Neuro-Nutritional Approach to Neuropathic Pain Management: A Critical Review
Abstract
1. Introduction
Neuropathogenic Mechanisms, Gender, and Age as Important Determinants of Pain
2. Acute Versus Chronic Pain
3. Mechanisms of Neuropathic Pain Induction
3.1. Inflammation and Its Involvement in Pain Transmission
3.1.1. A Brief Description of the Most Important Pain Targets in Inflammatory Pathways
Chemokines and Nerve Growth Factor
3.2. Inflammation and Aging
3.3. Oxidative Stress and Its Involvement in Pain Transmission
3.3.1. A Brief Description of the Most Important Pain Targets in Oxidative Stress Pathways for Neuropathic Pain
Transient Receptor Potential Channels
Nuclear Factor Erythroid 2-Related Factor
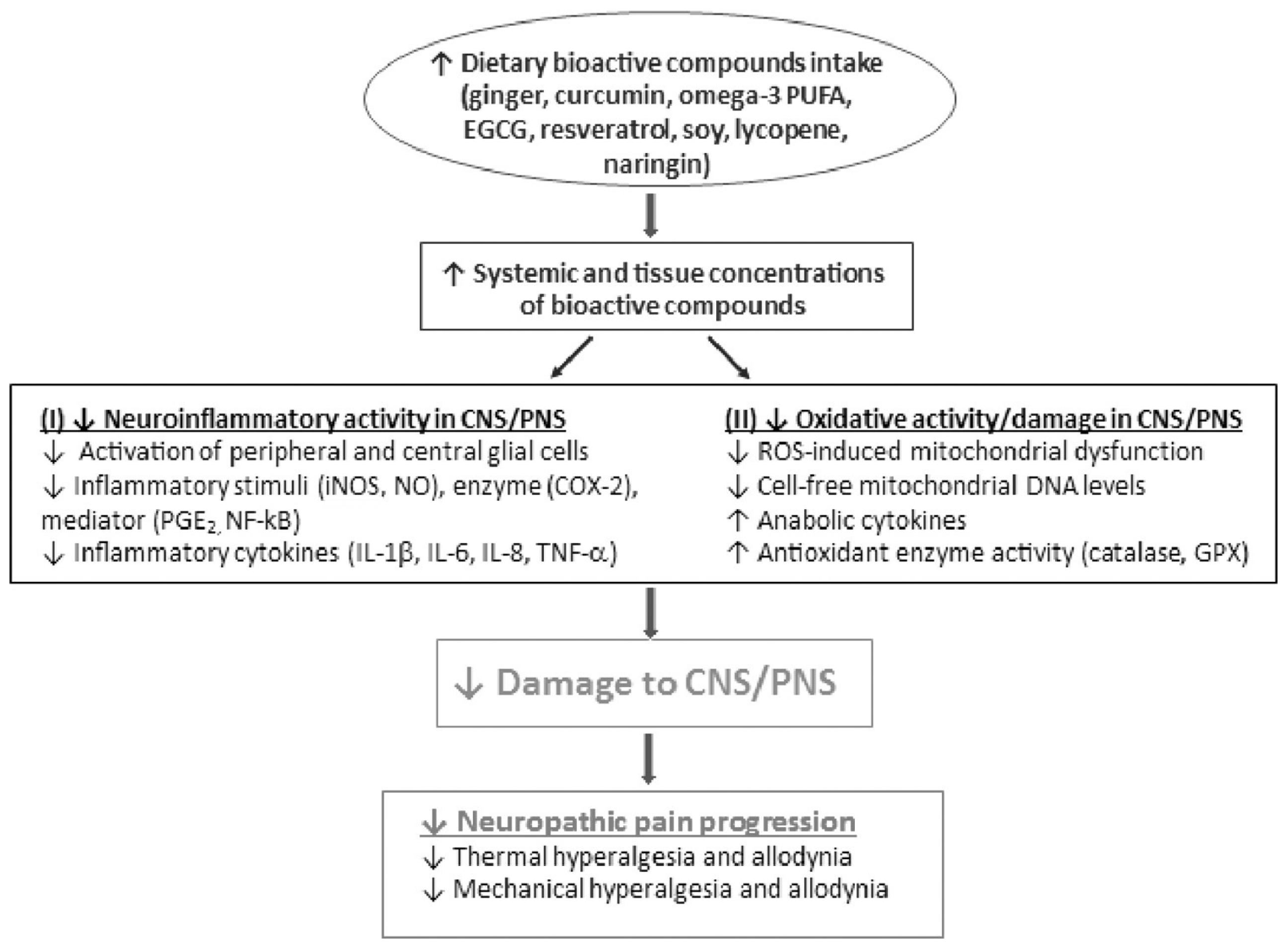
4. Nutrition and Neuropathic Pain
4.1. Melatonin: Secretion and Effects
4.1.1. Analgesic Effect of Melatonin in Human Studies
| Study | Pain Model | Study Design | Participants | Outcomes |
|---|---|---|---|---|
| Jallouli et al., 2025 [253] | Multiple sclerosis | Randomized controlled trial | 27 patients with multiple sclerosis | Improvements in dynamic postural stability and walking performance |
| Mehramiri et al., 2024 [263] | Migraines | Double-blind, randomized clinical trial | 60 patients with episodic migraines | Reduction of frequency and duration of migraine attacks |
| Alstadhaug et al., 2010 [314] | Migraines | Randomized, double-blind, placebo-controlled crossover study | Men and women, aged 18–65 years, with migraines but otherwise healthy, experiencing 2–7 attacks per month | No reduction in attack frequency and no improvement in sleep quality |
| Acuna-Castroviejo et al., 2006 [307] | Fibromyalgia | Open study | 4 patients who also received other medication, including chronic analgesics, antidepressants, sedative hypnotics, and in one case opioids | Improvements in the sleep/wake cycle and significant reduction of pain severity and fatigue |
| Lu et al., 2005 [309] | Irritable bowel syndrome | Double-blind placebo-controlled study | IBS patients (aged 20–64 years; 24 female) with sleep disturbances | Significant improvements in mean IBS scores after treatment with melatonin (3.9 ± 2.6) than with a placebo |
| Song et al., 2005 [308] | Irritable bowel syndrome | Randomized, double-blind, placebo-controlled study | 17 female patients satisfying the Rome II criteria for IBS | Decreased mean abdominal pain score and increased mean rectal pain threshold |
| Peres et al., 2004 [313] | Migraines | Open-label trial | 40 patients with episodic migraines with or without aura | Decreased headache frequency, headache intensity on a 0 to 10 scale, and duration in hours |
| Citera et al., 2000 [304] | Fibromyalgia | Open-label, randomized study | 21 female patients | Improvements in sleep quality, pain, fatigue, and depressive symptoms |
| Claustrat et al., 1997 [311] | Migraines | Open study | 6 migraine patients and 9 healthy controls | Significant pain relief and absence of side effects |
4.1.2. Melatonin and Dietary Sources
Sources of Melatonin and Benefits of Consuming MLT-Containing Foods
4.2. Overview of Different Dietary Interventions Against Neuropathic Pain
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldberg, D.S.; McGee, S.J. Pain as a Global Public Health Priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Magwood, K.; Smith, J.; Jenkins, M.R.; McGregor, A.J.; Quesnelle, K.M. Sex and Gender Differences in Pain Perception and Management in Clinical Settings. All Life 2024, 17, 2367421. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Primary Pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.R.; Davis, K.D. Sex and Gender Differences in Pain. Int. Rev. Neurobiol. 2022, 164, 277–307. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Loeser, J.D.; Treede, R.-D. The Kyoto Protocol of IASP Basic Pain Terminology. Pain 2008, 137, 473–477. [Google Scholar] [CrossRef]
- Fillingim, R.B. Individual Differences in Pain: Understanding the Mosaic That Makes Pain Personal. Pain 2017, 158 (Suppl. 1), S11–S18. [Google Scholar] [CrossRef]
- Leung, L. Pain Catastrophizing: An Updated Review. Indian J. Psychol. Med. 2012, 34, 204–217. [Google Scholar] [CrossRef]
- Verma, N.; Chouhan, D.; Meghana, A.; Tiwari, V. Heat Shock Proteins in Chronic Pain: From Molecular Chaperones to Pain Modulators. Neuropharmacology 2025, 266, 110263. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Silva, Á.J.C.; de Lavor, M.S.L. Nitroxidative Stress, Cell—Signaling Pathways, and Manganese Porphyrins: Therapeutic Potential in Neuropathic Pain. Int. J. Mol. Sci. 2025, 26, 2050. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. Neuroinflammation, Oxidative Stress and Their Interplay in Neuropathic Pain: Focus on Specialized pro-Resolving Mediators and NADPH Oxidase Inhibitors as Potential Therapeutic Strategies. Pharmacol. Res. 2020, 162, 105280. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Naziroglu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic Pain: Delving into the Oxidative Origin and the Possible Implication of Transient Receptor Potential Channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.A.; Rice, A.S.C.; Rief, W.; Sluka, A.K. Do We Need a Third Mechanistic Descriptor for Chronic Pain States? Pain 2016, 157, 1382–1386. [Google Scholar] [CrossRef]
- Pulminskas, A.; Hojjatie, R.; Karatas, T.B.; Li, Y.H.; Orenstein, L.A.V. Hidradenitis Suppurativa Symptom Relief: Pain and Itch. Dermatol. Clin. 2025, 43, 247–260. [Google Scholar] [CrossRef]
- Wood, S.; Coxon, L.; Glyn-Jones, S.; Barker, K.L. Neuropathic Pain Is a Feature in Patients with Symptomatic Femoral Acetabular Impingement. Physiotherapy 2024, 124, 135–142. [Google Scholar] [CrossRef]
- Pedersen, T.R.; Berendt, M.; Rusbridge, C. Neuroanatomy of Spinal Nociception and Pain in Dogs and Cats: A Practical Review for the Veterinary Clinician. Front. Vet. Sci. 2025, 12, 1534685. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Lascelles, B.D.X.; Murrell, J.; Robertson, S.; Steagall, P.V.M.; Wright, B. 2022 WSAVA Guidelines for the Recognition, Assessment and Treatment of Pain. J. Small Anim. Pract. 2023, 64, 177–254. [Google Scholar] [CrossRef]
- Terminology|International Association for the Study of Pain. Available online: https://www.iasp-pain.org/resources/terminology/#pain (accessed on 2 April 2025).
