Effects of Caffeine Intake on Self-Administered Sleeping Quality and Wearable Monitoring of Sleep in a Cohort of Young Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
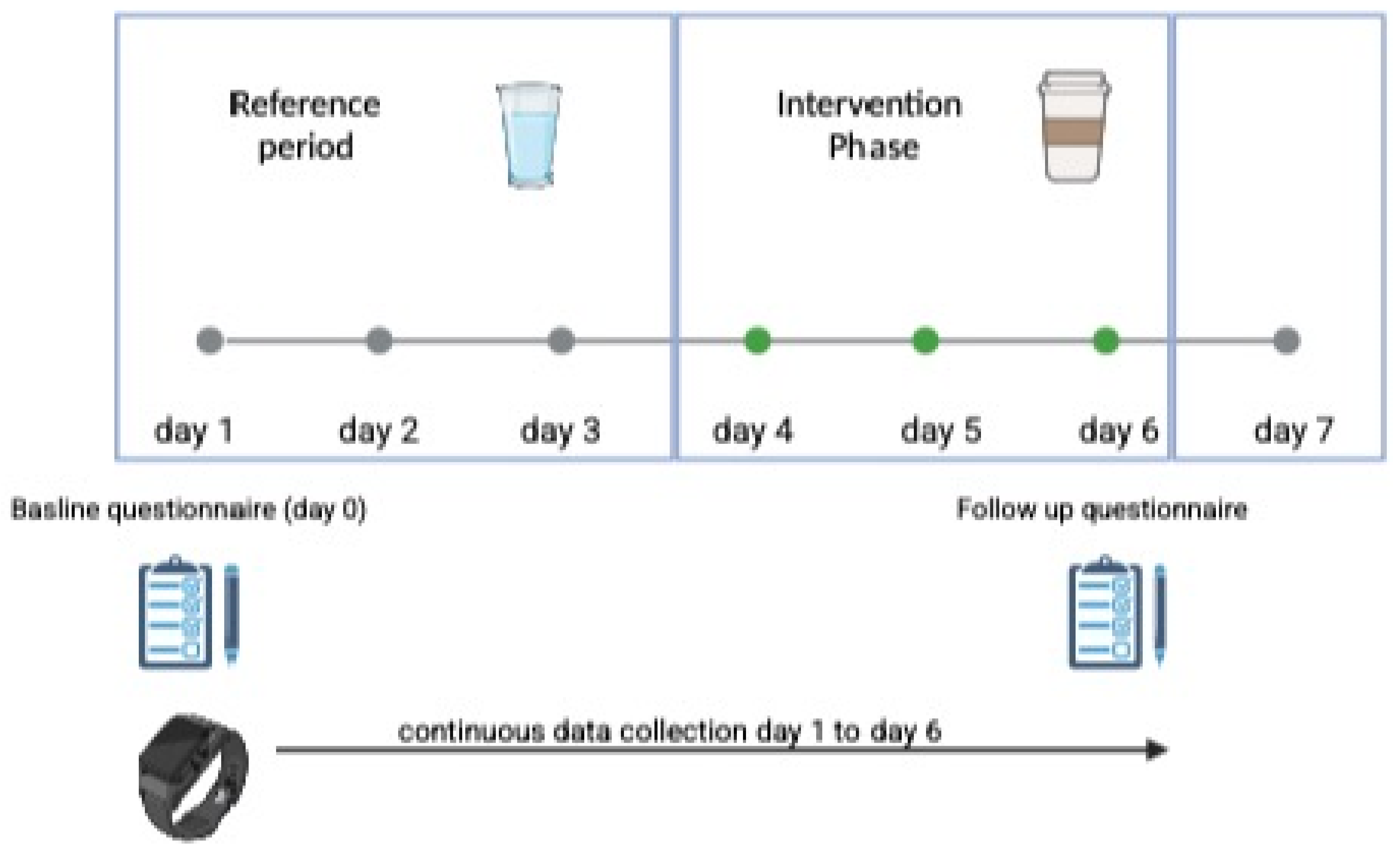
2.1. Study Setting
- Intervention Group 1: Caffeine intake via coffee consumption
- Intervention Group 2: Caffeine intake via energy drink consumption
- A self-administered questionnaire at baseline (Day 1) and on the last day of the intervention (Day 7).
