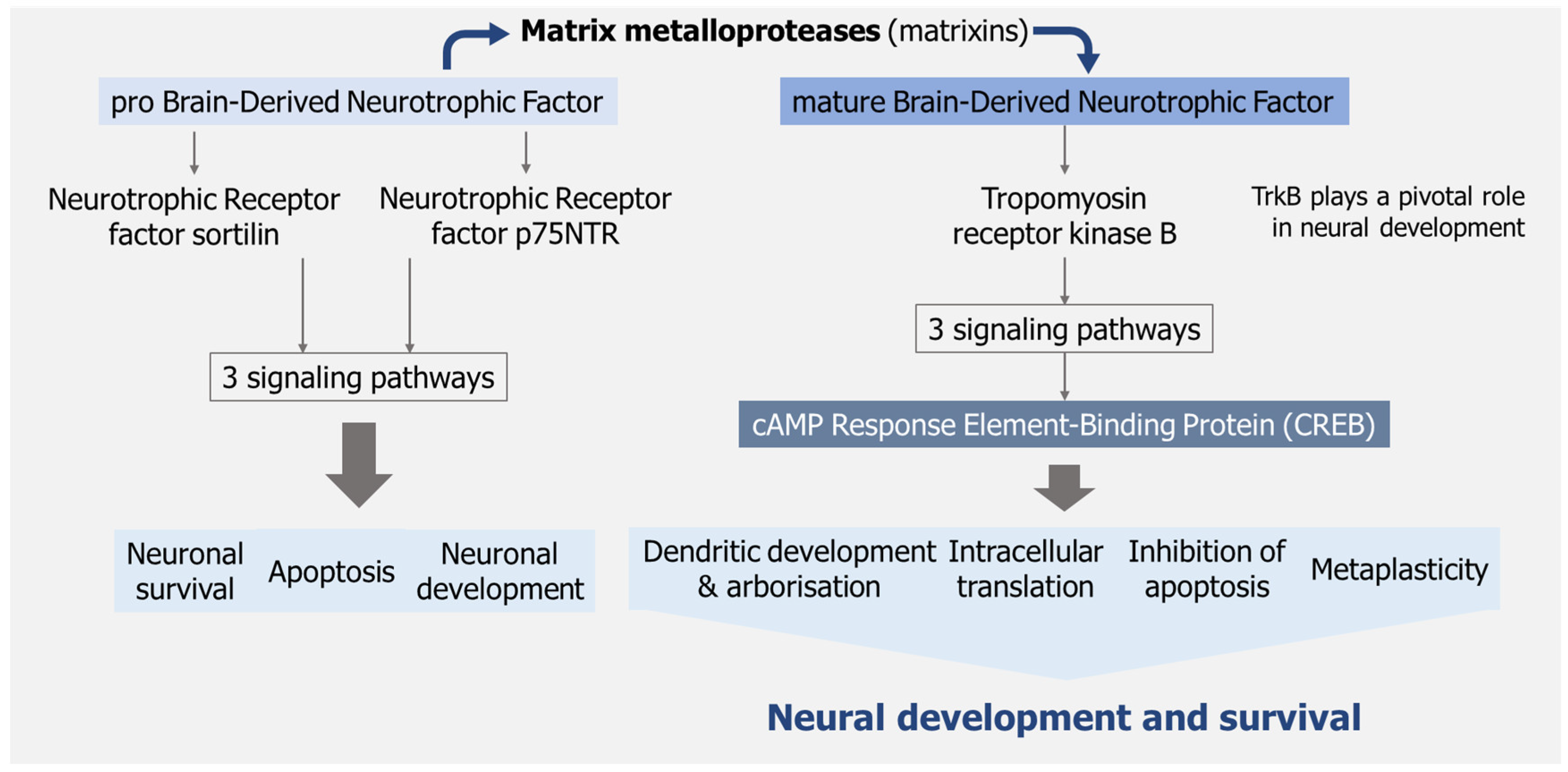
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Schirò, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef]
- Vacaras, V.; Paraschiv, A.C.; Iluț, S.; Vacaras, C.; Nistor, C.; Marin, G.E.; Schiopu, A.M.; Nistor, D.T.; Vesa, Ș.C.; Mureșanu, D.F. Brain-Derived Neurotrophic Factor in Multiple Sclerosis Disability: A Prospective Study. Brain Sci. 2024, 14, 243. [Google Scholar] [CrossRef]
- Marino-Puertas, L.; Goulas, T.; Gomis-Rüth, F.X. Matrix Metalloproteinases Outside Vertebrates. Biochim. Biophys. Acta—Mol. Cell Res. 2017, 1864, 2026–2035. [Google Scholar] [CrossRef]
- von Bohlen und Halbach, O.; Klausch, M. The Neurotrophin System in the Postnatal Brain-An Introduction. Biology 2024, 13, 558. [Google Scholar] [CrossRef]
- Pisani, A.; Paciello, F.; Del Vecchio, V.; Malesci, R.; De Corso, E.; Cantone, E.; Fetoni, A.R. The Role of BDNF as a Biomarker in Cognitive and Sensory Neurodegeneration. J. Pers. Med. 2023, 13, 652. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. An Interaction between Brain-Derived Neurotrophic Factor and Stress-Related Glucocorticoids in the Pathophysiology of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 1596. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. The Role of Brain-Derived Neurotrophic Factor as an Essential Mediator in Neuronal Functions and the Therapeutic Potential of Its Mimetics for Neuroprotection in Neurologic and Psychiatric Disorders. Molecules 2025, 30, 848. [Google Scholar] [CrossRef]
- Alway, E.; Reicher, N.; Bohórquez, D.V. Deciphering Visceral Instincts: A Scientific Quest to Unravel Food Choices from Molecules to Mind. Genes Dev. 2024, 38, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician Guidelines for the Treatment of Psychiatric Disorders with Nutraceuticals and Phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Mulè, S.; Ferrari, S.; Rosso, G.; Galla, R.; Battaglia, S.; Curti, V.; Molinari, C.; Uberti, F. The Combined Effect of Green Tea, Saffron, Resveratrol, and Citicoline against Neurodegeneration Induced by Oxidative Stress in an In Vitro Model of Cognitive Decline. Oxidative Med. Cell. Longev. 2024, 2024, 7465045. [Google Scholar] [CrossRef] [PubMed]
- Horovitz, O. Nutritional Psychology: Review the Interplay Between Nutrition and Mental Health. Nutr. Rev. 2025, 83, 562–576. [Google Scholar] [CrossRef]
- Hiltensperger, R.; Neher, J.; Böhm, L.; Mueller-Stierlin, A.S. Mapping the Scientific Research on Nutrition and Mental Health: A Bibliometric Analysis. Nutrients 2025, 17, 399. [Google Scholar] [CrossRef]
- Firth, J.; Gangwisch, J.E.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Wootton, R.E.; Wootton, R.E.; Mayer, E.A.; Mayer, E.A. Food and Mood: How Do Diet and Nutrition Affect Mental Wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef]
- Global Nutrition Target Collaborators. Global, Regional, and National Progress towards the 2030 Global Nutrition Targets and Forecasts to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 404, 2543–2583. [Google Scholar] [CrossRef]
- Selvaraj, R.; Selvamani, T.Y.; Zahra, A.; Malla, J.; Dhanoa, R.K.; Venugopal, S.; Shoukrie, S.I.; Hamouda, R.K.; Hamid, P. Association Between Dietary Habits and Depression: A Systematic Review. Cureus 2022, 14, e32359. [Google Scholar] [CrossRef]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional Psychiatry: Towards Improving Mental Health by What You Eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef]
