Feasibility of a Structured Calorie-Restricted Dietary Intervention in Korean Adults with Early Type 2 Diabetes and Obesity: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
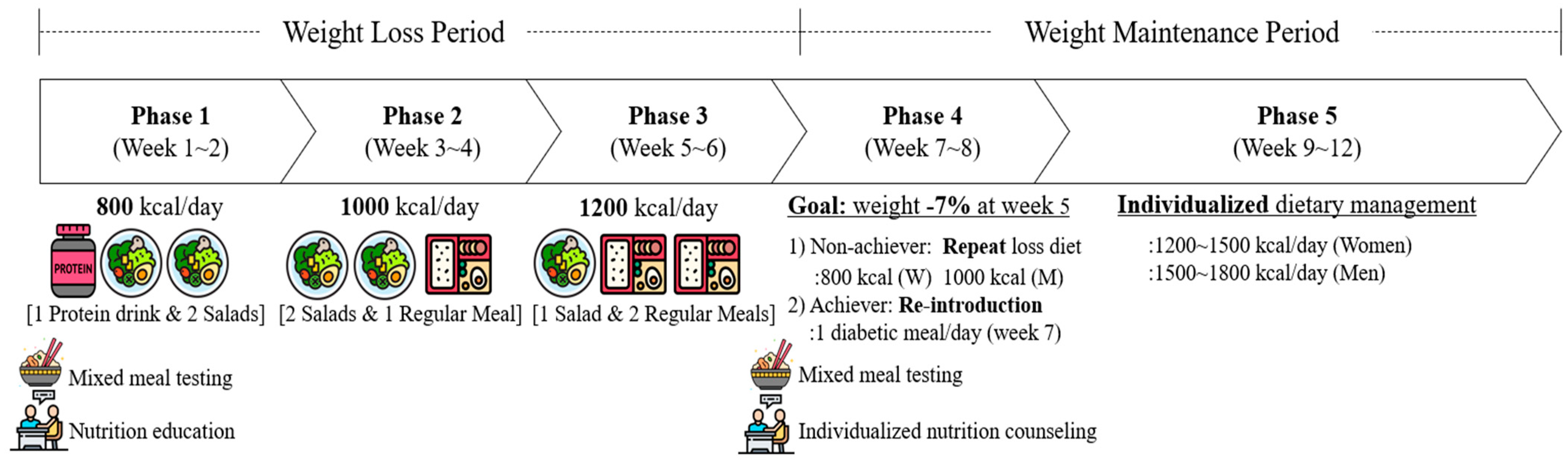
2.2. Dietary Protocol
2.2.1. Weight Loss Period
2.2.2. Weight Maintenance Period
2.3. Outcome Measures
2.3.1. Anthropometric Measures and Body Composition
2.3.2. Nutrient Intake and Dietary Adherence
2.3.3. Postprandial Glycemic Response of Mixed Meal
2.3.4. Other Measures
2.4. Statistical Analysis
3. Results
3.1. Participants
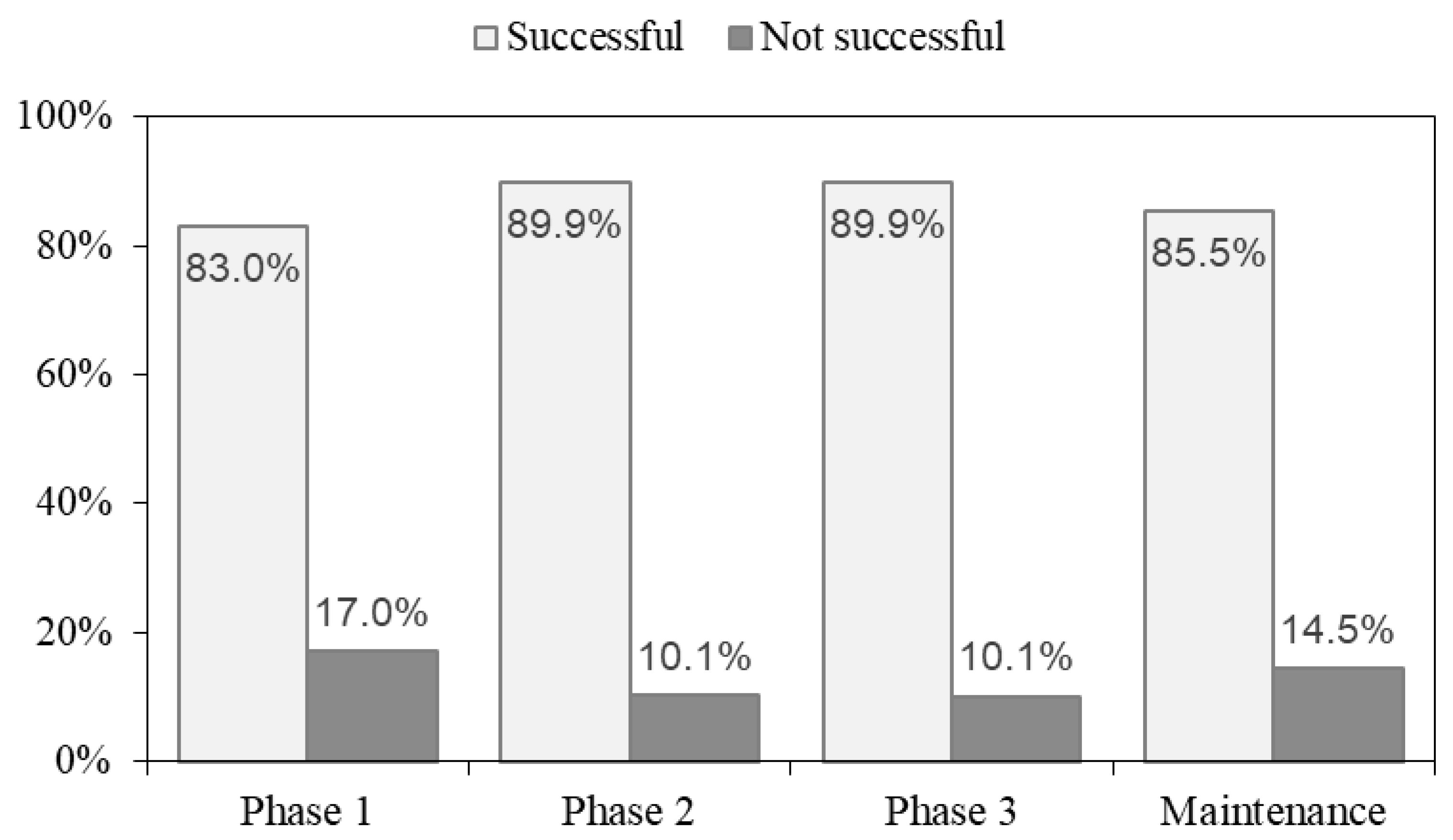
3.2. Nutrient Intake and Dietary Adherence
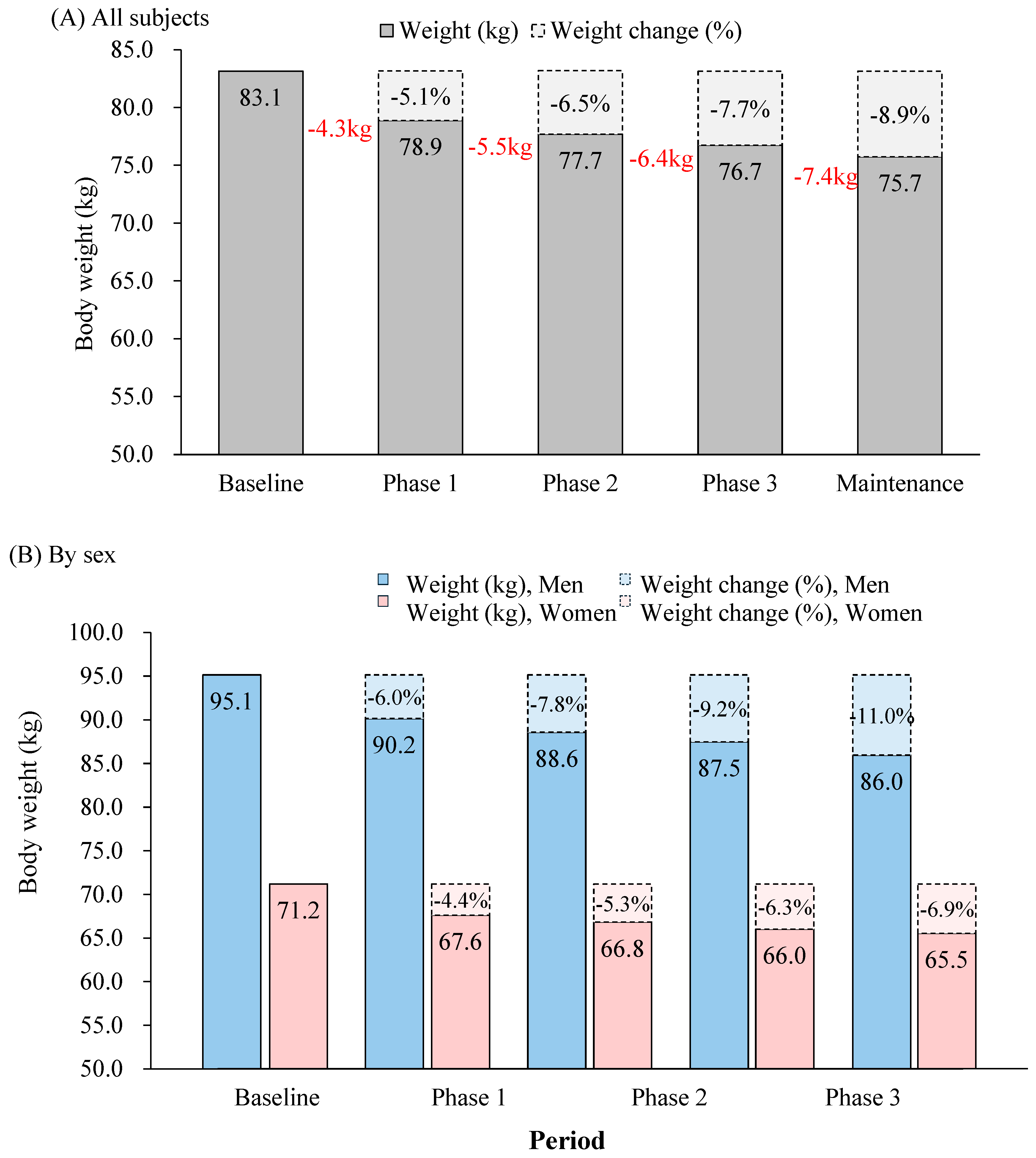
3.3. Changes in Body Weight
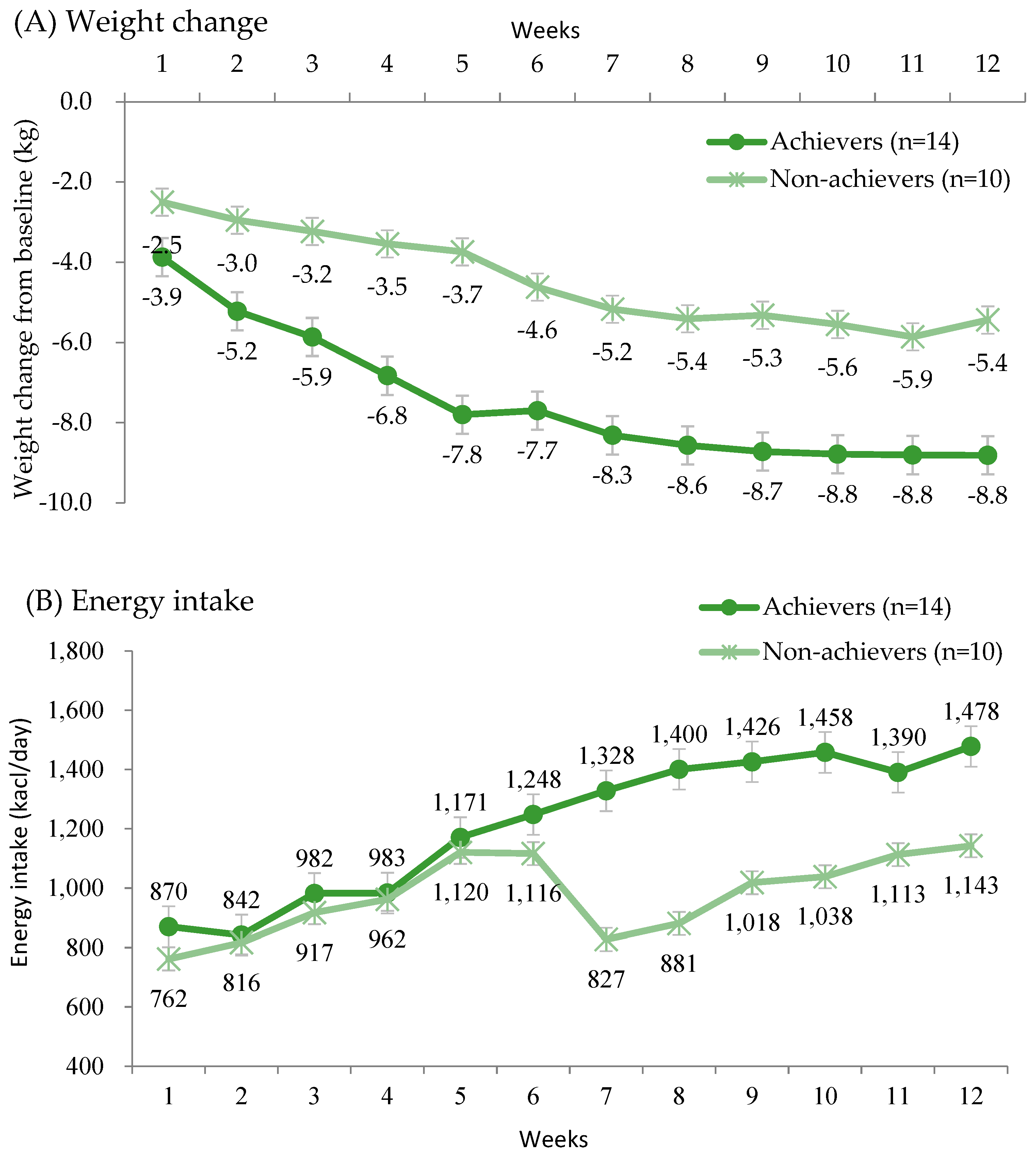
3.4. Weight Change and Nutrient Intake by Achievement of Weight Reduction Goals
3.5. Postprandial Glycemic Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 800 kcal | 1000 kcal | 1200 kcal | |
|---|---|---|---|
| Day 1 | Protein Drink | Chicken Breast Salad | Vietnamese Spring Rolls Salad |
| Fruit Cashew nut Salad | Mapa eggplant with Palbo rice | Hot Chicken Bulgogi and Brown Chickpeas Rice | |
| Stir-fried pork and Sweet potato barley rice | Champignon Gravy Chicken Set | Soft Tofu Gang-doenjang and Green tea Seagrass Rice | |
| Day 2 | Protein Drink | Protein Drink | Grilled Potato Cheese Salad |
| Yangjanpi Salad | Taco Salad | Ssamjang Fried Pork and Brown Barley Rice | |
| Mapa eggplant with Palbo rice | Sichuan Steamed Zucchini Set | Yangjanpi Salad | |
| Day 3 | Protein Drink | Poke Bowls Salad | Taco Salad |
| Basil Webfood Octopus Salad | Bacon Kimchi Fried Rice | Stir-fried Squid and Brown Oat Rice | |
| Gyudong with Brown Oat rice | Sichuan Eggplant Rice Set | Egg and Bulgogi Salad | |
| Day 4 | Protein Drink | Protein Drink | Poke Bowls Salad |
| Bun Cha Salad | Chicken Steak Salad | Mediterranean Rice Set | |