- Data from the wearable device.
2.2. Study Population
- Age > 18 years
- No pre-existing medical conditions
- No contradiction between daily caffeine consumption
- Singed consent for study participation
2.3. Outcome Measures and Endpoints
- Self-assessed questionnaires containing validated questions on:
- Smartwatch tracking (Withings ScanWatch)
Primary and Secondary Endpoints
2.4. Statistical Analyses
3. Results
3.1. Demographics and Baseline Data
3.2. Primary Endpoint: Duration of Sleep (See Table 1)
| Baseline | Coffee | Energy Drink | Baseline vs. Coffee vs. Energy Drink p-Value | |
|---|---|---|---|---|
| Duration of sleep | ||||
| mean ± SD [hours] | 6.9 ± 1.0 | 6.9 ± 1.3 | 6.7 ± 1.1 | 0.183 |
3.3. Secondary Endpoints
- Sleep measures from wearable data (see Table 2A)
- 2.
- Self-Assessment Data (see Table 2B,C)
- 3.
- PSQI Assessment (see Table 2C)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMU | Ludwig-Maximilians-Universität |
| PSQI | Pittsburgh Sleep Quality Index |
| RefC | Reference Period Coffee |
| RefE | Reference Period Energy Drink |
| sd | standard deviation |
References
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, Y.; Morikawa, Y.; Nakamura, K.; Sakurai, M.; Miura, K.; Ishizaki, M.; Kido, T.; Naruse, Y.; Suwazono, Y.; Nakagawa, H. The effects of sleep duration on the incidence of cardiovascular events among middle-aged male workers in Japan. Scand. J. Work. Environ. Health 2011, 37, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar]
- Gobin, C.M.; Banks, J.B.; Fins, A.I.; Tartar, J.L. Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. J. Sleep Res. 2015, 24, 535–542. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Stathakis, G.; Koloi, A.; Bakiri, E.; Moudatsaki, M.; Pouladaki, E.; Moniaki, V.; Mauroudi, E.; Schiza, S. Prevalence and risk factors of poor sleep quality and fatigue during exam periods in university students. ERJ Open Res. 2017, 3, P2. [Google Scholar] [CrossRef]
- Crispim, C.A.; Zimberg, I.Z.; dos Reis, B.G.; Diniz, R.M.; Tufik, S.; de Mello, M.T. Relationship between food intake and sleep pattern in healthy individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef]
- Sakal, C.; Zhao, W.; Xu, W.; Li, X. Effects of caffeine on accelerometer measured sleep and physical activity among older adults under free-living conditions. BMC Public Health 2024, 24, 3299. [Google Scholar] [CrossRef]