- Zielińska, M.; Łuszczki, E.; Michońska, I.; Dereń, K. The Mediterranean Diet and the Western Diet in Adolescent Depression-Current Reports. Nutrients 2022, 14, 4390. [Google Scholar] [CrossRef]
- Chopra, C.; Mandalika, S.; Kinger, N. Does Diet Play a Role in the Prevention and Management of Depression among Adolescents? A Narrative Review. Nutr. Health 2021, 27, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Baklizi, G.S.; Bruce, B.C.; Santos, A.C. de C.P. Neuronutrição Na Depressão e Transtorno de Ansiedade. Res. Soc. Dev. 2021, 10, e52101724454. [Google Scholar] [CrossRef]
- Caroni, D.; Rodrigues, J.S.; Santos, A.L. Influence of Diet on the Prevention and Treatment of Alzheimer: An Integrative Review. Res. Soc. Dev. 2023, 12, e14812541677. [Google Scholar] [CrossRef]
- Offor, S.J.; Orish, C.N.; Frazzoli, C.; Orisakwe, O.E. Augmenting Clinical Interventions in Psychiatric Disorders: Systematic Review and Update on Nutrition. Front. Psychiatry 2021, 12, 565583. [Google Scholar] [CrossRef]
- Rebouças, F.d.C.; Barbosa, L.L.; Nascimento, L.F.d.; Ferreira, J.C.d.S.; Freitas, F.M.N.d.O. A Influência Da Nutrição No Tratamento e Prevenção Dos Transtornos Mentais: Ansiedade e Depressão. Res. Soc. Dev. 2022, 11, e57111537078. [Google Scholar] [CrossRef]
- Reily, N.M.; Tang, S.; Negrone, A.; Gan, D.Z.Q.; Sheanoda, V.; Christensen, H. Omega-3 Supplements in the Prevention and Treatment of Youth Depression and Anxiety Symptoms: A Scoping Review. PLoS ONE 2023, 18, e0284057. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Mo, L.; Luo, J.; Shen, Q.; Quan, W. Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2024, 16, 125. [Google Scholar] [CrossRef]
- Suárez-López, L.M.; Bru-Luna, L.M.; Martí-Vilar, M. Influence of Nutrition on Mental Health: Scoping Review. Healthcare 2023, 11, 2183. [Google Scholar] [CrossRef]
- Zielińska, M.; Łuszczki, E.; Dereń, K. Dietary Nutrient Deficiencies and Risk of Depression (Review Article 2018–2023). Nutrients 2023, 15, 2433. [Google Scholar] [CrossRef]
- Wiss, D.A.; LaFata, E.M. Ultra-Processed Foods and Mental Health: Where Do Eating Disorders Fit into the Puzzle? Nutrients 2024, 16, 1955. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. [Google Scholar] [CrossRef] [PubMed]
- Key, M.N.; Szabo-Reed, A.N. Impact of Diet and Exercise Interventions on Cognition and Brain Health in Older Adults: A Narrative Review. Nutrients 2023, 15, 2495. [Google Scholar] [CrossRef] [PubMed]
- Roszczenko, P.; Szewczyk-Roszczenko, O.K.; Gornowicz, A.; Iwańska, I.A.; Bielawski, K.; Wujec, M.; Bielawska, A. The Anticancer Potential of Edible Mushrooms: A Review of Selected Species from Roztocze, Poland. Nutrients 2024, 16, 2849. [Google Scholar] [CrossRef]
- Rathor, P.; Ch, R. The Impacts of Dietary Intervention on Brain Metabolism and Neurological Disorders: A Narrative Review. Dietetics 2024, 3, 289–307. [Google Scholar] [CrossRef]
- Hassan, A.; Khan, M.K.I.; Hasan, A.; Fordos, S.; Naeem, M.Z.; Usman, A. Investigating the Relationship between Food Quality and Mental Health. Biol. Life Sci. Forum 2023, 26, 104. [Google Scholar] [CrossRef]