| Walnut Bibimbap | Greek Braised Pork Set | Bulgogi and Korean Vegetables and Brown Barley Rice | |
| Day 5 | Protein Drink | Protein Drink | Root Vegetables Chicken Salad |
| Tofu Salad | Yuringi Salad | Bulgogi and Korean Vegetables and Brown Barley Rice | |
| Bacon kimchi fried rice | Japanese Hamburg Curry Set | Sriracha sauce Chicken Salad | |
| Day 6 | Protein Drink | Short-fruit Pimpinella Meat Pasta Salad | Conchiglie Jambon Salad |
| Potato Blueberry Compote Salad | Walnut Bibimbap | Vegan Hamburg Steak and Grilled Emergency crop | |
| Pumpkin Bean cream Risotto and Fried chicken | Jambon Tortilla Salad | Grilled Chicken and Brown Chickpeas Rice | |
| Day 7 | Protein Drink | Protein Drink | Blueberry Compote Salad |
| Ricotta Granola Salad | Blueberry Compote Salad | Abalone Rice and Beef bone Dried Pollak Soup | |
| Chicken Cashew nut Salad and Brown Oat rice | Chickpeas Cream Chicken Steak and Taco Pilaf | Chicken Casew nut Curry and Brown Oat Rice |
| Nutrients | Phase 4 | Phase 5 | ||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 12) | Women (n = 12) | Men (n = 12) | Women (n = 12) | |||||
| Achiever (n = 8) | Non-Achiever (n = 4) | Achiever (n = 6) | Non-Achiever (n = 6) | Achiever (n = 8) | Non-Achiever (n = 4) | Achiever (n = 6) | Non-Achiever (n = 6) | |
| Energy (kcal/day) | 1345 ± 246.4 | 983.8 ± 68 | 1350.2 ± 316.4 | 764 ± 68 | 1524.9 ± 308.7 | 1169.9 ± 186.2 | 1319.6 ± 282.4 | 1016.5 ± 216.1 |
| Carbohydrate (g) | 152.0 ± 35.9 | 131.9 ± 6.5 | 156.3 ± 46.3 | 103.8 ± 7.1 | 163.0 ± 37.5 | 129.4 ± 22.5 | 157.7 ± 38.9 | 102.0 ± 42.6 |
| Sugar (g) | 24.9 ± 17.1 | 18.8 ± 1.1 | 25.6 ± 11.9 | 18.7 ± 3.8 | 23.4 ± 8.1 | 17.0 ± 10.0 | 22.2 ± 9.0 | 13.1 ± 6.3 |
| Protein (g) | 67.1 ± 14.4 | 48.4 ± 3.2 | 74.8 ± 10.5 | 37.8 ± 7.2 | 78.5 ± 14.0 | 56.1 ± 12.8 | 67.5 ± 10.8 | 53.7 ± 12.3 |