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Kankowski, S.; Grothe, C.; Haastert-Talini, K. Neuropathic Pain: Spotlighting Anatomy, Experimental Models, Mechanisms, and Therapeutic Aspects. Eur. J. Neurosci. 2021, 54, 4475–4496. [Google Scholar] [CrossRef] [PubMed]
- Hilgart, D.R.; Iversen, M.M.; Peters, A.Y.; Zabriskie, M.S.; Hoareau, G.L.; Vapniarsky, N.; Clark, G.A.; Shah, L.M.; Rieke, V. Non-Invasive Central Nervous System Assessment of a Porcine Model of Neuropathic Pain Demonstrates Increased Latency of Somatosensory-Evoked Potentials. J. Neurosci. Methods 2023, 396, 109934. [Google Scholar] [CrossRef]
- Woolf, C.J.; Mannion, R.J. Neuropathic Pain: Aetiology, Symptoms, Mechanisms, and Management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Ghanbari, A. Beneficial Effects of Exercise in Neuropathic Pain: An Overview of the Mechanisms Involved. Pain Res. Manag. 2025, 2025, 3432659. [Google Scholar] [CrossRef]
- Tonye-Geoffroy, L.; Mauboussin Carlos, S.; Tuffet, S.; Fromentin, H.; Berard, L.; Leblanc, J.; Laroche, F. Efficacy of a Combination of Hypnosis and Transcutaneous Electrical Nerve Stimulation for Chronic Non-Cancer Pain: A Randomized Controlled Trial. J. Adv. Nurs. 2021, 77, 2875–2886. [Google Scholar] [CrossRef]
- Leoni, M.L.G.; Mercieri, M.; Viswanath, O.; Cascella, M.; Rekatsina, M.; Pasqualucci, A.; Caruso, A.; Varrassi, G. Neuropathic Pain: A Comprehensive Bibliometric Analysis of Research Trends, Contributions, and Future Directions. Curr. Pain Headache Rep. 2025, 29, 73. [Google Scholar] [CrossRef]
- Devigili, G.; Di Stefano, G.; Donadio, V.; Frattale, I.; Grazzi, L.; Mantovani, E.; Nolano, M.; Provitera, V.; Quitadamo, S.G.; Tamburin, S.; et al. Therapeutic Approach to Fibromyalgia: A Consensus Statement on Pharmacological and Non-Pharmacological Treatment from the Neuropathic Pain Special Interest Group of the Italian Neurological Society. Neurol. Sci. 2025, 46, 2263–2288. [Google Scholar] [CrossRef]
- Frediani, J.K.; Lal, A.A.; Kim, E.; Leslie, S.L.; Boorman, D.W.; Singh, V. The Role of Diet and Non-Pharmacologic Supplements in the Treatment of Chronic Neuropathic Pain: A Systematic Review. Pain Pract. 2024, 24, 186–210. [Google Scholar] [CrossRef]
- Xu, H.; Yin, T. Effective Acupuncture in Treating Decade-Long Occipital Neuralgia in an Elderly Patient. Am. J. Case Rep. 2024, 25, e945546. [Google Scholar] [CrossRef]
- Petrini, L.; Arendt-Nielsen, L. Understanding Pain Catastrophizing: Putting Pieces Together. Front. Psychol. 2020, 11, 603420. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A. Reason and Emotion in Psychotherapy; Lyle Stuart: New York, NY, USA, 1962; ISBN 0818401222, 9780818401220. Available online: https://it.scribd.com/doc/277829817/Reason-and-Emotion-in-Psychotherapy-Albert-Ellis-Ph-D (accessed on 11 April 2025).
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and Validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Thorn, B.; Haythornthwaite, J.A.; Keefe, F.; Martin, M.; Bradley, L.A.; Lefebvre, J.C. Theoretical Perspectives on the Relation Between Catastrophizing and Pain. Clin. J. Pain 2001, 17, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Kim, M.-E. Catastrophizing as a Key Factor for Understanding Posttraumatic Trigeminal Neuropathy: A Preliminary Study. J. Oral Med. Pain 2023, 48, 45–55. [Google Scholar] [CrossRef]
- Heutink, M.; Post, M.W.M.; Bongers-Janssen, H.M.H.; Dijkstra, C.A.; Snoek, G.J.; Spijkerman, D.C.M.; Lindeman, E. The CONECSI Trial: Results of a Randomized Controlled Trial of a Multidisciplinary Cognitive Behavioral Program for Coping with Chronic Neuropathic Pain after Spinal Cord Injury. Pain 2012, 153, 120–128. [Google Scholar] [CrossRef]
- Miziara, I.D.; Filho, B.C.A.; Oliveira, R.; Rodrigues dos Santos, R.M. Group Psychotherapy: An Additional Approach to Burning Mouth Syndrome. J. Psychosom. Res. 2009, 67, 443–448. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Q.; Zhang, Y.; Ren, C.; Ou, C. Comparison of Two Different Neuromodulation Treatments in Patients With Acute Zoster-Related Trigeminal Neuropathic Pain and Pain Catastrophizing. Neuromodulation 2025, in press. [Google Scholar] [CrossRef]
- Martin, A.M.; Ketchum, J.M.; Agtarap, S.; Hammond, F.M.; Sevigny, M.; Peckham, M.; Dams-O’Connor, K.; Corrigan, J.D.; Walker, W.C.; Hoffman, J.M. Characterizing Extreme Phenotypes for Pain Catastrophizing in Persons With Chronic Pain Following Mild to Severe Traumatic Brain Injury Requiring Inpatient Rehabilitation: A NIDILRR and VA TBI Model Systems Collaborative Project. J. Head Trauma. Rehabil. 2025, 39, 31–42. [Google Scholar] [CrossRef]
- Brodin, E.; Ernberg, M.; Olgart, L. Neurobiology: General Considerations—From Acute to Chronic Pain. Den. Norske Tann. Tid. 2016, 126, 28–33. [Google Scholar] [CrossRef]
- Kaushik, A.S.; Strath, L.J.; Sorge, R.E. Dietary Interventions for Treatment of Chronic Pain: Oxidative Stress and Inflammation. Pain Ther. 2020, 9, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Field, R.; Pourkazemi, F.; Hashempur, M.H.; Thiruvengadam, M.; Rooney, K. Editorial: Diet, Nutrition, and Functional Foods for Chronic Pain. Front. Nutr. 2024, 11, 1456706. [Google Scholar] [CrossRef] [PubMed]
- Elma, Ö.; Brain, K.; Dong, H.J. The Importance of Nutrition as a Lifestyle Factor in Chronic Pain Management: A Narrative Review. J. Clin. Med. 2022, 11, 5950. [Google Scholar] [CrossRef]
- Philpot, U.; Johnson, M.I. Diet Therapy in the Management of Chronic Pain: Better Diet Less Pain? Pain Manag. 2019, 9, 335–338. [Google Scholar] [CrossRef]
- Kaur, T.; Shyu, B.C. Melatonin: A New-Generation Therapy for Reducing Chronic Pain and Improving Sleep Disorder-Related Pain. Adv. Exp. Med. Biol. 2018, 1099, 229–251. [Google Scholar] [CrossRef]
- Curatolo, P.; Moavero, R. Use of Nutraceutical Ingredient Combinations in the Management of Tension-Type Headaches with or without Sleep Disorders. Nutrients 2021, 13, 1631. [Google Scholar] [CrossRef]
- Mishra, G.; Singh, P.; Molla, M.; Shumet Yimer, Y.; Ewunetie, A.; Yimer Tadesse, T.; Mengie Ayele, T.; Kefale, B. Nutraceuticals: A Source of Benefaction for Neuropathic Pain and Fibromyalgia. J. Funct. Foods 2022, 97, 105260. [Google Scholar] [CrossRef]
- Cuomo, A.; Parascandolo, I. Role of Nutrition in the Management of Patients with Chronic Musculoskeletal Pain. J. Pain Res. 2024, 17, 2223–2238. [Google Scholar] [CrossRef]
- Elma, Ö.; Tümkaya Yılmaz, S.; Nijs, J.; Clarys, P.; Coppieters, I.; Mertens, E.; Malfliet, A.; Deliens, T. Impaired Carbohydrate Metabolism among Women with Chronic Low Back Pain and the Role of Dietary Carbohydrates: A Randomized Controlled Cross-Over Experiment. J. Clin. Med. 2024, 13, 2155. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic Hyperglycemia Mediated Physiological Alteration and Metabolic Distortion Leads to Organ Dysfunction, Infection, Cancer Progression and Other Pathophysiological Consequences: An Update on Glucose Toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Glucose and Reactive Oxygen Species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: A Novel Regulator of Pain. J. Neural Transm. 2019, 127, 445–465. [Google Scholar] [CrossRef]
- D’Egidio, F.; Lombardozzi, G.; Kacem Ben Haj M’Barek, H.E.; Mastroiacovo, G.; Alfonsetti, M.; Cimini, A. The Influence of Dietary Supplementations on Neuropathic Pain. Life 2022, 12, 1125. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, J.; Zhang, W.; Doherty, M.; Zhang, Y.; Xie, H.; Li, W.; Wang, N.; Lei, G.; Zeng, C. Gut Dysbiosis in Rheumatic Diseases: A Systematic Review and Meta-Analysis of 92 Observational Studies. EBioMedicine 2022, 80, 104055. [Google Scholar] [CrossRef]
- Shabani, M.; Hasanpour, E.; Mohammadifar, M.; Bahmani, F.; Talaei, S.A.; Aghighi, F. Evaluating the Effects of Probiotic Supplementation on Neuropathic Pain and Oxidative Stress Factors in an Animal Model of Chronic Constriction Injury of the Sciatic Nerve. Basic. Clin. Neurosci. 2023, 14, 375–384. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Jiang, B.C.; Gao, Y.J. Chemokines in Neuron–Glial Cell Interaction and Pathogenesis of Neuropathic Pain. Cell. Mol. Life Sci. 2017, 74, 3275–3291. [Google Scholar] [CrossRef]
- Corriero, A.; Giglio, M.; Inchingolo, F.; Moschetta, A.; Varrassi, G.; Puntillo, F. Gut Microbiota Modulation and Its Implications on Neuropathic Pain: A Comprehensive Literature Review. Pain Ther. 2023, 13, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The Function of Gut Microbiota in Immune-Related Neurological Disorders: A Review. J. Neuroinflammation 2022, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Zhang, C.; Jia, Y.; Zhou, H.; He, C.; Song, H. Investigating the Causal Impact of Gut Microbiota on Trigeminal Neuralgia: A Bidirectional Mendelian Randomization Study. Front. Microbiol. 2025, 16, 1420978. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Pan, H.; Zhang, Y.; Ye, Z.; Zhou, Y.; Zou, H.; Wang, K. Electroacupuncture Alleviates Neuropathic Pain and Negative Emotion in Mice by Regulating Gut Microbiota. J. Pain Res. 2025, 18, 341–352. [Google Scholar] [CrossRef]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-Style Diet for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef]
- Polli, A.; Nijs, J.; Ickmans, K.; Velkeniers, B.; Godderis, L. Linking Lifestyle Factors to Complex Pain States: 3 Reasons Why Understanding Epigenetics May Improve the Delivery of Patient-Centered Care. J. Orthop. Sports Phys. Ther. 2019, 49, 683–687. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Nutritional Factors in Chronic Musculoskeletal Pain: Unravelling the Underlying Mechanisms. Br. J. Anaesth. 2020, 125, e231–e233. [Google Scholar] [CrossRef]
- Nijs, J.; Malfliet, A.; Roose, E.; Lahousse, A.; Van Bogaert, W.; Johansson, E.; Runge, N.; Goossens, Z.; Labie, C.; Bilterys, T.; et al. Personalized Multimodal Lifestyle Intervention as the Best-Evidenced Treatment for Chronic Pain: State-of-the-Art Clinical Perspective. J. Clin. Med. 2024, 13, 644. [Google Scholar] [CrossRef]