- Marcus, J.B. (Ed.) Chapter 7—Vitamin and Mineral Basics: The ABCs of Healthy Foods and Beverages, Including Phytonutrients and Functional Foods: Healthy Vitamin and Mineral Choices, Roles and Applications in Nutrition, Food Science and the Culinary Arts. In Culinary Nutrition; Academic Press: Cambridge, MA, USA, 2013; pp. 279–331. ISBN 9780123918826. [Google Scholar] [CrossRef]
- ‘Aqilah, N.M.N.; Rovina, K.; Felicia, W.X.L.; Vonnie, J.M. A Review on the Potential Bioactive Components in Fruits and Vegetable Wastes as Value-Added Products in the Food Industry. Molecules 2023, 28, 2631. [Google Scholar] [CrossRef]
- Bell, V.; Silva, C.R.P.G.; Guina, J.; Fernandes, T.H. Mushrooms as Future Generation Healthy Foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Al Qutaibi, M.; Kagne, S.R. Exploring the Phytochemical Compositions, Antioxidant Activity, and Nutritional Potentials of Edible and Medicinal Mushrooms. Int. J. Microbiol. 2024, 2024, 6660423. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An Insight into the Nutritional and Medicinal Value of Edible Mushrooms: A Natural Treasury for Human Health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef]
- Yimam, M.A.; Andreini, M.; Carnevale, S.; Muscaritoli, M. The Role of Algae, Fungi, and Insect-Derived Proteins and Bioactive Peptides in Preventive and Clinical Nutrition. Front. Nutr. 2024, 11, 1461621. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Nemeș, S.A.; Fărcaș, A.; Socaciu, C.; Semeniuc, C.A.; Socaciu, M.I.; Socaci, S. Bioactive Secondary Metabolites in Mushrooms: A Focus on Polyphenols, Their Health Benefits and Applications. Food Biosci. 2024, 62, 105166. [Google Scholar] [CrossRef]
- Paterska, M.; Czerny, B.; Cielecka-Piontek, J. Macrofungal Extracts as a Source of Bioactive Compounds for Cosmetical Anti-Aging Therapy: A Comprehensive Review. Nutrients 2024, 16, 2810. [Google Scholar] [CrossRef]
- Licastro, F.; Porcellini, E. Activation of Endogenous Retrovirus, Brain Infections and Environmental Insults in Neurodegeneration and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7263. [Google Scholar] [CrossRef]
- Jarosz, A.S.; Halo, J.V. Transcription of Endogenous Retroviruses: Broad and Precise Mechanisms of Control. Viruses 2024, 16, 1312. [Google Scholar] [CrossRef]
- Fan, J.; Qin, Z. Roles of Human Endogenous Retrovirus-K-Encoded Np9 in Human Diseases: A Small Protein with Big Functions. Viruses 2024, 16, 581. [Google Scholar] [CrossRef]
- Kozubek, P.; Kuźniar, J.; Czaja, M.; Sitka, H.; Kochman, U.; Leszek, J. Human Endogenous Retroviruses and Their Putative Role in Pathogenesis of Alzheimer’s Disease, Inflammation, and Senescence. Biomedicines 2025, 13, 59. [Google Scholar] [CrossRef]
- Adler, G.L.; Le, K.; Fu, Y.H.; Kim, W.S. Human Endogenous Retroviruses in Neurodegenerative Diseases. Genes 2024, 15, 745. [Google Scholar] [CrossRef]
- Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. J. Fungi 2024, 10, 394. [Google Scholar] [CrossRef]
- Guo, R.; Pang, J.; Zhao, J.; Xiao, X.; Li, J.; Li, J.; Wang, W.; Zhou, S.; Zhao, Y.; Zhang, Z.; et al. Unveiling the Neuroprotective Potential of Dietary Polysaccharides: A Systematic Review. Front. Nutr. 2023, 10, 1299117. [Google Scholar] [CrossRef] [PubMed]