| Fat (g) | 50.0 ± 12.1 | 33.5 ± 6.3 | 49.6 ± 15.6 | 25.6 ± 3.3 | 57.5 ± 12.5 | 46.1 ± 11.6 | 47.1 ± 13.0 | 41.8 ± 14.5 |
| Saturated fat (g) | 12.1 ± 3.4 | 6.9 ± 2.1 | 11.2 ± 2.6 | 5 ± 0.7 | 14.8 ± 3.6 | 12.7 ± 4 | 11.8 ± 2.6 | 10.2 ± 5.8 |
| Percent from Energy | ||||||||
| Carbohydrate (%) | 47.0 ± 7.3 | 52.1 ± 3.1 | 45.6 ± 4.8 | 52.6 ± 3 | 44.3 ± 5.2 | 47 ± 6.1 | 48.2 ± 4.2 | 42.1 ± 14.1 |
| Sugar (%) | 6.7 ± 3.1 | 7.5 ± 0.4 | 7.6 ± 2.4 | 9.3 ± 1.5 | 6.1 ± 1.6 | 6.1 ± 3.6 | 6.8 ± 2.1 | 5.1 ± 1.8 |
| Protein (%) | 20.3 ± 2.8 | 18.7 ± 0.6 | 22.8 ± 6.0 | 18.7 ± 2.2 | 21.8 ± 2.1 | 19.3 ± 2.1 | 21.1 ± 2.8 | 22.1 ± 5.5 |
| Fat (%) | 32.7 ± 5.3 | 29.2 ± 3.5 | 31.6 ± 2.3 | 28.7 ± 2.2 | 33.9 ± 3.6 | 33.7 ± 4.2 | 30.7 ± 3.2 | 35.7 ± 9.3 |
| Saturated fat (%) | 7.7 ± 1.5 | 5.9 ± 1.2 | 7.3 ± 0.7 | 5.6 ± 0.5 | 8.6 ± 1.0 | 8.9 ± 1.4 | 7.7 ± 0.5 | 8.5 ± 2.7 |
References
- Kim, Y.; Nho, S.J.; Woo, G.; Kim, H.; Park, S.; Kim, Y.; Park, O.; Oh, K. Trends in the prevalence and management of major metabolic risk factors for chronic disease over 20 years: Findings from the 1998–2018 Korea National Health and Nutrition Examination Survey. Epidemiol. Health 2021, 43, e2021028. [Google Scholar]
- Park, S.E.; Ko, S.H.; Kim, J.Y.; Kim, K.; Moon, J.H.; Kim, N.H.; Han, K.D.; Choi, S.H.; Cha, B.S. Diabetes fact sheet in Korea 2024. Diabetes Metab. J. 2025, 49, 24–33. [Google Scholar]
- Yang, Y.S.; Han, B.-D.; Han, K.; Jung, J.-H.; Son, J.W. Obesity fact sheet in Korea, 2021: Trends in obesity prevalence and obesity-related comorbidity incidence stratified by age from 2009 to 2019. J. Obes. Metab. Syndr. 2022, 31, 169. [Google Scholar]
- Hu, F.B. Globalization of Diabetes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar]
- Ha, K.H.; Kim, D.J. Trends in the Diabetes Epidemic in Korea. Endocrinol. Metab. 2015, 30, 142. [Google Scholar]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar]