- Klowak, M.; Lau, R.; Mohammed, M.N.; Birago, A.; Samson, B.; Ahmed, L.; Renee, C.; Meconnen, M.; Sam, M.; Boggild, A.K. A Systematic Review of Dietary Lifestyle Interventions for Neuropathic Pain. J. Clin. Med. 2024, 13, 6766. [Google Scholar] [CrossRef]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.; Homs, J.; Boadas-Vaello, P. Physiological Changes and Pathological Pain Associated with Sedentary Lifestyle-Induced Body Systems Fat Accumulation and Their Modulation by Physical Exercise. Int. J. Environ. Res. Public Health 2021, 18, 13333. [Google Scholar] [CrossRef] [PubMed]
- Abafita, B.J.; Singh, A.; Aitken, D.; Ding, C.; Moonaz, S.; Palmer, A.J.; Blizzard, L.; Inglis, A.; Drummen, S.J.J.; Jones, G.; et al. Yoga or Strengthening Exercise for Knee Osteoarthritis. JAMA Netw. Open 2025, 8, e253698. [Google Scholar] [CrossRef]
- Rouhi, S.; Egorova-Brumley, N.; Jordan, A.S. Chronic Sleep Deficiency and Its Impact on Pain Perception in Healthy Females. J. Sleep Res. 2024, 34, e14284. [Google Scholar] [CrossRef]
- Haack, M.; Simpson, N.; Sethna, N.; Kaur, S.; Mullington, J. Sleep Deficiency and Chronic Pain: Potential Underlying Mechanisms and Clinical Implications. Neuropsychopharmacology 2020, 45, 205–216. [Google Scholar] [CrossRef]
- Odo, M.; Koh, K.; Takada, T.; Yamashita, A.; Narita, M.; Kuzumaki, N.; Ikegami, D.; Sakai, H.; Iseki, M.; Inada, E.; et al. Changes in Circadian Rhythm for MRNA Expression of Melatonin 1A and 1B Receptors in the Hypothalamus under a Neuropathic Pain-like State. Synapse 2014, 68, 153–158. [Google Scholar] [CrossRef]
- Aigner, C.J.; Cinciripini, P.M.; Anderson, K.O.; Baum, G.P.; Gritz, E.R.; Lam, C.Y. The Association of Pain With Smoking and Quit Attempts in an Electronic Diary Study of Cancer Patients Trying to Quit. Nicotine Tob. Res. 2016, 18, 1449–1455. [Google Scholar] [CrossRef]
- Richardson, E.J.; Ness, T.J.; Redden, D.T.; Stewart, C.C.; Richards, J.S. Effects of Nicotine on Spinal Cord Injury Pain Vary among Subtypes of Pain and Smoking Status: Results from a Randomized, Controlled Experiment. J. Pain 2012, 13, 1206–1214. [Google Scholar] [CrossRef]
- Dragan, S.; Șerban, M.C.; Damian, G.; Buleu, F.; Valcovici, M.; Christodorescu, R. Dietary Patterns and Interventions to Alleviate Chronic Pain. Nutrients 2020, 12, 2510. [Google Scholar] [CrossRef]
- Ilari, S.; Proietti, S.; Milani, F.; Vitiello, L.; Muscoli, C.; Russo, P.; Bonassi, S. Dietary Patterns, Oxidative Stress, and Early Inflammation: A Systematic Review and Meta-Analysis Comparing Mediterranean, Vegan, and Vegetarian Diets. Nutrients 2025, 17, 548. [Google Scholar] [CrossRef]
- Veronese, N.; Ragusa, F.S.; Dominguez, L.J.; Cusumano, C.; Barbagallo, M. Mediterranean Diet and Osteoarthritis: An Update. Aging Clin. Exp. Res. 2024, 36, 231. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome with Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Argento, F.R.; Becatti, M.; Fiorillo, C.; Coccia, M.E.; Fatini, C. Mediterranean Diet and Oxidative Stress: A Relationship with Pain Perception in Endometriosis. Int. J. Mol. Sci. 2023, 24, 14601. [Google Scholar] [CrossRef]
- Ciapała, K.; Mika, J. Advances in Neuropathic Pain Research: Selected Intracellular Factors as Potential Targets for Multidirectional Analgesics. Pharmaceuticals 2023, 16, 1624. [Google Scholar] [CrossRef]
- Brightwell, J.J.; Taylor, B.K. Noradrenergic Neurons in the Locus Coeruleus Contribute to Neuropathic Pain. Neuroscience 2009, 160, 174–185. [Google Scholar] [CrossRef]
- Ni, H.; Yao, M.; Huang, B.; Xu, L.; Zheng, Y.; Chu, Y.; Wang, H.; Liu, M.; Xu, S.; Li, H. Glial Activation in the Periaqueductal Gray Promotes Descending Facilitation of Neuropathic Pain through the P38 MAPK Signaling Pathway. J. Neurosci. Res. 2016, 94, 50–61. [Google Scholar] [CrossRef]
- Samineni, V.K.; Premkumar, L.S.; Faingold, C.L. Neuropathic Pain-Induced Enhancement of Spontaneous and Pain-Evoked Neuronal Activity in the Periaqueductal Gray That Is Attenuated by Gabapentin. Pain 2017, 158, 1241–1253. [Google Scholar] [CrossRef]
- Corder, G.; Ahanonu, B.; Grewe, B.F.; Wang, D.; Schnitzer, M.J.; Scherrer, G. An Amygdalar Neural Ensemble That Encodes the Unpleasantness of Pain. Science 2019, 363, 276–281. [Google Scholar] [CrossRef]
- Huang, J.; Gadotti, V.M.; Chen, L.; Souza, I.A.; Huang, S.; Wang, D.; Ramakrishnan, C.; Deisseroth, K.; Zhang, Z.; Zamponi, G.W. A Neuronal Circuit for Activating Descending Modulation of Neuropathic Pain. Nat. Neurosci. 2019, 22, 1659–1668. [Google Scholar] [CrossRef]
- Busa, P.; Kuthati, Y.; Huang, N.; Wong, C.S. New Advances on Pathophysiology of Diabetes Neuropathy and Pain Management: Potential Role of Melatonin and DPP-4 Inhibitors. Front. Pharmacol. 2022, 13, 864088. [Google Scholar] [CrossRef]
- AK, S.; CF, N.; RC, R.; JG, C.; JM, C. Diabetic Neuropathic Pain: Physiopathology and Treatment. World J. Diabetes 2015, 6, 432. [Google Scholar] [CrossRef]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of Neuropathic Pain: Focus on Diabetic Neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef]
- Compton, S.L.E.; Yang, S.; Madere, J.; Weltzien Ba, E.K.; Caan Drph, B.J.; Meyerhardt, J.A.; Schmitz, K.H.; Brown Phd, J.C.; Brown, J.C. Dietary Quality and Chemotherapy-Induced Peripheral Neuropathy in Colon Cancer. Cancer 2025, 131, e35599. [Google Scholar] [CrossRef]
- Aghili, M.; Taherioun, M.; Jafari, F.; Azadvari, M.; Lashkari, M.; Kolahdouzan, K.; Ghalehtaki, R.; Abdshah, A. Duloxetine to Prevent Neuropathy in Breast Cancer Patients under Paclitaxel Chemotherapy (a Double-Blind Randomized Trial). Support. Care Cancer 2024, 32, 493. [Google Scholar] [CrossRef]
- Bae, E.H.; Greenwald, M.K.; Schwartz, A.G. Chemotherapy-Induced Peripheral Neuropathy: Mechanisms and Therapeutic Avenues. Neurotherapeutics 2021, 18, 2384–2396. [Google Scholar] [CrossRef]
- Galley, H.F.; McCormick, B.; Wilson, K.L.; Lowes, D.A.; Colvin, L.; Torsney, C. Melatonin Limits Paclitaxel-Induced Mitochondrial Dysfunction in Vitro and Protects against Paclitaxel-Induced Neuropathic Pain in the Rat. J. Pineal Res. 2017, 63, e12444. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, Y.Z.; Cui, W.; Li, L.B.; Zhang, Z.C.; Tian, B.P.; Zhang, G.S. Melatonin Attenuates Pain Hypersensitivity and Decreases Astrocyte-Mediated Spinal Neuroinflammation in a Rat Model of Oxaliplatin-Induced Pain. Inflammation 2017, 40, 2052–2061. [Google Scholar] [CrossRef]
- Cohen, S.P.; Wang, E.J.; Doshi, T.L.; Vase, L.; Cawcutt, K.A.; Tontisirin, N. Chronic Pain and Infection: Mechanisms, Causes, Conditions, Treatments, and Controversies. BMJ Med. 2022, 1, e000108. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Ai, Z.; Guo, R.; Cheng, H.; Wang, Y. Exploring Viral Neuropathic Pain: Molecular Mechanisms and Therapeutic Implications. PLoS Pathog. 2024, 20, e1012397. [Google Scholar] [CrossRef]
- Alsaloum, M.; Estacion, M.; Almomani, R.; Gerrits, M.M.; Bönhof, G.J.; Ziegler, D.; Malik, R.; Ferdousi, M.; Lauria, G.; Merkies, I.S.J.; et al. A Gain-of-Function Sodium Channel Β2-Subunit Mutation in Painful Diabetic Neuropathy. Mol. Pain 2019, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boadas-Vaello, P.; Castany, S.; Homs, J.; Álvarez-Pérez, B.; Deulofeu, M.; Verdú, E. Neuroplasticity of Ascending and Descending Pathways after Somatosensory System Injury: Reviewing Knowledge to Identify Neuropathic Pain Therapeutic Targets. Spinal Cord 2016, 54, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of Pain and Its Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef]
- Acioglu, C.; Heary, R.F.; Elkabes, S. Roles of Neuronal Toll-like Receptors in Neuropathic Pain and Central Nervous System Injuries and Diseases. Brain Behav. Immun. 2022, 102, 163–178. [Google Scholar] [CrossRef]
- Vallerand, A.H.; Polomano, R.C. The Relationship of Gender to Pain. Pain Manag. Nurs. 2000, 1, 8–15. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex Differences in Pain: A Brief Review of Clinical and Experimental Findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender Differences in Pain and Its Relief. Ann. Ist. Super. Sanita 2016, 52, 184–189. [Google Scholar] [CrossRef]
- Templeton, K.J. Sex and Gender Issues in Pain Management. J. Bone Jt. Surg. 2020, 102, 32–35. [Google Scholar] [CrossRef]
- Keogh, E. Sex and Gender Differences in Pain: Past, Present, and Future. Pain 2022, 163, S108–S116. [Google Scholar] [CrossRef]
- Goodin, B.R.; McGuire, L.; Allshouse, M.; Stapleton, L.; Haythornthwaite, J.A.; Burns, N.; Mayes, L.A.; Edwards, R.R. Associations Between Catastrophizing and Endogenous Pain-Inhibitory Processes: Sex Differences. J. Pain 2009, 10, 180–190. [Google Scholar] [CrossRef]
- Elklit, A.; Jones, A. The Association Between Anxiety and Chronic Pain After Whiplash Injury. Clin. J. Pain 2006, 22, 487–490. [Google Scholar] [CrossRef]
- Rivest, K.; Côté, J.N.; Dumas, J.-P.; Sterling, M.; De Serres, S.J. Relationships between Pain Thresholds, Catastrophizing and Gender in Acute Whiplash Injury. Man. Ther. 2010, 15, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Elklit, A. The Association between Gender, Coping Style and Whiplash Related Symptoms in Sufferers of Whiplash Associated Disorder. Scand. J. Psychol. 2007, 48, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mayer, E.A.; Fling, C.; Labus, J.S.; Naliboff, B.D.; Hong, J.Y.; Kilpatrick, L.A. Sex-Based Differences in Brain Alterations across Chronic Pain Conditions. J. Neurosci. Res. 2017, 95, 604–616. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in Neuropathic Pain: Cellular and Molecular Mechanisms and Therapeutic Potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Coraggio, V.; Guida, F.; Boccella, S.; Scafuro, M.; Paino, S.; Romano, D.; Maione, S.; Luongo, L. Neuroimmune-Driven Neuropathic Pain Establishment: A Focus on Gender Differences. Int. J. Mol. Sci. 2018, 19, 281. [Google Scholar] [CrossRef]