- Behrad, S.; Pourranjbar, S.; Pourranjbar, M.; Abbasi-Maleki, S.; Mehr, S.R.; Salmani, R.H.G.; Moradikor, N. Grifola Frondosa Polysaccharides Alleviate Alzheimer’s Disease in Rats. Asian Pac. J. Trop. Biomed. 2024, 14, 500–506. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Paola, R.D.; Siracusa, R.; Fusco, R.; Ontario, M.; Cammilleri, G.; Pantano, L.; Scuto, M.; Tomasello, M.; Spanò, S.; Salinaro, A.T.; et al. Redox Modulation of Meniere Disease by Coriolus Versicolor Treatment, a Nutritional Mushroom Approach with Neuroprotective Potential. Curr. Neuropharmacol. 2023, 22, 2079. [Google Scholar] [CrossRef]
- Sharika, R.; Mongkolpobsin, K.; Rangsinth, P.; Prasanth, M.I.; Nilkhet, S.; Pradniwat, P.; Tencomnao, T.; Chuchawankul, S. Experimental Models in Unraveling the Biological Mechanisms of Mushroom-Derived Bioactives against Aging- and Lifestyle-Related Diseases: A Review. Nutrients 2024, 16, 2682. [Google Scholar] [CrossRef]
- Roodveldt, C.; Bernardino, L.; Oztop-Cakmak, O.; Dragic, M.; Fladmark, K.E.; Ertan, S.; Busra, A.; Pita, C.; Ciglar, L.; Garraux, G.; et al. The Immune System in Parkinson’s Disease: What We Know so Far. Brain 2024, 147, 3306–3324. [Google Scholar] [CrossRef]
- Lau, B.F.; Abdullah, N. Sclerotium-Forming Mushrooms as an Emerging Source of Medicinals: Current Perspectives. In Mushroom Biotechnology: Developments and Applications; Petre, M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 111–136. ISBN 9780128027943. [Google Scholar]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P.C. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium Erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- Wang, S.; Dong, K.; Zhang, J.; Chen, C.; Shuai, H.; Yu, X. Raw Inonotus Obliquus Polysaccharide Counteracts Alzheimer’s Disease in a Transgenic Mouse Model by Activating the Ubiquitin-Proteosome System. Nutr. Res. Pract. 2023, 17, 1128–1142. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Feng, X.; Li, L.; Hao, J.; Wang, D.; Wang, Q. Mushroom Polysaccharides as Potential Candidates for Alleviating Neurodegenerative Diseases. Nutrients 2022, 14, 4833. [Google Scholar] [CrossRef]
- Martínez-Mármol, R.; Chai, Y.J.; Conroy, J.N.; Khan, Z.; Hong, S.M.; Kim, S.B.; Gormal, R.S.; Lee, D.H.; Lee, J.K.; Coulson, E.J.; et al. Hericerin Derivatives Activates a Pan-Neurotrophic Pathway in Central Hippocampal Neurons Converging to ERK1/2 Signaling Enhancing Spatial Memory. J. Neurochem. 2023, 165, 791–808. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, G.; Liu, W.; Zhang, F.; Linhardt, R.J.; Wang, X.; Zhang, A. Bioactive Substances in Hericium Erinaceus and Their Biological Properties: A Review. Food Sci. Hum. Wellness 2024, 13, 1825–1844. [Google Scholar] [CrossRef]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective Metabolites of Hericium Erinaceus Promote Neuro-Healthy Aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Scuto, M.; Zappalà, A.; Ontario, M.L.; Petralia, A.; Abid-Essefi, S.; Maiolino, L.; Signorile, A.; Salinaro, A.T.; Calabrese, V. Hericium Erinaceus Prevents Dehp-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2020, 21, 2138. [Google Scholar] [CrossRef] [PubMed]