- Gardiner, C.; Weakley, J.; Burke, L.M.; Roach, G.D.; Sargent, C.; Maniar, N.; Townshend, A.; Halson, S.L. The effect of caffeine on subsequent sleep: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 69, 101764. [Google Scholar] [CrossRef]
- Higbee, M.R.; Gipson, C.S.; El-Saidi, M. Caffeine Consumption Habits, Sleep Quality, Sleep Quantity, and Perceived Stress of Undergraduate Nursing Students. Nurse Educ. 2022, 47, 120–124. [Google Scholar] [CrossRef]
- Gardiner, C.L.; Weakley, J.; Burke, L.M.; Fernandez, F.; Johnston, R.D.; Leota, J.; Russell, S.; Munteanu, G.; Townshend, A.; Halson, S.L. Dose and timing effects of caffeine on subsequent sleep: A randomised clinical crossover trial. Sleep 2024, 48, zsae230. [Google Scholar] [CrossRef]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine effects on sleep taken 0, 3, or 6 h before going to bed. J. Clin. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Doyle, J.; Smith, S.; Crabtree, D.R.; Fraser, M. Measurement of Heart Rate Using the Withings ScanWatch Device During Free-living Activities: Validation Study. JMIR Form. Res. 2022, 6, e34280. [Google Scholar] [CrossRef]
- Frija, J.; Mullaert, J.; Abensur Vuillaume, L.; Grajoszex, M.; Wanono, R.; Benzaquen, H.; Kerzabi, F.; Geoffroy, P.A.; Matrot, B.; Trioux, T.; et al. Metrology of two wearable sleep trackers against polysomnography in patients with sleep complaints. J. Sleep Res. 2025, 34, e14235. [Google Scholar] [CrossRef]
- Edwards, B.A.; O’Driscoll, D.M.; Ali, A.; Jordan, A.S.; Trinder, J.; Malhotra, A. Aging and sleep: Physiology and pathophysiology. Semin. Respir. Crit. Care Med. 2010, 31, 618–633. [Google Scholar] [CrossRef]
- Swift, C.G.; Tiplady, B. The effects of age on the response to caffeine. Psychopharmacology 1988, 94, 29–31. [Google Scholar] [CrossRef]
- Weibel, J.; Lin, Y.S.; Landolt, H.P.; Kistler, J.; Rehm, S.; Rentsch, K.M.; Slawik, H.; Borgwardt, S.; Cajochen, C.; Reichert, C.F. The impact of daily caffeine intake on nighttime sleep in young adult men. Sci. Rep. 2021, 11, 4668. [Google Scholar] [CrossRef]
- Rauh, R.; Burkert, M.; Siepmann, M.; Mueck-Weymann, M. Acute effects of caffeine on heart rate variability in habitual caffeine consumers. Clin. Physiol. Funct. Imaging 2006, 26, 163–166. [Google Scholar] [CrossRef]
- Newcombe, P.F.; Renton, K.W.; Rautaharju, P.M.; Spencer, C.A.; Montague, T.J. High-dose caffeine and cardiac rate and rhythm in normal subjects. Chest 1988, 94, 90–94. [Google Scholar] [CrossRef]