- Salis, F.; Sardo, S.; Finco, G.; Gessa, G.L.; Franconi, F.; Agabio, R. Sex-Gender Differences Are Completely Neglected in Treatments for Neuropathic Pain. Pharmaceuticals 2024, 17, 838. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Nees, F.; Marziniak, M.; Evers, S.; Flor, H.; Knecht, S. Pain Catastrophizing and Pain-Related Emotions. Clin. J. Pain 2011, 27, 578–586. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Namane, M.; Cicuttini, F. Osteoarthritis. Lancet 2025, 405, 71–85. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular Inflammation: Underpinnings of Aging and Age-Related Diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Klowak, M.; Boggild, A.K. The Efficacy of a Whole Foods, Plant-Based Dietary Lifestyle Intervention for the Treatment of Peripheral Neuropathic Pain in Leprosy: A Randomized Control Trial Protocol. Front. Nutr. 2023, 10, 1196470. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, H.; Radfar, M.; Soheily, S.; Shamsi, S.A.; Khalkhali, H.R. Effect of Lifestyle Interventions on Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes, Result of a Randomized Clinical Trial. Algology 2018, 30, 165–170. [Google Scholar] [CrossRef]
- Voscopoulos, C.; Lema, M. When Does Acute Pain Become Chronic? Br. J. Anaesth. 2010, 105 (Suppl. 1), i69–i85. [Google Scholar] [CrossRef]
- Fotio, Y.; Jung, K.M.; Palese, F.; Obenaus, A.; Tagne, A.M.; Lin, L.; Rashid, T.I.; Pacheco, R.; Jullienne, A.; Ramirez, J.; et al. NAAA-Regulated Lipid Signaling Governs the Transition from Acute to Chronic Pain. Sci. Adv. 2021, 7, eabi8834. [Google Scholar] [CrossRef]
- Bai, Q.; Liu, S.; Shu, H.; Tang, Y.; George, S.; Dong, T.; Schmidt, B.L.; Tao, F. TNFα in the Trigeminal Nociceptive System Is Critical for Temporomandibular Joint Pain. Mol. Neurobiol. 2019, 56, 278–291. [Google Scholar] [CrossRef]
- Zeng, J.; Li, S.; Zhang, C.; Huang, G.; Yu, C. The Mechanism of Hyperalgesia and Anxiety Induced by Remifentanil: Phosphorylation of GluR1 Receptors in the Anterior Cingulate Cortex. J. Mol. Neurosci. 2018, 65, 93–101. [Google Scholar] [CrossRef]
- Price, T.J.; Ray, P.R. Recent Advances toward Understanding the Mysteries of the Acute to Chronic Pain Transition. Curr. Opin. Physiol. 2019, 11, 42. [Google Scholar] [CrossRef]
- Torebjörk, H.E.; Lundberg, L.E.; LaMotte, R.H. Central Changes in Processing of Mechanoreceptive Input in Capsaicin-Induced Secondary Hyperalgesia in Humans. J. Physiol. 1992, 448, 765–780. [Google Scholar] [CrossRef]
- Jaffal, S.M. Neuroplasticity in Chronic Pain: Insights into Diagnosis and Treatment. Korean J. Pain 2025, 38, 89–102. [Google Scholar] [CrossRef]
- Wani, P.; Anand, R. Neuroplasticity and Pain Perception: Exploring the Complexities of Temporomandibular Disorders. Cureus 2025, 17, e79098. [Google Scholar] [CrossRef] [PubMed]
- Torrance, N.; Smith, B.H.; Bennett, M.I.; Lee, A.J. The Epidemiology of Chronic Pain of Predominantly Neuropathic Origin. Results from a General Population Survey. J. Pain 2006, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Toth, C.; Brady, S.; Hatfield, M. The Importance of Catastrophizing for Successful Pharmacological Treatment of Peripheral Neuropathic Pain. J. Pain Res. 2014, 7, 327–338. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Vacca, V.; Pavone, F.; Marinelli, S. Impact of Caloric Restriction on Peripheral Nerve Injury-Induced Neuropathic Pain during Ageing in Mice. Eur. J. Pain 2020, 24, 374–382. [Google Scholar] [CrossRef]
- Casale, R.; Symeonidou, Z.; Ferfeli, S.; Micheli, F.; Scarsella, P.; Paladini, A. Food for Special Medical Purposes and Nutraceuticals for Pain: A Narrative Review. Pain Ther. 2021, 10, 225–242. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, Y.; Zhang, W.; Sun, Y.E.; Jiang, M.; Gu, W.J.; Ma, Z.L.; Gu, X.P. Anti-Nociceptive Effects of Caloric Restriction on Neuropathic Pain in Rats Involves Silent Information Regulator 1. Br. J. Anaesth. 2018, 120, 807–817. [Google Scholar] [CrossRef]
- Lee, S.; Notterpek, L. Dietary Restriction Supports Peripheral Nerve Health by Enhancing Endogenous Protein Quality Control Mechanisms. Exp. Gerontol. 2012, 48, 1085. [Google Scholar] [CrossRef]
- Areti, A.; Komirishetty, P.; Akuthota, M.; Malik, R.A.; Kumar, A. Melatonin Prevents Mitochondrial Dysfunction and Promotes Neuroprotection by Inducing Autophagy during Oxaliplatin-Evoked Peripheral Neuropathy. J. Pineal Res. 2017, 62, e12393. [Google Scholar] [CrossRef]
- Petrikonis, K.; Bernatoniene, J.; Kopustinskiene, D.M.; Casale, R.; Davinelli, S.; Saso, L. The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives. Pharmaceutics 2024, 16, 1068. [Google Scholar] [CrossRef]
- Gold, M.S.; Gebhart, G.F. Peripheral Pain Mechanisms and Nociceptor Sensitization. In Bonica’s Management of Pain; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Ghazisaeidi, S.; Muley, M.M.; Salter, M.W. Neuropathic Pain: Mechanisms, Sex Differences, and Potential Therapies for a Global Problem. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 565–583. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.S.; Lorenzo, L.E.; Lee, K.Y.; Gauthier, C.; Muley, M.M.; De Koninck, Y.; Prescott, S.A.; Salter, M.W. Chloride Dysregulation through Downregulation of KCC2 Mediates Neuropathic Pain in Both Sexes. Cell Rep. 2019, 28, 590–596.e4. [Google Scholar] [CrossRef] [PubMed]
- Westlund, K.N.; Montera, M.A.; Goins, A.E.; Alles, S.R.A.; Suri, N.; McIlwrath, S.L.; Bartel, R.; Durvasula, R.V.; Kunamneni, A. Single-Dose P2 X4R Single-Chain Fragment Variable Antibody Permanently Reverses Chronic Pain in Male Mice. Int. J. Mol. Sci. 2021, 22, 13612. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.R.; Campana, W.M.; Shubayev, V.I. The Role of Neuroinflammation in Neuropathic Pain: Mechanisms and Therapeutic Targets. Drug Discov. Today 2006, 11, 8–20. [Google Scholar] [CrossRef]
- Omoigui, S. The Biochemical Origin of Pain: The Origin of All Pain Is Inflammation and the Inflammatory Response. Part 2 of 3—Inflammatory Profile of Pain Syndromes. Med. Hypotheses 2007, 69, 1169–1178. [Google Scholar] [CrossRef]
- Kidd, B.L.; Urban, L.A. Mechanisms of Inflammatory Pain. Br. J. Anaesth. 2001, 87, 3–11. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. Pathophysiology of Neuropathic Pain: Inflammatory Mediators. In Neuropathic Pain; Cambridge University Press: Cambridge, UK, 2013; pp. 77–89. [Google Scholar]
- Staehelin Jensen, T. Pathophysiology of Pain: From Theory to Clinical Evidence. Eur. J. Pain. Suppl. 2008, 2, 13–17. [Google Scholar] [CrossRef]
- Sommer, C.; Leinders, M.; Üçeyler, N. Inflammation in the Pathophysiology of Neuropathic Pain. Pain 2018, 159, 595–602. [Google Scholar] [CrossRef]
- Muir, W.W. Physiology and Pathophysiology of Pain. Am. Assoc. Bov. Pract. Conf. Proc. 2003, 36, 33–35. [Google Scholar] [CrossRef]
- Schaible, H.G.; Richter, F. Pathophysiology of Pain. Langenbecks Arch. Surg. 2004, 389, 237–243. [Google Scholar] [CrossRef]
- Fong, A.; Schug, S.A. Pathophysiology of Pain: A Practical Primer. Plast. Reconstr. Surg. 2014, 134, 8S–14S. [Google Scholar] [CrossRef]
- Smith, P.A. The Biology of Neuropathic Pain. Fiziolohichnyi Zhurnal 2023, 69, 54–67. [Google Scholar] [CrossRef]
- Zelenka, M.; Schäfers, M.; Sommer, C. Intraneural Injection of Interleukin-1beta and Tumor Necrosis Factor-Alpha into Rat Sciatic Nerve at Physiological Doses Induces Signs of Neuropathic Pain. Pain 2005, 116, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, W.; Magnus, T.; Martin, B.; Keselman, A.; Mattson, M.P.; Maudsley, S. Targeting TNF-α Receptors for Neurotherapeutics. Trends Neurosci. 2008, 31, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF–TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Akassoglou, K.; Douni, E.; Bauer, J.; Lassmann, H.; Kollias, G.; Probert, L. Exclusive Tumor Necrosis Factor (TNF) Signaling by the P75TNF Receptor Triggers Inflammatory Ischemia in the CNS of Transgenic Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 709–714. [Google Scholar] [CrossRef]
- Chen, G.; Goeddel, D.V. TNF-R1 Signaling: A Beautiful Pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef]
- Tang, F.; Tang, G.; Xiang, J.; Dai, Q.; Rosner, M.R.; Lin, A. The Absence of NF-ΚB-Mediated Inhibition of c-Jun N-Terminal Kinase Activation Contributes to Tumor Necrosis Factor Alpha-Induced Apoptosis. Mol. Cell Biol. 2002, 22, 8571–8579. [Google Scholar] [CrossRef]
- Varfolomeev, E.E.; Ashkenazi, A. Tumor Necrosis Factor: An Apoptosis JuNKie? Cell 2004, 116, 491–497. [Google Scholar] [CrossRef]
- Borghi, A.; Verstrepen, L.; Beyaert, R. TRAF2 Multitasking in TNF Receptor-Induced Signaling to NF-ΚB, MAP Kinases and Cell Death. Biochem. Pharmacol. 2016, 116, 1–10. [Google Scholar] [CrossRef]
- Del Rivero, T.; Fischer, R.; Yang, F.; Swanson, K.A.; Bethea, J.R. Tumor Necrosis Factor Receptor 1 Inhibition Is Therapeutic for Neuropathic Pain in Males but Not in Females. Pain 2019, 160, 922–931. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.-R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors Are Interleukin-1β Sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.C.; Stemkowski, P.L.; Smith, P.A. Long-Term Actions of Interleukin-1β on K+, Na+ and Ca2+ Channel Currents in Small, IB4-Positive Dorsal Root Ganglion Neurons; Possible Relevance to the Etiology of Neuropathic Pain. J. Neuroimmunol. 2019, 332, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Stemkowski, P.L.; Noh, M.C.; Chen, Y.; Smith, P.A. Increased Excitability of Medium-Sized Dorsal Root Ganglion Neurons by Prolonged Interleukin-1β Exposure Is K+ Channel Dependent and Reversible. J. Physiol. 2015, 593, 3739–3755. [Google Scholar] [CrossRef]
- Vasudeva, K.; Vodovotz, Y.; Azhar, N.; Barclay, D.; Janjic, J.M.; Pollock, J.A. In Vivo and Systems Biology Studies Implicate IL-18 as a Central Mediator in Chronic Pain. J. Neuroimmunol. 2015, 283, 43–49. [Google Scholar] [CrossRef]
- Sommer, C.; Schmidt, C.; George, A.; Toyka, K.V. A Metalloprotease-Inhibitor Reduces Pain Associated Behavior in Mice with Experimental Neuropathy. Neurosci. Lett. 1997, 237, 45–48. [Google Scholar] [CrossRef]
- Miyoshi, K.; Obata, K.; Kondo, T.; Okamura, H.; Noguchi, K. Interleukin-18-Mediated Microglia/Astrocyte Interaction in the Spinal Cord Enhances Neuropathic Pain Processing after Nerve Injury. J. Neurosci. 2008, 28, 12775–12787. [Google Scholar] [CrossRef]
- Sun, J.H.; Yang, B.; Donnelly, D.F.; Ma, C.; LaMotte, R.H. MCP-1 Enhances Excitability of Nociceptive Neurons in Chronically Compressed Dorsal Root Ganglia. J. Neurophysiol. 2006, 96, 2189–2199. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Zychowska, M.; Mika, J. Pharmacological Blockade of CXCR3 by (±)-NBI-74330 Reduces Neuropathic Pain and Enhances Opioid Effectiveness—Evidence from in Vivo and in Vitro Studies. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3418–3437. [Google Scholar] [CrossRef]