- Yanshree; Yu, W.S.; Fung, M.L.; Lee, C.W.; Lim, L.W.; Wong, K.H. The Monkey Head Mushroom and Memory Enhancement in Alzheimer’s Disease. Cells 2022, 11, 2284. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox Modulation of Cellular Stress Response and Lipoxin A4 Expression by Hericium Erinaceus in Rat Brain: Relevance to Alzheimer’s Disease Pathogenesis. Immun. Ageing 2016, 13, 23. [Google Scholar] [CrossRef]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving Effects of the Mushroom Yamabushitake (Hericium erinaceus) on Mild Cognitive Impairment: A Double-Blind Placebo-Controlled Clinical Trial. Phyther. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Cha, S.; Bell, L.; Shukitt-Hale, B.; Williams, C.M. A Review of the Effects of Mushrooms on Mood and Neurocognitive Health across the Lifespan. Neurosci. Biobehav. Rev. 2024, 158, 105548. [Google Scholar] [CrossRef]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium Erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef]
- Li, I.C.; Lee, L.Y.; Tzeng, T.T.; Chen, W.P.; Chen, Y.P.; Shiao, Y.J.; Chen, C.C. Neurohealth Properties of Hericium Erinaceus Mycelia Enriched with Erinacines. Behav. Neurol. 2018, 5802634. [Google Scholar] [CrossRef]
- Shirvani, M.; Nouri, F.; Sarihi, A.; Habibi, P.; Mohammadi, M. Neuroprotective Effects of Dehydroepiandrosterone and Hericium Erinaceus in Scopolamine-Induced Alzheimer’s Diseases-like Symptoms in Male Rats. Cell Biochem. Biophys. 2024, 82, 2853–2864. [Google Scholar] [CrossRef]
- Grozier, C.; Alves, V.; Kilen, L.; O’Neal, E.; Simpson, F.; Waldman, H. Four Weeks of Hericium Erinaceus Supplementation Does Not Impact Markers of Metabolic Flexibility or Cognition. Int. J. Exerc. Sci. 2022, 15, 1366–1380. [Google Scholar] [CrossRef] [PubMed]
- Docherty, S.; Doughty, F.L.; Smith, E.F. The Acute and Chronic Effects of Lion’s Mane Mushroom Supplementation on Cognitive Function, Stress and Mood in Young Adults: A Double-Blind, Parallel Groups, Pilot Study. Nutrients 2023, 15, 4842. [Google Scholar] [CrossRef]
- Feng, L.; Cheah, I.K.M.; Ng, M.M.X.; Li, J.; Chan, S.M.; Lim, S.L.; Mahendran, R.; Kua, E.H.; Halliwell, B. The Association between Mushroom Consumption and Mild Cognitive Impairment: A Community-Based Cross-Sectional Study in Singapore. J. Alzheimer’s Dis. 2019, 68, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Bell, L.; Williams, C.M. The Relationship between Mushroom Intake and Cognitive Performance: An Epidemiological Study in the European Investigation of Cancer—Norfolk Cohort (EPIC-Norfolk). Nutrients 2024, 16, 353. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, J.; Cock, I.; Fourie, P.; Gulati, V.; Rosenzweig, J.; Cock, I.; Fourie, P.; Gulati, V. Inhibitory Activity of Hericium Erinaceus Extracts against Some Bacterial Triggers of Multiple Sclerosis and Selected Autoimmune Diseases. In Proceedings of the Abstracts of the 4th International Electronic Conference on Nutrients (IECN 2024), Online, 16–18 October 2024; MDPI: Basel, Switzerland, 2024. [Google Scholar]
- Sharma, H.; Sharma, N.; An, S.S.A. Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents. Nutrients 2024, 16, 102. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Medicinal Fungus Cordyceps with Its Nutraceutical and Therapeutic Potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.M.; Proestos, C.; Brennan, C.; Zeng, M.; Tomasevic, I.; Elobeid, T.; Çadırcı, K.; Bayrak, M.; et al. Exploring the Potential Medicinal Benefits of Ganoderma Lucidum: From Metabolic Disorders to Coronavirus Infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef]