- Moon, J.S.; Kang, S.; Choi, J.H.; Lee, K.A.; Moon, J.H.; Chon, S.; Kim, D.; Kim, H.J.; Seo, J.A.; Kim, M.K.; et al. 2023 clinical practice guidelines for diabetes management in Korea: Full version recommendation of the Korean diabetes association. Diabetes Metab. J. 2024, 48, 546–708. [Google Scholar]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; A Johnson, J.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. bmj 2021, 372, m4743. [Google Scholar]
- Malandrucco, I.; Pasqualetti, P.; Giordani, I.; Manfellotto, D.; De Marco, F.; Alegiani, F.; Sidoti, A.M.; Picconi, F.; Di Flaviani, A.; Frajese, G.; et al. Very-low-calorie diet: A quick therapeutic tool to improve β cell function in morbidly obese patients with type 2 diabetes. Am. J. Clin. Nutr. 2012, 95, 609–613. [Google Scholar]
- Sathananthan, M.; Shah, M.; Edens, K.L.; Grothe, K.B.; Piccinini, F.; Farrugia, L.P.; Micheletto, F.; Man, C.D.; Cobelli, C.; A Rizza, R.; et al. Six and 12 weeks of caloric restriction increases β cell function and lowers fasting and postprandial glucose concentrations in people with type 2 diabetes. J. Nutr. 2015, 145, 2046–2051. [Google Scholar]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. 2018, 69, 63–683. [Google Scholar]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar]
- Steven, S.; Taylor, R. Restoring normoglycaemia by use of a very low calorie diet in long-and short-duration Type 2 diabetes. Diabet. Med. 2015, 32, 1149–1155. [Google Scholar]
- Goday, A.; Bellido, D.; Sajoux, I.; Crujeiras, A.B.; Burguera, B.; García-Luna, P.P.; Oleaga, A.; Moreno, B.; Casanueva, F.F. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr. Diabetes 2016, 6, e230. [Google Scholar]