- Sandy-Hindmarch, O.; Bennett, D.L.; Wiberg, A.; Furniss, D.; Baskozos, G.; Schmid, A.B. Systemic Inflammatory Markers in Neuropathic Pain, Nerve Injury, and Recovery. Pain 2022, 163, 526–537. [Google Scholar] [CrossRef]
- Honjoh, K.; Nakajima, H.; Hirai, T.; Watanabe, S.; Matsumine, A. Relationship of Inflammatory Cytokines From M1-Type Microglia/Macrophages at the Injured Site and Lumbar Enlargement With Neuropathic Pain After Spinal Cord Injury in the CCL21 Knockout (Plt) Mouse. Front. Cell Neurosci. 2019, 13, 525. [Google Scholar] [CrossRef]
- Boakye, P.A.; Tang, S.-J.; Smith, P.A. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β. Front. Pain Res. 2021, 2, 698157. [Google Scholar] [CrossRef] [PubMed]
- Mesquida-Veny, F.; Martínez-Torres, S.; Del Rio, J.A.; Hervera, A. Nociception-Dependent CCL21 Induces Dorsal Root Ganglia Axonal Growth via CCR7-ERK Activation. Front. Immunol. 2022, 13, 880647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Cao, D.L.; Zhang, X.; Ji, R.R.; Gao, Y.J. Chemokine Contribution to Neuropathic Pain: Respective Induction of CXCL1 and CXCR2 in Spinal Cord Astrocytes and Neurons. Pain 2013, 154, 2185–2197. [Google Scholar] [CrossRef]
- da Silva Junior, C.A.; de Castro Junior, C.J.; Pereira, E.M.R.; Binda, N.S.; da Silva, J.F.; do Nascimento Cordeiro, M.; Diniz, D.M.; Cecilia, F.S.; Ferreira, J.; Gomez, M.V. The Inhibitory Effect of Phα1β Toxin on Diabetic Neuropathic Pain Involves the CXCR4 Chemokine Receptor. Pharmacol. Rep. 2020, 72, 47–54. [Google Scholar] [CrossRef]
- Silva, R.L.; Lopes, A.H.; Guimarães, R.M.; Cunha, T.M. CXCL1/CXCR2 Signaling in Pathological Pain: Role in Peripheral and Central Sensitization. Neurobiol. Dis. 2017, 105, 109–116. [Google Scholar] [CrossRef]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and Modulators of Pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Fornasari, D. Pain Mechanisms in Patients with Chronic Pain. Clin. Drug Investig. 2012, 32 (Suppl. 1), 45–52. [Google Scholar] [CrossRef]
- Singh, S.P.; Guindon, J.; Mody, P.H.; Ashworth, G.; Kopel, J.; Chilakapati, S.; Adogwa, O.; Neugebauer, V.; Burton, M.D. Pain and Aging: A Unique Challenge in Neuroinflammation and Behavior. Mol. Pain. 2023, 19, 1–18. [Google Scholar] [CrossRef]
- Haidar, O.; O’Neill, N.; Staunton, C.A.; Bavan, S.; O’Brien, F.; Zouggari, S.; Sharif, U.; Mobasheri, A.; Kumagai, K.; Barrett-Jolley, R. Pro-Inflammatory Cytokines Drive Deregulation of Potassium Channel Expression in Primary Synovial Fibroblasts. Front. Physiol. 2020, 11, 226. [Google Scholar] [CrossRef]
- Yu, B.P. Aging and Oxidative Stress: Modulation by Dietary Restriction. Free Radic. Biol. Med. 1996, 21, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Vejapi, M.; Knezevic, N.N. The Role of Nitric Oxide and Neuroendocrine System in Pain Generation. Mol. Cell Endocrinol. 2024, 591, 112270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Mei, W.; Tian, X.B.; Tian, Y.K.; Liu, D.Q.; Ye, D.W. The Therapeutic Potential of Nrf2 Inducers in Chronic Pain: Evidence from Preclinical Studies. Pharmacol. Ther. 2021, 225, 107846. [Google Scholar] [CrossRef] [PubMed]
- Silva Santos Ribeiro, P.; Willemen, H.L.D.M.; Eijkelkamp, N. Mitochondria and Sensory Processing in Inflammatory and Neuropathic Pain. Front. Pain Res. 2022, 3, 1013577. [Google Scholar] [CrossRef]
- Bittar, A.; Jun, J.; La, J.-H.; Wang, J.; Leem, J.W.; Chung, J.M. Reactive Oxygen Species Affect Spinal Cell Type-Specific Synaptic Plasticity in a Model of Neuropathic Pain. Pain 2017, 158, 2137–2146. [Google Scholar] [CrossRef]
- Yowtak, J.; Lee, K.Y.; Kim, H.Y.; Wang, J.; Kim, H.K.; Chung, K.; Chung, J.M. Reactive Oxygen Species Contribute to Neuropathic Pain by Reducing Spinal GABA Release. Pain 2011, 152, 844–852. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Qu, X.; Sun, G.; Fu, Q.; Han, G. Stress/Cell Death Pathways, Neuroinflammation, and Neuropathic Pain. Immunol. Rev. 2024, 321, 33–51. [Google Scholar] [CrossRef]
- Ye, D.; Fairchild, T.J.; Vo, L.; Drummond, P.D. Painful Diabetic Peripheral Neuropathy: Role of Oxidative Stress and Central Sensitisation. Diabet. Med. 2022, 39, e14729. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Sima, A.; Stevens, M. Diabetic Neuropathy and Oxidative Stress. Diabetes Metab. Res. Rev. 2006, 22, 257–273. [Google Scholar] [CrossRef]
- Uchida, K.; Dezaki, K.; Yoneshiro, T.; Watanabe, T.; Yamazaki, J.; Saito, M.; Yada, T.; Tominaga, M.; Iwasaki, Y. Involvement of Thermosensitive TRP Channels in Energy Metabolism. J. Physiol. Sci. 2017, 67, 549–560. [Google Scholar] [CrossRef]
- Simon, F.; Leiva-Salcedo, E.; Armisén, R.; Riveros, A.; Cerda, O.; Varela, D.; Eguiguren, A.L.; Olivero, P.; Stutzin, A. Hydrogen Peroxide Removes TRPM4 Current Desensitization Conferring Increased Vulnerability to Necrotic Cell Death. J. Biol. Chem. 2010, 285, 37150–37158. [Google Scholar] [CrossRef] [PubMed]
- Nesic, O.; Lee, J.; Unabia, G.C.; Johnson, K.; Ye, Z.; Vergara, L.; Hulsebosch, C.E.; Perez-Polo, J.R. Aquaporin 1—A Novel Player in Spinal Cord Injury. J. Neurochem. 2008, 105, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Kiani, A.; Jalili, C.; Abbaszadeh, F.; Piri, S.; Farzaei, M.H.; Rastegari-Pouyani, M.; Mohammadi-Noori, E.; Khan, H. Intrathecal Administration of Melatonin Ameliorates the Neuroinflammation- Mediated Sensory and Motor Dysfunction in A Rat Model of Compression Spinal Cord Injury. Curr. Mol. Pharmacol. 2021, 14, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Averitt, D.L.; Maier, C.; Basu, A. The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) Activation in Preclinical Models of Peripheral Neuropathic Pain. Antioxidants 2022, 11, 430. [Google Scholar] [CrossRef]
- Luan, Y.; Luo, Y.; Deng, M. New Advances in Nrf2-Mediated Analgesic Drugs. Phytomedicine 2023, 110, 154598. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The Emerging Role of Nrf2 in Mitochondrial Function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Fan, Z.; Wirth, A.-K.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 Pathway Promotes Cell Proliferation and Diminishes Ferroptosis. Oncogenesis 2017, 6, e371. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase to Regulate Proteasomal Degradation of Nrf2. Mol. Cell Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB Protein Is an Adaptor That Bridges Nrf2 to a Cul3-Based E3 Ligase: Oxidative Stress Sensing by a Cul3-Keap1 Ligase. Mol. Cell Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Salvemini, D. Mini-Review: Mitochondrial Dysfunction and Chemotherapy-Induced Neuropathic Pain. Neurosci. Lett. 2021, 760, 136087. [Google Scholar] [CrossRef]
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells 2022, 11, 706. [Google Scholar] [CrossRef]
- Shilovsky, G.A.; Ashapkin, V.V. Transcription Factor Nrf2 and Mitochondria—Friends or Foes in the Regulation of Aging Rate. Biochemistry 2022, 87, 1477–1486. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Wang, J.Q.; Wu, Y.H. MG53 Ameliorates Nerve Injury Induced Neuropathic Pain through the Regulation of Nrf2/HO-1 Signaling in Rats. Behav. Brain Res. 2023, 449, 114489. [Google Scholar] [CrossRef]
- Elsayed, H.R.H.; Ali, E.M.T.; Rabei, M.R.; El Nashar, E.M.; Alghamdi, M.A.; Al-Zahrani, N.S.; Alshehri, S.H.; Aldahhan, R.A.; Morsy, A.I. Angiotensin II Type 1 Receptor Blockade Attenuates the Neuropathological Changes in the Spinal Cords of Diabetic Rats with Modulation of Nuclear Factor Erythroid 2-Related Factor 2/Heme Oxygenase 1 System. Tissue Cell 2024, 88, 102420. [Google Scholar] [CrossRef]
- Miao, H.; Xu, J.; Xu, D.; Ma, X.; Zhao, X.; Liu, L. Nociceptive Behavior Induced by Chemotherapeutic Paclitaxel and Beneficial Role of Antioxidative Pathways. Physiol. Res. 2019, 68, 491–500. [Google Scholar] [CrossRef]
- Chen, H.; Xie, K.; Chen, Y.; Wang, Y.; Wang, Y.; Lian, N.; Zhang, K.; Yu, Y. Nrf2/HO-1 Signaling Pathway Participated in the Protection of Hydrogen Sulfide on Neuropathic Pain in Rats. Int. Immunopharmacol. 2019, 75, 105746. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Nijs, J.; Elma, Ö.; Yilmaz, S.T.; Mullie, P.; Vanderweeën, L.; Clarys, P.; Deliens, T.; Coppieters, I.; Weltens, N.; Van Oudenhove, L.; et al. Nutritional Neurobiology and Central Nervous System Sensitisation: Missing Link in a Comprehensive Treatment for Chronic Pain? Br. J. Anaesth. 2019, 123, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Zhao, L.; Hwang, D.H. Modulation of Pattern Recognition Receptor-Mediated Inflammation and Risk of Chronic Diseases by Dietary Fatty Acids. Nutr. Rev. 2010, 68, 38–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Myung, A.K.; Cho, I.H.; Mi, S.K.; Lee, S.; Jo, E.K.; Choi, S.Y.; Park, K.; Jong, S.K.; Akira, S.; et al. A Critical Role of Toll-like Receptor 2 in Nerve Injury-Induced Spinal Cord Glial Cell Activation and Pain Hypersensitivity. J. Biol. Chem. 2007, 282, 14975–14983. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.X.; Liu, H.Y.; Yue, N.; Du, Y.R.; Che, L.M.; Yu, J.S. Research Progress on the Mechanism of Chronic Neuropathic Pain. IBRO Neurosci. Rep. 2023, 14, 80–85. [Google Scholar] [CrossRef]
- Sesti, F.; Capozzolo, T.; Pietropolli, A.; Collalti, M.; Bollea, M.R.; Piccione, E. Dietary Therapy: A New Strategy for Management of Chronic Pelvic Pain. Nutr. Res. Rev. 2011, 24, 31–38. [Google Scholar] [CrossRef]
- Kahya, M.C.; Nazıroğlu, M.; Övey, İ.S. Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium. Mol. Neurobiol. 2017, 54, 2345–2360. [Google Scholar] [CrossRef]
- Oveissi, V.; Ram, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Naseri, R.; Belwal, T.; Devkota, H.P.; Abbasabadi, Z.; Farzaei, M.H. Medicinal Plants and Their Isolated Phytochemicals for the Management of Chemotherapy-Induced Neuropathy: Therapeutic Targets and Clinical Perspective. DARU J. Pharm. Sci. 2019, 27, 389–406. [Google Scholar] [CrossRef]
- Sic, A.; Manzar, A.; Knezevic, N.N. The Role of Phytochemicals in Managing Neuropathic Pain: How Much Progress Have We Made? Nutrients 2024, 16, 4342. [Google Scholar] [CrossRef]