- Lian, W.; Yang, X.; Duan, Q.; Li, J.; Zhao, Y.; Yu, C.; He, T.; Sun, T.; Zhao, Y.; Wang, W. The Biological Activity of Ganoderma Lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules 2024, 29, 2516. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma Lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef]
- Ma, F.; Wang, J.; Jiang, W.; Luo, J.; Yang, R.; Zhang, L.; Han, C. Ganoderic Acid A: A Potential Natural Neuroprotective Agent for Neurological Disorders: A Review. Int. J. Med. Mushrooms 2024, 26, 11–23. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Li, G.; Jiang, Y.; Zhang, G.; Ling, J. A Novel Promising Neuroprotective Agent: Ganoderma Lucidum Polysaccharide. Int. J. Biol. Macromol. 2023, 229, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F.; Alsayegh, A.A.; Ahmad, F.A.; Akhtar, M.S.; Alavudeen, S.S.; Bantun, F.; Wahab, S.; Ahmed, A.; Ali, M.; Elbendary, E.Y.; et al. Ganoderma Lucidum: Insight into Antimicrobial and Antioxidant Properties with Development of Secondary Metabolites. Heliyon 2024, 10, e25607. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, Z.; Wu, S.; Chen, M.; Huang, R.; Wang, J.; Wu, Q.; Ding, Y. Preparation of Antioxidant Protein Hydrolysates from Pleurotus Geesteranus and Their Protective Effects on H2O2 Oxidative Damaged PC12 Cells. Molecules 2020, 25, 5408. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, M.; Liao, X.; Huang, R.; Wang, J.; Xie, Y.; Hu, H.; Zhang, J.; Wu, Q.; Ding, Y. Protein Hydrolysates from Pleurotus Geesteranus Obtained by Simulated Gastrointestinal Digestion Exhibit Neuroprotective Effects in H2O2 -Injured PC12 Cells. J. Food Biochem. 2022, 46, e13879. [Google Scholar] [CrossRef]
- Trovato, A.; Pennisi, M.; Crupi, R.; Di Paola, R.; Alario, A.; Modafferi, S.; Di Rosa, G.; Fernandes, T.; Signorile, A.; Maiolino, L.; et al. Neuroinflammation and Mitochondrial Dysfunction in the Pathogenesis of Alzheimer’s Disease: Modulation by Coriolus Versicolor (Yun-Zhi) Nutritional Mushroom. J. Neurol. Neuromed. 2017, 2, 19–28. [Google Scholar]
- Li, N.; Li, H.; Liu, Z.; Feng, G.; Shi, C.; Wu, Y. Unveiling the Therapeutic Potentials of Mushroom Bioactive Compounds in Alzheimer’s Disease. Foods 2023, 12, 2972. [Google Scholar] [CrossRef]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic Applications of Mushrooms and Their Biomolecules along with a Glimpse of in Silico Approach in Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef]
- Kou, R.W.; Xia, B.; Han, R.; Li, Z.Q.; Yang, J.R.; Yin, X.; Gao, Y.Q.; Gao, J.M. Neuroprotective Effects of a New Triterpenoid from Edible Mushroom on Oxidative Stress and Apoptosis through the BDNF/TrkB/ERK/CREB and Nrf2 Signaling Pathway in Vitro and in Vivo. Food Funct. 2022, 13, 12121–12134. [Google Scholar] [CrossRef]
- Lee, O.Y.A.; Wong, A.N.N.; Ho, C.Y.; Tse, K.W.; Chan, A.Z.; Leung, G.P.H.; Kwan, Y.W.; Yeung, M.H.Y. Potentials of Natural Antioxidants in Reducing Inflammation and Oxidative Stress in Chronic Kidney Disease. Antioxidants 2024, 13, 751. [Google Scholar] [CrossRef]
- Ferrão, J.; Bell, V.; Calabrese, V.; Pimentel, L.; Pintado, M.; Fernandes, T. Impact of Mushroom Nutrition on Microbiota and Potential for Preventative Health. J. Food Nutr. Res. 2017, 5, 226–233. Available online: https://pubs.sciepub.com/jfnr/5/4/4/index.html (accessed on 1 March 2025).