- Claydon, E.A.; Kahwash, J.M.; Lilly, C.L.; Alamir, Y.; Zullig, K.J. Subjective Sleep Quality, Caffeine, and Dieting Behaviors Among University-Attending Young Adults. Nat. Sci. Sleep 2023, 15, 737–747. [Google Scholar] [CrossRef]
- Costantino, A.; Maiese, A.; Lazzari, J.; Casula, C.; Turillazzi, E.; Frati, P.; Fineschi, V. The Dark Side of Energy Drinks: A Comprehensive Review of Their Impact on the Human Body. Nutrients 2023, 15, 3922. [Google Scholar] [CrossRef]
- Kerpershoek, M.L.; Antypa, N.; Van den Berg, J.F. Evening use of caffeine moderates the relationship between caffeine consumption and subjective sleep quality in students. J. Sleep Res. 2018, 27, e12670. [Google Scholar] [CrossRef] [PubMed]
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Coffee | RefC | Coffee vs. RefC p-Value | Energy Drink | RefE | Energy Drink vs. Ref p-Value | Coffee vs. Energy Drink p-Value | |
| Wearable data [mean ± sd] | |||||||
| Light sleep [min] | 242.3 ± 4.0 | 255.7 ± 4.3 | 0.308 | 237.5 ± 4.0 | 261.3 ± 4.4 | 0.086 | 0.095 |
| Deep sleep [min] | 189.3 ± 3.2 | 198.7 ± 3.3 | 0.562 | 192.3 ± 3.2 | 195.8 ± 3.3 | 0.733 | 0.790 |
| Awake [min] | 22.7 ± 0.4 | 23.4 ± 0.4 | 0.587 | 21.3 ± 0.4 | 18.9 ± 0.3 | 0.241 | 0.604 |
| Duration to sleep [min] | 2.8 ± 0.1 | 2.9 ± 0.1 | 0.803 | 2.5 ± 0.04 | 2.4 ± 0.04 | 0.895 | 0.062 |
| Duration to wake up [min] | 4.2 ± 0.1 | 4.0 ± 0.1 | 0.920 | 4.4 ± 0.1 | 2.7 ± 0.1 | 0.161 | 0.393 |
| Average heart rate [bpm] | 62.2 ± 8.5 | 62.1 ± 10.2 | 0.650 | 62.6 ± 9.4 | 62.3 ± 8.4 | 0.787 | 0.934 |
| (B) | |||||||
| Baseline | Coffee | Energy Drink | Baseline vs. Coffee vs. Energy Drink p-Value | ||||
| Time to fall asleep | |||||||
| mean ± SD [min] | 23.5 ± 15.5 | 27.4 ± 33.2 | 27.3 ± 25.4 | 0.559 | |||
| (C) | |||||||
| Baseline | Coffee | Energy Drink | Baseline vs. Coffee vs. Energy Drink p-Value | ||||
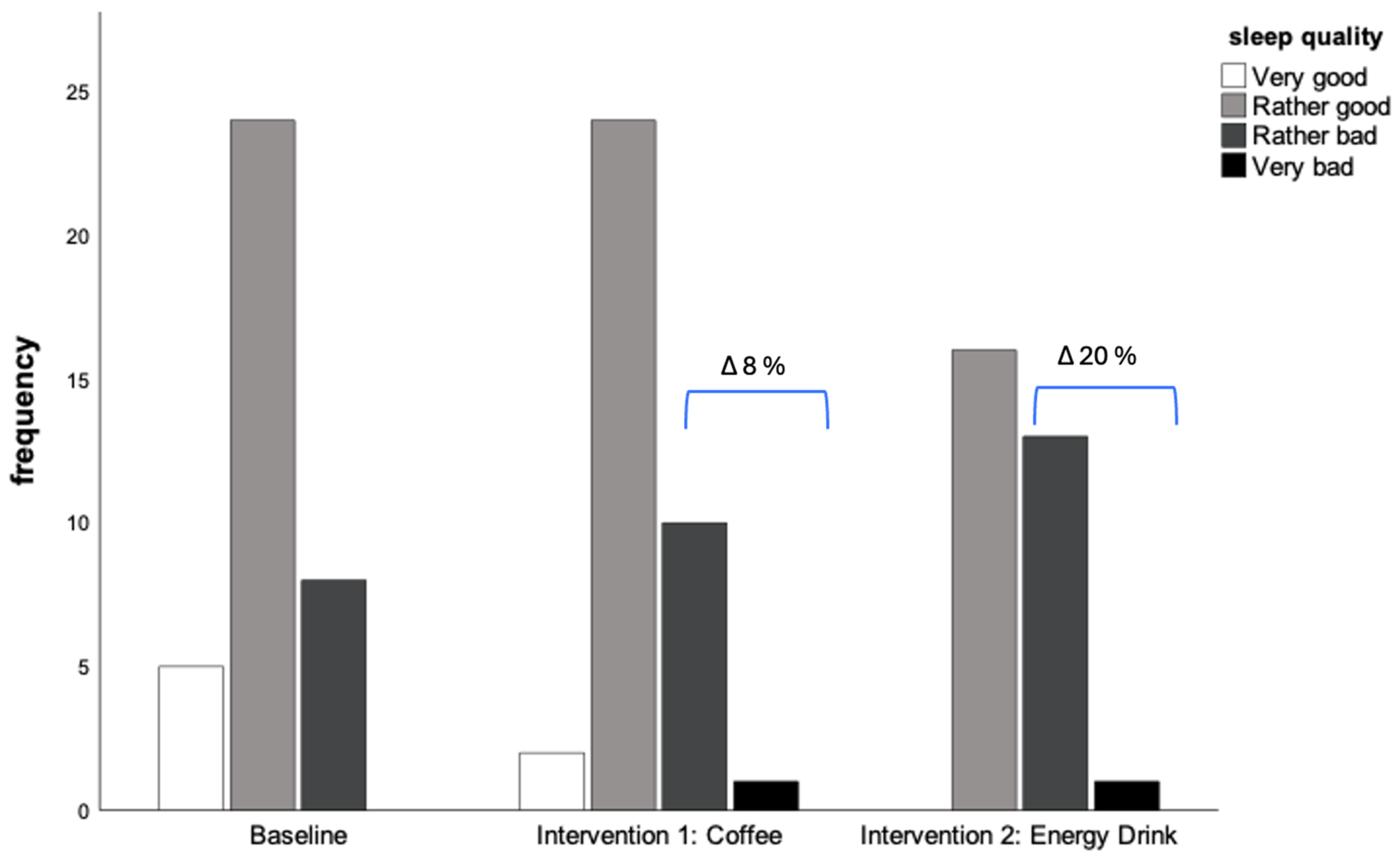
| Self-assessed sleeping quality | |||||||
| Very good | 13.5 (5) | 5.4 (2) | - | 0.173 | |||
| Rather good | 64.9 (24) | 64.9 (24) | 53.3 (16) | ||||
| Rather bad | 21.6 (8) | 27.0 (10) | 43.3 (13) | ||||
| Very bad | - | 2.7 (1) | 3.3 (1) | ||||
| PSQI | |||||||
| Mean ± SD | 6.7 ± 2.6 | 6.5 ± 2.6 | 0.948 | ||||
| Baseline n = 37 | Coffee n = 37 | Energy Drinks n = 31 | p-Value | |
|---|---|---|---|---|
| Problems to fall asleep | ||||
| Never | 16.2 (6) | 18.9 (7) | 16.1 (5) | 0.317 |
| Rarely | 40.5 (15) | 37.8 (14) | 19.4 (6) | |
| Sometimes | 29.7 (11) | 16.2 (6) | 32.3 (10) | |