- Akbar, S.; Subhan, F.; Karim, N.; Shahid, M.; Ahmad, N.; Ali, G.; Mahmood, W.; Fawad, K. 6-Methoxyflavanone Attenuates Mechanical Allodynia and Vulvodynia in the Streptozotocin-Induced Diabetic Neuropathic Pain. Biomed. Pharmacother. 2016, 84, 962–971. [Google Scholar] [CrossRef]
- Takeda, M.; Sashide, Y.; Toyota, R.; Ito, H. The Phytochemical, Quercetin, Attenuates Nociceptive and Pathological Pain: Neurophysiological Mechanisms and Therapeutic Potential. Molecules 2024, 29, 3957. [Google Scholar] [CrossRef]
- Zurowski, D.; Nowak, L.; Machowska, A.; Wordliczek, J.; Thor, P.J. Exogenous Melatonin Abolishes Mechanical Allodynia but Not Thermal Hyperalgesia in Neuropathic Pain. The Role of the Opioid System and Benzodiazepine-Gabaergic Mechanism. J. Physiol. Pharmacol. 2012, 63, 641–647. [Google Scholar] [PubMed]
- Allison, D.J.; Thomas, A.; Beaudry, K.; Ditor, D.S. Targeting Inflammation as a Treatment Modality for Neuropathic Pain in Spinal Cord Injury: A Randomized Clinical Trial. J. Neuroinflammation 2016, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.; Hal Scofield, R.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Dydyk, A.M.; Conermann, T. Chronic Pain; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024; Bookshelf ID: NBK553030. [Google Scholar] [PubMed]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Do Nutritional Factors Interact with Chronic Musculoskeletal Pain? A Systematic Review. J. Clin. Med. 2020, 9, 702. [Google Scholar] [CrossRef]
- Tick, H. Nutrition and Pain. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 309–320. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Miccono, A.; Naso, M.; Nichetti, M.; Riva, A.; Guerriero, F.; De Gregori, M.; Peroni, G.; Perna, S. Food Pyramid for Subjects with Chronic Pain: Foods and Dietary Constituents as Anti-Inflammatory and Antioxidant Agents. Nutr. Res. Rev. 2018, 31, 131–151. [Google Scholar] [CrossRef]
- Brain, K.; Burrows, T.L.; Rollo, M.E.; Chai, L.K.; Clarke, E.D.; Hayes, C.; Hodson, F.J.; Collins, C.E. A Systematic Review and Meta-Analysis of Nutrition Interventions for Chronic Noncancer Pain. J. Hum. Nutr. Diet. 2019, 32, 198–225. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, X.; Liu, H.; Shao, X.; Chu, S.; Gong, X.; Ma, Z.; Gu, X. Misaligned Feeding May Aggravate Pain by Disruption of Sleep-Awake Rhythm. Anesth. Analg. 2018, 127, 255–260. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Badaeva, A.V.; Danilov, A.B.; Clayton, P.; Moskalev, A.A.; Karasev, A.V.; Tarasevich, A.F.; Vorobyeva, Y.D.; Novikov, V.N. Perspectives on Neuronutrition in Prevention and Treatment of Neurological Disorders. Nutrients 2023, 15, 2505. [Google Scholar] [CrossRef]
- Shen, C.L.; Castro, L.; Fang, C.Y.; Castro, M.; Sherali, S.; White, S.; Wang, R.; Neugebauer, V. Bioactive Compounds for Neuropathic Pain: An Update on Preclinical Studies and Future Perspectives. J. Nutr. Biochem. 2022, 104, 108979. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, Function and Physiological Role of Melatonin in the Lower Gut. World J. Gastroenterol. 2011, 17, 3888–3898. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, E.; Calvo, J.R.; Maldonado-Aibar, M.D.; del Millan-Linares, M.C.; Montserrat-de la Paz, S. Mediterranean Diet and Melatonin: A Systematic Review. Antioxidants 2023, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin- A Pleiotropic, Orchestrating Regulator Molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the Skin: Synthesis, Metabolism and Functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Sciupokas, A.; Kopustinskiene, D.M.; Petrikonis, K. Novel Drug Targets and Emerging Pharmacotherapies in Neuropathic Pain. Pharmaceutics 2023, 15, 1799. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; de Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
- Garofoli, F.; Franco, V.; Accorsi, P.; Albertini, R.; Angelini, M.; Asteggiano, C.; Aversa, S.; Ballante, E.; Borgatti, R.; Cabini, R.F.; et al. Fate of Melatonin Orally Administered in Preterm Newborns: Antioxidant Performance and Basis for Neuroprotection. J. Pineal Res. 2024, 76, e12932. [Google Scholar] [CrossRef]
- Franco, C.; Sciatti, E.; Favero, G.; Bonomini, F.; Vizzardi, E.; Rezzani, R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. Int. J. Mol. Sci. 2022, 23, 14489. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Spence, D.W.; Moscovitch, A.; Trakht, I.; Brown, G.M.; Cardinali, D.P. Potential Use of Melatonergic Drugs in Analgesia: Mechanisms of Action. Brain Res. Bull. 2010, 81, 362–371. [Google Scholar] [CrossRef]
- Chang, A.M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening Use of Light-Emitting EReaders Negatively Affects Sleep, Circadian Timing, and next-Morning Alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef]
- Maldonado, M.; Romero-Aibar, J.; Calvo, J. The Melatonin Contained in Beer Can Provide Health Benefits, Due to Its Antioxidant, Anti-Inflammatory and Immunomodulatory Properties. J. Sci. Food Agric. 2023, 103, 3738–3747. [Google Scholar] [CrossRef] [PubMed]
- Sieminski, M.; Reimus, M.; Kałas, M.; Stępniewska, E. Antioxidant and Anti-Inflammatory Properties of Melatonin in Secondary Traumatic Brain Injury. Antioxidants 2024, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Harofteh, F.Z.; Sahebnasagh, A.; Ghaneei, N.; Ardakani, M.E.Z.; Saghafi, F. Efficacy and Safety of Topical Rosuvastatin & Melatonin vs. Placebo in Patients with Mild to Moderate Plaque Psoriasis: A Preliminary Randomized Double-Blinded Clinical Trial. Skin. Res. Technol. 2024, 30, e13689. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Fenger, A.Q.; Petersen, M.C.; Rosenberg, J.; Werner, M.U. Analgesic and Antihyperalgesic Effects of Melatonin in a Human Inflammatory Pain Model: A Randomized, Double-Blind, Placebo-Controlled, Three-Arm Crossover Study. Pain 2015, 156, 2286–2294. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, Q.; Chen, J.; Wang, Y.; Liu, X.; Zhang, X.; Shi, Y.; Zhan, H.; Zhong, X.; Yao, M.; et al. Melatonin Improves Mitochondrial Dysfunction and Attenuates Neuropathic Pain by Regulating SIRT1 in Dorsal Root Ganglions. Neuroscience 2023, 534, 29–40. [Google Scholar] [CrossRef]
- Mokhtari, T.; Yue, L.P.; Hu, L. Exogenous Melatonin Alleviates Neuropathic Pain-Induced Affective Disorders by Suppressing NF-ΚB/NLRP3 Pathway and Apoptosis. Sci. Rep. 2023, 13, 2111. [Google Scholar] [CrossRef]
- Jallouli, S.; Ghroubi, S.; Damak, M.; Sakka, S.; Elleuch, M.H.; Mhiri, C.; Yahia, A.; Driss, T.; de Marco, G.; Hammouda, O. 12-Week Melatonin Supplementation Improved Dynamic Postural Stability and Walking Performance in Persons Living with Multiple Sclerosis: A Randomized Controlled Trial. Behav. Brain Res. 2025, 476, 115191. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tang, Y.R.; Gao, X.; Liu, J.; Zhang, N.N.; Liang, Z.J.; Li, Y.; Pan, L.X. The Anti-Inflammatory and Analgesic Effects of Intraperitoneal Melatonin after Spinal Nerve Ligation Are Mediated by Inhibition of the NF-ΚB/NLRP3 Inflammasome Signaling Pathway. Brain Res. Bull. 2021, 169, 156–166. [Google Scholar] [CrossRef]
- De Gregorio, D.; Comai, S. Acute and Chronic Pain Preclinical Models to Study the Analgesic Properties of Melatonergic Compounds. Methods Mol. Biol. 2022, 2550, 453–461. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological Effects in Humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin Treatment Improves Blood Pressure, Lipid Profile, and Parameters of Oxidative Stress in Patients with Metabolic Syndrome. J. Pineal Res. 2011, 50, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Franco, M.; Planells, E.; Quintero, B.; Acuña-Castroviejo, D.; Rusanova, I.; Escames, G.; Molina-López, J. Effect of Melatonin Supplementation on Antioxidant Status and DNA Damage in High Intensity Trained Athletes. Int. J. Sports Med. 2017, 38, 1117–1125. [Google Scholar] [CrossRef]
- Abadi, S.H.M.H.; Shirazi, A.; Alizadeh, A.M.; Changizi, V.; Najafi, M.; Khalighfard, S.; Nosrati, H. The Effect of Melatonin on Superoxide Dismutase and Glutathione Peroxidase Activity, and Malondialdehyde Levels in the Targeted and the Non-Targeted Lung and Heart Tissues after Irradiation in Xenograft Mice Colon Cancer. Curr. Mol. Pharmacol. 2018, 11, 326–335. [Google Scholar] [CrossRef]
- Mistraletti, G.; Paroni, R.; Umbrello, M.; D’Amato, L.; Sabbatini, G.; Taverna, M.; Formenti, P.; Finati, E.; Favero, G.; Bonomini, F.; et al. Melatonin Pharmacological Blood Levels Increase Total Antioxidant Capacity in Critically Ill Patients. Int. J. Mol. Sci. 2017, 18, 759. [Google Scholar] [CrossRef]
- Kuthati, Y.; Lin, S.H.; Chen, I.J.; Wong, C.S. Melatonin and Their Analogs as a Potential Use in the Management of Neuropathic Pain. J. Formos. Med. Assoc. 2019, 118, 1177–1186. [Google Scholar] [CrossRef]
- Mehramiri, A.; Shalilahmadi, D.; Mohamadianinejad, S.E.; Kouti, L.; Hosseinpour, Y. The Effect of Melatonin on Reducing the Frequency and Severity of Migraine Attacks: A Double-Blind, Randomized Clinical Trial. Iran. J. Med. Sci. 2024, 49, 313–321. [Google Scholar] [CrossRef]
- Thomson, D.M.; Mitchell, E.J.; Openshaw, R.L.; Pratt, J.A.; Morris, B.J. Mice Lacking Melatonin MT2 Receptors Exhibit Attentional Deficits, Anxiety and Enhanced Social Interaction. J. Psychopharmacol. 2021, 35, 1265–1276. [Google Scholar] [CrossRef]
- Repova, K.; Baka, T.; Krajcirovicova, K.; Stanko, P.; Aziriova, S.; Reiter, R.J.; Simko, F. Melatonin as a Potential Approach to Anxiety Treatment. Int. J. Mol. Sci. 2022, 23, 16187. [Google Scholar] [CrossRef]
- Alavez-Pérez, N.; Patiño-Camacho, I.S.; Granados-Soto, V.; Déciga-Campos, M. Melatonin Synergizes with the Antinociceptive Effect of N-Palmitoylethanolamide and Paracetamol. Pharmazie 2022, 77, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A Review of the Molecular Aspects of Melatonin’s Anti-Inflammatory Actions: Recent Insights and New Perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. Antiinflammatory Activity of Melatonin in Central Nervous System. Curr. Neuropharmacol. 2010, 8, 228–242. [Google Scholar] [CrossRef]