- Ferreiro, E.; Pita, I.R.; Mota, S.I.; Valero, J.; Ferreira, N.R.; Fernandes, T.; Calabrese, V.; Fontes-Ribeiro, C.A.; Pereira, F.C.; Rego, A.C. Coriolus Versicolor Biomass Increases Dendritic Arborization of Newly-Generated Neurons in Mouse Hippocampal Dentate Gyrus. Oncotarget 2018, 9, 32929–32942. [Google Scholar] [CrossRef] [PubMed]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and Neurohormesis in the Pathogenesis of Alzheimer’s Disease and Alzheimer-Linked Pathologies: Modulation by Nutritional Mushrooms. Immun. Ageing 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Modafferi, S.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; et al. Natural Compounds Such as Hericium Erinaceus and Coriolus Versicolor Modulate Neuroinflammation, Oxidative Stress and Lipoxin A4 Expression in Rotenone-Induced Parkinson’s Disease in Mice. Biomedicines 2022, 10, 2505. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.J. A Review on Mushrooms as a Versatile Therapeutic Agent with Emphasis on Its Bioactive Constituents for Anticancer and Antioxidant Potential. Explor. Med. 2024, 5, 312–330. [Google Scholar] [CrossRef]
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–Medicinal Mushrooms: A Review of Organic Compounds and Bioelements with Antioxidant Activity. Eur. Food Res. Technol. 2021, 247, 513–533. [Google Scholar] [CrossRef]
- Hassan, M.; Shahzadi, S.; Ransom, R.F.; Kloczkowski, A. Nature’s Own Pharmacy: Mushroom-Based Chemical Scaffolds and Their Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 5596. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant Compounds from Edible Mushrooms as Potential Candidates for Treating Age-Related Neurodegenerative Diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Sharma, E.; Bairwa, R.; Lal, P.; Pattanayak, S.; Chakrapani, K.; Poorvasandhya, R.; Kumar, A.; Altaf, M.A.; Tiwari, R.K.; Lal, M.K.; et al. Edible Mushrooms Trending in Food: Nutrigenomics, Bibliometric, from Bench to Valuable Applications. Heliyon 2024, 10, e36963. [Google Scholar] [CrossRef]
- Gajendra, K.; Pratap, G.K.; Poornima, D.V.; Shantaram, M.; Ranjita, G. Natural Acetylcholinesterase Inhibitors: A Multi-Targeted Therapeutic Potential in Alzheimer’s Disease. Eur. J. Med. Chem. Rep. 2024, 11, 100154. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Jędrejko, K.; Sułkowska-Ziaja, K.; Ziaja, M.; Kała, K.; Muszyńska, B. Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder. Foods 2022, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Chenghom, O.; Suksringar, J.; Morakot, N. Mineral Composition and Germanium Contents in Some Phellinus Mushrooms in the Northeast of Thailand. Curr. Res. Chem. 2010, 2, 24–34. [Google Scholar] [CrossRef]
- Ferrão, J.; Bell, V.; Chaquisse, E.; Garrine, C.; Fernandes, T. The Synbiotic Role of Mushrooms: Is Germanium a Bioactive Prebiotic Player? A Review Article. Am. J. Food Nutr. 2019, 7, 26–35. [Google Scholar]
- Luo, X.; Sun, J.; Kong, D.; Lei, Y.; Gong, F.; Zhang, T.; Shen, Z.; Wang, K.; Luo, H.; Xu, Y. The Role of Germanium in Diseases: Exploring Its Important Biological Effects. J. Transl. Med. 2023, 21, 795. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health? Microorganisms 2024, 12, 1570. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-KappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Nasiry, D.; Khalatbary, A.R. Natural Polyphenols for the Management of Autism Spectrum Disorder: A Review of Efficacy and Molecular Mechanisms. Nutr. Neurosci. 2024, 27, 241–251. [Google Scholar] [CrossRef]