- Kashyap, A.; Mackay, A.; Carter, B.; Fyfe, C.L.; Johnstone, A.M.; Myint, P.K. Investigating the Effectiveness of Very Low-Calorie Diets and Low-Fat Vegan Diets on Weight and Glycemic Markers in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4870. [Google Scholar]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar]
- Taheri, S.; Zaghloul, H.; Chagoury, O.; Elhadad, S.; Ahmed, S.H.; El Khatib, N.; Amona, R.A.; El Nahas, K.; Suleiman, N.; Alnaama, A.; et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): An open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 477–489. [Google Scholar]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar]
- Song, Y.; Joung, H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 456–462. [Google Scholar]
- Kim, M.K.; Kim, J.; Park, S.; Song, Y.; Kwon, H. Impact of caloric restriction on diabetes remission in Korean adults with obesity (CREDO-K study). Diabetes Obes. Metab. 2025, 27, 1609–1613. [Google Scholar]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar]
- Atkinson, R.L.; Dietz, W.H.; Foreyt, J.P.; Goodwin, N.J.; Hill, J.O.; Hirsch, J.; Pi-Sunyer, X.; Weinsier, R.L.; Wing, R.; Yanovsko, S.Z.; et al. Very low-calorie diets. JAMA 1993, 270, 967–974. [Google Scholar]
- Heilbronn, L.K. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar]
- Flanagan, E.W.; Spann, R.; Berry, S.E.; Berthoud, H.; Broyles, S.; Foster, G.D.; Krakoff, J.; Loos, R.J.F.; Lowe, M.R.; Ostendorf, D.M.; et al. New insights in the mechanisms of weight-loss maintenance: Summary from a Pennington symposium. Obesity 2023, 31, 2895–2908. [Google Scholar]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. A very-low-calorie diet (VLCD) intervention for the management of prediabetes and early Type 2 diabetes mellitus in a multi-ethnic cohort in Aotearoa New Zealand: The PROGRESS NZ feasibility study. Asia Pac. J. Clin. Nutr. 2024, 33, 200. [Google Scholar]