- Papagiannidou, E.; Skene, D.J.; Ioannides, C. Potential Drug Interactions with Melatonin. Physiol. Behav. 2014, 131, 17–24. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, Y.K.; Agarwal, S.; Aneja, S.; Kalaivani, M.; Kohli, K. Effects of Add-on Melatonin Administration on Antioxidant Enzymes in Children with Epilepsy Taking Carbamazepine Monotherapy: A Randomized, Double-Blind, Placebo-Controlled Trial. Epilepsia 2004, 45, 1636–1639. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef]
- Kinker, G.S.; Ostrowski, L.H.; Ribeiro, P.A.C.; Chanoch, R.; Muxel, S.M.; Tirosh, I.; Spadoni, G.; Rivara, S.; Martins, V.R.; Santos, T.G.; et al. MT1 and MT2 Melatonin Receptors Play Opposite Roles in Brain Cancer Progression. J. Mol. Med. 2021, 99, 289–301. [Google Scholar] [CrossRef]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and Cellular Localization of Melatonin MT1 and MT2 Receptors in the Adult Rat Brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef]
- Xie, S.; Fan, W.; He, H.; Huang, F. Role of Melatonin in the Regulation of Pain. J. Pain Res. 2020, 13, 331–343. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Lai, C.Y.; Lin, L.T.; Chou, D.; Yeh, C.M.; Cheng, J.K.; Wang, H.H.; Lin, K.H.; Lin, T.B.; Peng, H.Y. Melatonin Relieves Paclitaxel-Induced Neuropathic Pain by Regulating PNEK2-Dependent Epigenetic Pathways in DRG Neurons. ACS Chem. Neurosci. 2023, 14, 4227–4239. [Google Scholar] [CrossRef]
- Lin, J.J.; Lin, Y.; Zhao, T.Z.; Zhang, C.K.; Zhang, T.; Chen, X.L.; Ding, J.Q.; Chang, T.; Zhang, Z.; Sun, C.; et al. Melatonin Suppresses Neuropathic Pain via MT2-Dependent and -Independent Pathways in Dorsal Root Ganglia Neurons of Mice. Theranostics 2017, 7, 2015–2032. [Google Scholar] [CrossRef]
- Kuthati, Y.; Davuluri, V.N.G.; Yang, C.P.; Chang, H.C.; Chang, C.P.; Wong, C.S. Melatonin MT2 Receptor Agonist IIK-7 Produces Antinociception by Modulation of ROS and Suppression of Spinal Microglial Activation in Neuropathic Pain Rats. J. Pain Res. 2019, 12, 2473–2485. [Google Scholar] [CrossRef]
- Lopez-Canul, M.; Palazzo, E.; Dominguez-Lopez, S.; Luongo, L.; Lacoste, B.; Comai, S.; Angeloni, D.; Fraschini, F.; Boccella, S.; Spadoni, G.; et al. Selective Melatonin MT2 Receptor Ligands Relieve Neuropathic Pain through Modulation of Brainstem Descending Antinociceptive Pathways. Pain 2015, 156, 305–317. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Liu, X.; Hu, S.; Li, H.; Feng, Y.; Ke, J.; Long, X. Melatonin Abates TMJOA Chronic Pain by MT2R in Trigeminal Ganglion Neurons. J. Dent. Res. 2022, 101, 111–119. [Google Scholar] [CrossRef]
- Huang, C.T.; Chen, S.H.; Chang, C.F.; Lin, S.C.; Lue, J.H.; Tsai, Y.J. Melatonin Reduces Neuropathic Pain Behavior and Glial Activation through MT2 Melatonin Receptor Modulation in a Rat Model of Lysophosphatidylcholine-Induced Demyelination Neuropathy. Neurochem. Int. 2020, 140, 104827. [Google Scholar] [CrossRef]
- Chiang, R.P.Y.; Huang, C.T.; Tsai, Y.J. Melatonin Reduces Median Nerve Injury-Induced Mechanical Hypersensitivity via Inhibition of Microglial P38 Mitogen-Activated Protein Kinase Activation in Rat Cuneate Nucleus. J. Pineal Res. 2013, 54, 232–244. [Google Scholar] [CrossRef]
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin Receptors: Distribution in Mammalian Brain and Their Respective Putative Functions. Brain Struct. Funct. 2017, 222, 2921–2939. [Google Scholar] [CrossRef]
- Posa, L.; De Gregorio, D.; Gobbi, G.; Comai, S. Targeting Melatonin MT2 Receptors: A Novel Pharmacological Avenue for Inflammatory and Neuropathic Pain. Curr. Med. Chem. 2018, 25, 3866–3882. [Google Scholar] [CrossRef]
- Kuthati, Y.; Wong, C.S. The Melatonin Type 2 Receptor Agonist IIK7 Attenuates and Reverses Morphine Tolerance in Neuropathic Pain Rats Through the Suppression of Neuroinflammation in the Spinal Cord. Pharmaceuticals 2024, 17, 1638. [Google Scholar] [CrossRef] [PubMed]
- Mini, L.J.; Wang-Weigand, S.; Zhang, J. Self-Reported Efficacy and Tolerability of Ramelteon 8 Mg in Older Adults Experiencing Severe Sleep-Onset Difficulty. Am. J. Geriatr. Pharmacother. 2007, 5, 177–184. [Google Scholar] [CrossRef]
- Afolalu, E.F.; Ramlee, F.; Tang, N.K.Y. Effects of Sleep Changes on Pain-Related Health Outcomes in the General Population: A Systematic Review of Longitudinal Studies with Exploratory Meta-Analysis. Sleep Med. Rev. 2018, 39, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Staffe, A.T.; Bech, M.W.; Clemmensen, S.L.K.; Nielsen, H.T.; Larsen, D.B.; Petersen, K.K. Total Sleep Deprivation Increases Pain Sensitivity, Impairs Conditioned Pain Modulation and Facilitates Temporal Summation of Pain in Healthy Participants. PLoS ONE 2019, 14, e0225849. [Google Scholar] [CrossRef]
- Huang, C.T.; Chiang, R.P.Y.; Chen, C.L.; Tsai, Y.J. Sleep Deprivation Aggravates Median Nerve Injury-Induced Neuropathic Pain and Enhances Microglial Activation by Suppressing Melatonin Secretion. Sleep 2014, 37, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Ameliorates Anxiety-like Behaviors Induced by Sleep Deprivation in Mice: Role of Oxidative Stress, Neuroinflammation, Autophagy and Apoptosis. Brain Res. Bull. 2021, 174, 161–172. [Google Scholar] [CrossRef]
- Landis, C.A. Is Melatonin the Next “New” Therapy to Improve Sleep and Reduce Pain? Sleep 2014, 37, 1405–1406. [Google Scholar] [CrossRef][Green Version]
- Chen, I.J.; Yang, C.P.; Lin, S.H.; Lai, C.M.; Wong, C.S. The Circadian Hormone Melatonin Inhibits Morphine-Induced Tolerance and Inflammation via the Activation of Antioxidative Enzymes. Antioxidants 2020, 9, 780. [Google Scholar] [CrossRef]
- Deng, Y.-K.; Ding, J.-F.; Liu, J.; Yang, Y.-Y. Analgesic Effects of Melatonin on Post-Herpetic Neuralgia. Int. J. Clin. Exp. Med. 2015, 8, 5004–5009. [Google Scholar]
- Ambriz-Tututi, M.; Rocha-González, H.I.; Cruz, S.L.; Granados-Soto, V. Melatonin: A Hormone That Modulates Pain. Life Sci. 2009, 84, 489–498. [Google Scholar] [CrossRef]
- Mantovani, M.; Kaster, M.P.; Pertile, R.; Calixto, J.B.; Rodrigues, A.L.S.; Santos, A.R.S. Mechanisms Involved in the Antinociception Caused by Melatonin in Mice. J. Pineal Res. 2006, 41, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Gobbi, G. Unveiling the Role of Melatonin MT2 Receptors in Sleep, Anxiety and Other Neuropsychiatric Diseases: A Novel Target in Psychopharmacology. J. Psychiatry Neurosci. 2014, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Meena, S.; Kalonia, H.; Gupta, A.; Kumar, P. Effect of Nitric Oxide in Protective Effect of Melatonin against Chronic Constriction Sciatic Nerve Injury Induced Neuropathic Pain in Rats. Indian J. Exp. Biol. 2011, 49, 664–671. [Google Scholar] [PubMed]
- Aydogan, S.; Betul Yerer, M.; Goktas, A. Melatonin and Nitric Oxide. J. Endocrinol. Investig. 2006, 29, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Buffoli, B.; Bonazza, V.; Reiter, R.J.; Rezzani, R.; Rodella, L.F. Single Administration of Melatonin Modulates the Nitroxidergic System at the Peripheral Level and Reduces Thermal Nociceptive Hypersensitivity in Neuropathic Rats. Int. J. Mol. Sci. 2017, 18, 2143. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.W.; Lutsch, E.F. Daily Susceptibility Rhythm to Morphine Analgesia. J. Pharm. Sci. 1969, 58, 374–376. [Google Scholar] [CrossRef]
- Hansen, M.V.; Halladin, N.L.; Rosenberg, J.; Gögenur, I.; Møller, A.M. Melatonin for Pre- and Postoperative Anxiety in Adults. Cochrane Database Syst. Rev. 2015, 2015, CD009861. [Google Scholar] [CrossRef]
- Rokhtabnak, F.; Ghodraty, M.R.; Kholdebarin, A.; Khatibi, A.; Alizadeh, S.S.S.; Koleini, Z.S.; Zamani, M.M.; Pournajafian, A. Comparing the Effect of Preoperative Administration of Melatonin and Passiflora Incarnata on Postoperative Cognitive Disorders in Adult Patients Undergoing Elective Surgery. Anesth. Pain Med. 2016, 7, e41238. [Google Scholar] [CrossRef]
- Wilhelmsen, M.; Amirian, I.; Reiter, R.J.; Rosenberg, J.; Gögenur, I. Analgesic Effects of Melatonin: A Review of Current Evidence from Experimental and Clinical Studies. J. Pineal Res. 2011, 51, 270–277. [Google Scholar] [CrossRef]
- Almay, B.G.L.; von Knorring, L.; Wetterberg, L. Melatonin in Serum and Urine in Patients with Idiopathic Pain Syndromes. Psychiatry Res. 1987, 22, 179–191. [Google Scholar] [CrossRef]
- Citera, G.; Arias, M.A.; Maldonado-Cocco, J.A.; Lázaro, M.A.; Rosemffet, M.G.; Brusco, L.I.; Scheines, E.J.; Cardinalli, D.P. The Effect of Melatonin in Patients with Fibromyalgia: A Pilot Study. Clin. Rheumatol. 2000, 19, 9–13. [Google Scholar] [CrossRef]
- Jallouli, S.; Ghroubi, S.; Sakka, S.; Ben Dhia, I.; Damak, M.; Yahia, A.; Driss, T.; Mhiri, C.; Elleuch, M.H.; Hammouda, O. Effects of a Nighttime Melatonin Ingestion on Dynamic Postural Balance and Muscle Strength the Following Morning in People Living with Multiple Sclerosis: A Preliminary Study. Clin. Neurol. Neurosurg. 2024, 238, 108165. [Google Scholar] [CrossRef]
- Hussain, S.A.R.; Al-Khalifa, I.I.; Jasim, N.A.; Gorial, F.I. Adjuvant Use of Melatonin for Treatment of Fibromyalgia. J. Pineal Res. 2011, 50, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Reiter, R.J. Melatonin Therapy in Fibromyalgia. J. Pineal Res. 2006, 40, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Song, G.H.; Leng, P.H.; Gwee, K.A.; Moochhala, S.M.; Ho, K.Y. Melatonin Improves Abdominal Pain in Irritable Bowel Syndrome Patients Who Have Sleep Disturbances: A Randomised, Double Blind, Placebo Controlled Study. Gut 2005, 54, 1402–1407. [Google Scholar] [CrossRef]
- Lu, W.Z.; Gwee, K.A.; Moochhalla, S.; Ho, K.Y. Melatonin Improves Bowel Symptoms in Female Patients with Irritable Bowel Syndrome: A Double-Blind Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2005, 22, 927–934. [Google Scholar] [CrossRef]
- Masruha, M.R.; de Souza Vieira, D.S.; Minett, T.S.C.; Cipolla-Neto, J.; Zukerman, E.; Vilanova, L.C.P.; Peres, M.F.P. Low Urinary 6-Sulphatoxymelatonin Concentrations in Acute Migraine. J. Headache Pain 2008, 9, 221–224. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Geoffriau, M.; Zaidan, R.; Mallo, C.; Chazot, G. Nocturnal Plasma Melatonin Profile and Melatonin Kinetics during Infusion in Status Migrainosus. Cephalalgia 1997, 17, 511–517. [Google Scholar] [CrossRef]
- Tabeeva, G.R.; Sergeev, A.V.; Gromova, S.A. Possibilities of Preventive Treatment of Migraine with the MT1- and MT2 Agonist and 5-HT2c Receptor Antagonist Agomelatin (Valdoxan). Zhurnal Nevrol. Psikhiatrii Im. S.S. Korsakova 2011, 111, 32–36. [Google Scholar]