- Al Mamun, A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Polyphenols Targeting NF-ΚB Pathway in Neurological Disorders: What We Know So Far? Int. J. Biol. Sci. 2024, 20, 1332–1355. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi). In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 175–199. ISBN 9781439807163. [Google Scholar]
- Uffelman, C.N.; Harold, R.; Hodson, E.S.; Chan, N.I.; Foti, D.; Campbell, W.W. Effects of Consuming White Button and Oyster Mushrooms within a Healthy Mediterranean-Style Dietary Pattern on Changes in Subjective Indexes of Brain Health or Cognitive Function in Healthy Middle-Aged and Older Adults. Foods 2024, 13, 2319. [Google Scholar] [CrossRef]
- Robinson-Agramonte, M.d.l.A.; García, E.N.; Guerra, J.F.; Hurtado, Y.V.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- Modafferi, S.; Lupo, G.; Tomasello, M.; Rampulla, F.; Ontario, M.; Scuto, M.; Salinaro, A.T.; Arcidiacono, A.; Anfuso, C.D.; Legmouz, M.; et al. Antioxidants, Hormetic Nutrition, and Autism. Curr. Neuropharmacol. 2023, 22, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Mihailovich, M.; Tolinački, M.; Soković Bajić, S.; Lestarevic, S.; Pejovic-Milovancevic, M.; Golić, N. The Microbiome-Genetics Axis in Autism Spectrum Disorders: A Probiotic Perspective. Int. J. Mol. Sci. 2024, 25, 12407. [Google Scholar] [CrossRef] [PubMed]
- Ba, D.M.; Gao, X.; Al-Shaar, L.; Muscat, J.; Chinchilli, V.M.; Ssentongo, P.; Beelman, R.B.; Richie, J. Mushroom Intake and Cognitive Performance among US Older Adults: The National Health and Nutrition Examination Survey, 2011–2014. Br. J. Nutr. 2022, 128, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A Mechanistic Review on Medicinal Mushrooms-Derived Bioactive Compounds: Potential Mycotherapy Candidates for Alleviating Neurological Disorders. Planta Med. 2020, 86, 1161–1175. [Google Scholar] [CrossRef]
- Calabrese, V.; Osakabe, N.; Siracusa, R.; Modafferi, S.; Di Paola, R.; Cuzzocrea, S.; Jacob, U.M.; Fritsch, T.; Abdelhameed, A.S.; Rashan, L.; et al. Transgenerational Hormesis in Healthy Aging and Antiaging Medicine from Bench to Clinics: Role of Food Components. Mech. Ageing Dev. 2024, 220, 111960. [Google Scholar] [CrossRef]
- Contato, A.G.; Conte-Junior, C.A. Lion’s Mane Mushroom (Hericium erinaceus): A Neuroprotective Fungus with Antioxidant, Anti-Inflammatory, and Antimicrobial Potential—A Narrative Review. Nutrients 2025, 17, 1307. [Google Scholar] [CrossRef]
| Genus Family | Species Name | Common Name | |
|---|---|---|---|
| Lentinula | Lentinula edodes | Shiitake mushroom |  |
| Grifola | Grifola frondosa | Maitake mushroom |  |
| Polypore | Coriolus versicolor | Turkey tail mushroom |  |
| Inonotus | Inonotus obliquus | Chaga mushroom |  |
| Hericium | Hericium erinaceus | Lion’s Mane mushroom |  |
| Ophiocordyceps | Cordyceps sinensis | Caterpillar mushroom |  |
| Ganoderma | Ganoderma lucidum | Reishi (Lingzhi) mushroom |  |
| Pleurotus | Pleurotus ostreatus | Oyster mushroom |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, V.; Dimitrov, P.; Fernandes, T. Supporting Neurologic Health with Mushroom Nutrition. Nutrients 2025, 17, 1568. https://doi.org/10.3390/nu17091568
Bell V, Dimitrov P, Fernandes T. Supporting Neurologic Health with Mushroom Nutrition. Nutrients. 2025; 17(9):1568. https://doi.org/10.3390/nu17091568
Chicago/Turabian StyleBell, Victoria, Palmen Dimitrov, and Tito Fernandes. 2025. "Supporting Neurologic Health with Mushroom Nutrition" Nutrients 17, no. 9: 1568. https://doi.org/10.3390/nu17091568
APA StyleBell, V., Dimitrov, P., & Fernandes, T. (2025). Supporting Neurologic Health with Mushroom Nutrition. Nutrients, 17(9), 1568. https://doi.org/10.3390/nu17091568