- Churuangsuk, C.; Hall, J.; Reynolds, A.; Griffin, S.J.; Combet, E.; Lean, M.E.J. Diets for weight management in adults with type 2 diabetes: An umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia 2022, 65, 14–36. [Google Scholar]
- Choi, J.H.; Min, S.H.; Lim, K.H.; Shin, U.J.; Kim, M.-S. Glucose-lowering effect of home-delivered therapeutic meals in patients with type 2 diabetes. J. Korean Diabetes 2020, 21, 46–54. [Google Scholar]
- Aller, E.E.J.G.; Larsen, T.M.; Claus, H.; Lindroos, A.K.; Kafatos, A.; Pfeiffer, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Stender, S.; et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: The DIOGENES trial 12-month results. Int. J. Obes. 2014, 38, 1511–1517. [Google Scholar]
- Kim, J.Y. Optimal diet strategies for weight loss and weight loss maintenance. J. Obes. Metab. Syndr. 2020, 30, 20. [Google Scholar]
- Ha, K.; Joung, H.; Song, Y. Low-carbohydrate diet and the risk of metabolic syndrome in Korean adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1122–1132. [Google Scholar]
- Goris, A.H.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am. J. Clin. Nutr. 2000, 71, 130–134. [Google Scholar]
- Kanellakis, S.; Sidiropoulou, S.; Apostolidou, E.; Skoufas, E.; Bountouvi, E.; Prelorentzou, T.; Manios, Y. Association of dietary intake underreporting with body image perception. Clin. Nutr. Open Sci. 2021, 40, 30–37. [Google Scholar]

| Total (n = 24) | Men (n = 12) | Women (n = 12) | p-Value 1 | |
|---|---|---|---|---|
| Age (years) | 42.2 ± 10.9 | 43.3 ± 10.2 | 41.1 ± 12.1 | 0.7069 |
| Body weight (kg) | 83.1 ± 15 | 95.1 ± 10 | 71.2 ± 7.6 | <0.0001 |
| BMI (kg/m2) | 29.6 ± 3.9 | 31.5 ± 4.3 | 27.8 ± 2.6 | 0.0464 |
| Waist circumference (cm) | 97.3 ± 11.3 | 105.6 ± 9 | 89.0 ± 6.0 | <0.0001 |
| Muscle mass (kg) | 29.7 ± 6.8 | 35.5 ± 3.5 | 23.9 ± 3.4 | <0.0001 |
| Fat mass (kg) | 29.7 ± 7.9 | 32 ± 9.6 | 27.3 ± 5 | 0.2252 |