| Often | 10.8 (4) | 24.3 (9) | 32.3 (10) | |
| Always | 2.7 (1) | 2.7 (1) | - | |
| Wake up at night | ||||
| Never | 40.5 (15) | 27.0 (10) | 38.7 (12) | 0.546 |
| Rarely | 35.1 (13) | 32.4 (12) | 22.6 (7) | |
| Sometimes | 18.9 (7) | 24.3 (9) | 19.4 (6) | |
| Often | 5.4 (3) | 13.5 (5) | 19.4 (6) | |
| Always | - | 2.7 (1) | - | |
| Problems to feel Rested | ||||
| Never | - | 8.1 (3) | - | 0.014 |
| Rarely | 13.5 (5) | 10.8 (4) | 22.6 (7) | |
| Sometimes | 13.5 (5) | 27.0 (10) | 41.9 (13) | |
| Often | 59.5 (22) | 37.8 (14) | 19.4 (6) | |
| Always | 13.5 (5) | 16.2 (6) | 16.1 (5) | |
| Sleep medication | ||||
| Never | 86.5 (32) | 89.2 (33) | 90.0 (27) | 0.676 |
| Less than once aweek | 5.4 (2) | 5.4 (2) | 10.0 (3) | |
| At least once a week | 8.1 (3) | 5.4 (4) | ||
| Problems to stay awake during daytime | ||||
| Never | 51.4 (19) | 54.1 (20) | 54.8 (17) | 0.338 |
| Less than one aweek | 35.1 (13) | 37.8 (14) | 16.7 (5) | |
| At least once a week | 13.5 (5) | 8.1 (3) | 26.7 (8) | |
| Problems to manage daily routine | ||||
| No Problems | 27.0 (10) | 32.4 (12) | 26.7 (8) | 0.965 |
| Rarely | 40.5 (15) | 40.5 (15) | 33.3 (10) | |
| Sometimes | 29.7 (11) | 24.3 (9) | 36.7 (11) | |
| Often | 2.7 (1) | 2.7 (1) | 3.3 (1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlichtiger, J.; Brunner, S.; Strüven, A.; Hoppe, J.M.; Stremmel, C. Effects of Caffeine Intake on Self-Administered Sleeping Quality and Wearable Monitoring of Sleep in a Cohort of Young Healthy Adults. Nutrients 2025, 17, 1503. https://doi.org/10.3390/nu17091503
Schlichtiger J, Brunner S, Strüven A, Hoppe JM, Stremmel C. Effects of Caffeine Intake on Self-Administered Sleeping Quality and Wearable Monitoring of Sleep in a Cohort of Young Healthy Adults. Nutrients. 2025; 17(9):1503. https://doi.org/10.3390/nu17091503
Chicago/Turabian StyleSchlichtiger, Jenny, Stefan Brunner, Anna Strüven, John Michael Hoppe, and Christopher Stremmel. 2025. "Effects of Caffeine Intake on Self-Administered Sleeping Quality and Wearable Monitoring of Sleep in a Cohort of Young Healthy Adults" Nutrients 17, no. 9: 1503. https://doi.org/10.3390/nu17091503
APA StyleSchlichtiger, J., Brunner, S., Strüven, A., Hoppe, J. M., & Stremmel, C. (2025). Effects of Caffeine Intake on Self-Administered Sleeping Quality and Wearable Monitoring of Sleep in a Cohort of Young Healthy Adults. Nutrients, 17(9), 1503. https://doi.org/10.3390/nu17091503