| Body fat percent (%) | 38.6 ± 13.9 | 39 ± 19.4 | 38.3 ± 5.1 | 0.2366 |
| Clinical measures | ||||
| Duration of diabetes (years) | 2.5 ± 2.0 | 1.8 ± 1.3 | 3.2 ± 2.3 | 0.1749 |
| Fasting blood glucose (mg/dL) | 127.8 ± 31.9 | 123.8 ± 38.6 | 131.8 ± 24.5 | 0.1407 |
| HbA1c (mmol/mol) | 53.1 ± 13.5 | 51.2 ± 14.5 | 54.9 ± 12.6 | 0.3545 |
| (HbA1c, %) | (7.0 ± 1.2) | (6.8 ± 1.3) | (7.2 ± 1.2) | |
| Total cholesterol (mg/dL) | 150 ± 24.9 | 143.7 ± 24.6 | 156.3 ± 24.6 | 0.2251 |
| Low-density lipoprotein cholesterol (mg/dL) | 80 ± 19.8 | 75.3 ± 22.8 | 84.7 ± 15.8 | 0.2034 |
| High-density lipoprotein cholesterol (mg/dL) | 53 ± 10.1 | 51.4 ± 11 | 54.5 ± 9.4 | 0.3708 |
| Triglycerides (mg/dL) | 138.5 ± 65.4 | 134.4 ± 65.5 | 142.6 ± 68 | 0.8625 |
| Systolic blood pressure (mmHg) | 130.2 ± 15.4 | 132 ± 17.4 | 128.3 ± 13.6 | 0.7503 |
| Diastolic blood pressure (mmHg) | 82.4 ± 11.5 | 84.2 ± 11.1 | 80.7 ± 12 | 0.4184 |
| Comorbidities | ||||
| Hypertension (n, %) | 11 (45.8) | 8 (66.7) | 3 (25.0) | 0.0486 |
| Dyslipidemia (n, %) | 23 (95.8) | 12 (100.0) | 11 (91.7) | 0.3593 |
| Nutrients | Weight Loss Period | Weight Maintenance Period | |||||
|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | |||
| Achiever (n = 14) | Non-Achiever (n = 10) | Achiever (n = 14) | Non-Achiever (n = 10) | ||||
| Energy (kcal/day) (Mean ± SD) | 828.0 ± 124.4 | 964.5 ± 84.7 | 1172.3 ± 133.7 | 1347.2 ± 266.8 | 851.9 ± 130.4 | 1436.9 ± 305.1 | 1077.9 ± 209.3 |
| Carbohydrate (g) | 104.3 ± 13.3 | 125.6 ± 17.9 | 154.4 ± 19.6 | 153.9 ± 39 | 115 ± 15.9 | 160.7 ± 36.7 | 113 ± 37.1 |
| Sugar (g) | 19.1 ± 3.5 | 20.6 ± 5.1 | 22.7 ± 6.5 | 25.2 ± 14.5 | 18.7 ± 2.9 | 22.9 ± 8.2 | 14.6 ± 7.7 |
| Protein (g) | 48.8 ± 9.9 | 46.8 ± 7.4 | 48.8 ± 8.6 | 70.4 ± 13 | 42 ± 7.9 | 73.8 ± 13.5 | 54.7 ± 11.8 |
| Fat (g) | 29 ± 5.8 | 35.7 ± 3.9 | 44.5 ± 6.4 | 49.8 ± 13.1 | 28.8 ± 6 | 53 ± 13.3 | 43.5 ± 12.9 |
| Saturated fat (g) | 5.5 ± 2.0 | 6.6 ± 1.2 | 8.5 ± 2.1 | 11.7 ± 3 | 5.8 ± 1.6 | 13.5 ± 3.5 | 11.2 ± 5.1 |
| Percent of energy | |||||||
| Carbohydrate (%) | 47.9 ± 3.5 | 49.5 ± 4.3 | 51 ± 3.2 | 46.4 ± 6.2 | 52.4 ± 2.9 | 46 ± 5.1 | 44.1 ± 11.4 |
| Sugar (%) | 8.8 ± 1.4 | 8.1 ± 1.5 | 7.4 ± 1.4 | 7.1 ± 2.8 | 8.6 ± 1.5 | 6.4 ± 1.8 | 5.5 ± 2.5 |
| Protein (%) | 22.2 ± 2.6 | 18.6 ± 3.1 | 16 ± 1.6 | 21.4 ± 4.4 | 18.7 ± 1.7 | 21.5 ± 2.4 | 21 ± 4.5 |
| Fat (%) | 29.9 ± 4.1 | 31.8 ± 2.6 | 33 ± 2.8 | 32.2 ± 4.2 | 28.9 ± 2.6 | 32.5 ± 3.7 | 34.9 ± 7.4 |
| Saturated fat (%) | 5.6 ± 1.5 | 5.9 ± 1.2 | 6.3 ± 1.3 | 7.5 ± 1.2 | 5.7 ± 0.8 | 8.2 ± 0.9 | 8.7 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Kim, M.K.; Kim, J.; Choi, J.-Y.; Song, Y.; Kwon, H.-S. Feasibility of a Structured Calorie-Restricted Dietary Intervention in Korean Adults with Early Type 2 Diabetes and Obesity: A Pilot Study. Nutrients 2025, 17, 1530. https://doi.org/10.3390/nu17091530
Park S-J, Kim MK, Kim J, Choi J-Y, Song Y, Kwon H-S. Feasibility of a Structured Calorie-Restricted Dietary Intervention in Korean Adults with Early Type 2 Diabetes and Obesity: A Pilot Study. Nutrients. 2025; 17(9):1530. https://doi.org/10.3390/nu17091530
Chicago/Turabian StylePark, Su-Jeong, Mee Kyung Kim, Jinyoung Kim, Ji-Yeon Choi, YoonJu Song, and Hyuk-Sang Kwon. 2025. "Feasibility of a Structured Calorie-Restricted Dietary Intervention in Korean Adults with Early Type 2 Diabetes and Obesity: A Pilot Study" Nutrients 17, no. 9: 1530. https://doi.org/10.3390/nu17091530
APA StylePark, S.-J., Kim, M. K., Kim, J., Choi, J.-Y., Song, Y., & Kwon, H.-S. (2025). Feasibility of a Structured Calorie-Restricted Dietary Intervention in Korean Adults with Early Type 2 Diabetes and Obesity: A Pilot Study. Nutrients, 17(9), 1530. https://doi.org/10.3390/nu17091530