- Peres, M.F.P.; Zukerman, E.; Da Cunha Tanuri, F.; Moreira, F.R.; Cipolla-Neto, J. Melatonin, 3 Mg, Is Effective for Migraine Prevention. Neurology 2004, 63, 757. [Google Scholar] [CrossRef]
- Alstadhaug, K.B.; Odeh, F.; Salvesen, R.; Bekkelund, S.I. Prophylaxis of Migraine with Melatonin: A Randomized Controlled Trial. Neurology 2010, 75, 1527–1532. [Google Scholar] [CrossRef]
- Manchishi, S.M.; Cui, R.J.; Zou, X.H.; Cheng, Z.Q.; Li, B.J. Effect of Caloric Restriction on Depression. J. Cell Mol. Med. 2018, 22, 2528–2535. [Google Scholar] [CrossRef]
- Mendonça, C.R.; Noll, M.; Castro, M.C.R.; Silveira, E.A. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients 2020, 12, 3075. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.N.; Vincent, H.K.; Newman, C.B.; Batsis, J.A.; Abbate, L.M.; Huffman, K.F.; Bodley, J.; Vos, N.; Callahan, L.F.; Shultz, S.P. Evidence-Based Dietary Practices to Improve Osteoarthritis Symptoms: An Umbrella Review. Nutrients 2023, 15, 3050. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodríguez, A.B. Detection and Quantification of Melatonin and Serotonin in Eight Sweet Cherry Cultivars (Prunus avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by High Performance Liquid Chromatography-Mass Spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Riga, P.; Medina, S.; García-Flores, L.A.; Gil-Izquierdo, Á. Melatonin Content of Pepper and Tomato Fruits: Effects of Cultivar and Solar Radiation. Food Chem. 2014, 156, 347–352. [Google Scholar] [CrossRef]
- Aguilera, Y.; Herrera, T.; Liébana, R.; Rebollo-Hernanz, M.; Sanchez-Puelles, C.; Martín-Cabrejas, M.A. Impact of Melatonin Enrichment during Germination of Legumes on Bioactive Compounds and Antioxidant Activity. J. Agric. Food Chem. 2015, 63, 7967–7974. [Google Scholar] [CrossRef]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a Phytomelatonin-Rich Extract from Cultured Plants with Excellent Biochemical and Functional Properties as an Alternative to Synthetic Melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Spectrofluorimetric Determination of Melatonin in Kernels of Four Different Pistacia Varieties after Ultrasound-Assisted Solid–Liquid Extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef]
- González-Flores, D.; Gamero, E.; Garrido, M.; Ramírez, R.; Moreno, D.; Delgado, J.; Valdés, E.; Barriga, C.; Rodríguez, A.B.; Paredes, S.D. Urinary 6-Sulfatoxymelatonin and Total Antioxidant Capacity Increase after the Intake of a Grape Juice Cv. Tempranillo Stabilized with HHP. Food Funct. 2012, 3, 34–39. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum Melatonin Levels and Antioxidant Capacities after Consumption of Pineapple, Orange, or Banana by Healthy Male Volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H. Bin Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Xian Tan, D.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and Quantification of the Antioxidant Melatonin in Montmorency and Balaton Tart Cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High Levels of Melatonin in the Seeds of Edible Plants: Possible Function in Germ Tissue Protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Aguilera, Y.; Rebollo-Hernanz, M.; Herrera, T.; Cayuelas, L.T.; Rodríguez-Rodríguez, P.; De Pablo, Á.L.L.; Arribas, S.M.; Martin-Cabrejas, M.A. Intake of Bean Sprouts Influences Melatonin and Antioxidant Capacity Biomarker Levels in Rats. Food Funct. 2016, 7, 1438–1445. [Google Scholar] [CrossRef]
- Yu, C.; Shi, Z.; Lv, J.; Guo, Y.; Bian, Z.; Du, H.; Chen, Y.; Tao, R.; Huang, Y.; Chen, J.; et al. Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank. Nutrients 2017, 9, 232. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and Its Relationship to Plant Hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional Roles of Melatonin in Plants, and Perspectives in Nutritional and Agricultural Science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of Dietary Sources of Melatonin on Sleep Quality: A Review. J. Food Sci. 2020, 85, 5–13. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of Oral and Intravenous Melatonin in Healthy Volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin Uptake and Growth Prevention in Rat Hepatoma 7288CTC in Response to Dietary Melatonin: Melatonin Receptor-Mediated Inhibition of Tumor Linoleic Acid Metabolism to the Growth Signaling Molecule 13-Hydroxyoctadecadienoic Acid and the Potential Role of Phytomelatonin. Carcinogenesis 2004, 25, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Parrott, K.A.; Ayres, J.W.; Sack, R.L. Development and Characterization of an Oral Controlled-Release Delivery System for Melatonin. Drug Dev. Ind. Pharm. 1996, 22, 269–274. [Google Scholar] [CrossRef]
- Yeleswaram, K.; McLaughlin, L.G.; Knipe, J.O.; Schabdach, D. Pharmacokinetics and Oral Bioavailability of Exogenous Melatonin in Preclinical Animal Models and Clinical Implications. J. Pineal Res. 1997, 22, 45–51. [Google Scholar] [CrossRef]
- Hirata, F.; Hayaishi, O.; Tokuyama, T.; Seno, S. In Vitro and in Vivo Formation of Two New Metabolites of Melatonin. J. Biol. Chem. 1974, 249, 1311–1313. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Mascio, P.D.; Martinez, G.R.; Prado, F.M.; Reiter, R.J.; Tan, X. Novel Rhythms of N1-Acetyl-N2-Formyl-5-Methoxykynuramine and Its Precursor Melatonin in Water Hyacinth: Importance for Phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One Molecule, Many Derivatives: A Never-Ending Interaction of Melatonin with Reactive Oxygen and Nitrogen Species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant Properties of the Melatonin Metabolite N1-Acetyl-5-Methoxykynuramine (AMK): Scavenging of Free Radicals and Prevention of Protein Destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef]
- Ghanavati, M.; Clark, C.C.T.; Bahrami, A.; Teymoori, F.; Movahed, M.; Sohrab, G.; Hejazi, E. Dietary Intake of Polyphenols and Total Antioxidant Capacity and Risk of Prostate Cancer: A Case-Control Study in Iranian Men. Eur. J. Cancer Care 2021, 30, e13364. [Google Scholar] [CrossRef]
- Rickards, L.; Lynn, A.; Harrop, D.; Baker, M.E.; Russell, M.; Ranchordas, M.K. Effect of Polyphenol-Rich Foods, Juices, and Concentrates on Recovery from Exercise Induced Muscle Damage: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2988. [Google Scholar] [CrossRef]
- Quintans, J.; Quintans Júnior, L.; Araújo, A.; Barreto, R.; Sluka, K. Β-caryophyllene-complexed in Β-cyclodextrin Produces Antihyperalgesic Activity in Animal Model for Fibromyalgia. FASEB J. 2015, 29, 616.4. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, C.K.; Hwang, S.W. Biological Roles of Resolvins and Related Substances in the Resolution of Pain. Biomed. Res. Int. 2015, 2015, 830930. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Andrew, C.W.H.; Shyu, B.C. Modulation of Melatonin in Pain Behaviors Associated with Oxidative Stress and Neuroinflammation Responses in an Animal Model of Central Post-Stroke Pain. Int. J. Mol. Sci. 2023, 24, 5413. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Q.; Nie, L.Y.; Qian, L.N.; Zhao, K. Efficacy and Safety of Polyphenols for Osteoarthritis Treatment: A Meta-Analysis. Clin. Ther. 2021, 43, e241–e253.e2. [Google Scholar] [CrossRef] [PubMed]
- Valsamidou, E.; Gioxari, A.; Amerikanou, C.; Zoumpoulakis, P.; Skarpas, G.; Kaliora, A.C. Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies. Nutrients 2021, 13, 1420. [Google Scholar] [CrossRef]
- Sirše, M. Effect of Dietary Polyphenols on Osteoarthritis—Molecular Mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef]
- Tao, L.; Ding, Q.; Gao, C.; Sun, X. Resveratrol Attenuates Neuropathic Pain through Balancing Pro-Inflammatory and Anti-Inflammatory Cytokines Release in Mice. Int. Immunopharmacol. 2016, 34, 165–172. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Odphp 2015–2020 Dietary Guidelines for Americans. 2015. Available online: https://odphp.health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 11 April 2025).
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Tang, L.; Zirpoli, G.R.; Guru, K.; Moysich, K.B.; Zhang, Y.; Ambrosone, C.B.; McCann, S.E. Consumption of Raw Cruciferous Vegetables Is Inversely Associated with Bladder Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2008, 17, 938–944. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Anatomy of Health Effects of Mediterranean Diet: Greek EPIC Prospective Cohort Study. BMJ 2009, 338, 26–28. [Google Scholar] [CrossRef]
- Slavin, J. Whole Grains and Human Health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Piscitelli, P.; Crupi, P.; Desantis, A.; Greco, E.; Severino, F.P.; Pulimeno, M.; Guazzini, A.; Kyriakides, T.C.; et al. Scientific Evidence Supporting the Newly Developed One-Health Labeling Tool “Med-Index”: An Umbrella Systematic Review on Health Benefits of Mediterranean Diet Principles and Adherence in a Planeterranean Perspective. J. Transl. Med. 2023, 21, 755. [Google Scholar] [CrossRef] [PubMed]
- Popovac, A.; Jaćimović, J.; Trichopoulou, A.; Peppa, E.; Kotrokois, K.; Stančić, I.; Milić-Lemić, A.; Kossioni, A. Mediterranean Diet and Oral Health: Is There an Association? A Scoping Review. Nutr. Res. Rev. 2024, 1–15. [Google Scholar] [CrossRef]
- Ortolá, R.; García-Esquinas, E.; Sotos-Prieto, M.; Struijk, E.A.; Caballero, F.F.D.S.; Lopez-Garcia, E.; Rodríguez-Artalejo, F. Mediterranean Diet and Changes in Frequency, Severity, and Localization of Pain in Older Adults: The Seniors-ENRICA Cohorts. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2022, 77, 122–130. [Google Scholar] [CrossRef]
- Prego-Dominguez, J.; Hadrya, F.; Takkouche, B. Polyunsaturated Fatty Acids and Chronic Pain: A Systematic Review and Meta-Analysis. Pain Physician 2016, 19, 521–535. [Google Scholar]
- Hennigar, S.R.; Kelley, A.M.; Anderson, B.J.; Armstrong, N.J.; McClung, H.L.; Berryman, C.E.; Karl, J.P.; McClung, J.P. Sensitivity and Reliability of Zinc Transporter and Metallothionein Gene Expression in Peripheral Blood Mononuclear Cells as Indicators of Zinc Status: Responses to Ex Vivo Zinc Exposure and Habitual Zinc Intake in Humans. Br. J. Nutr. 2021, 125, 361–368. [Google Scholar] [CrossRef]
- Abdelrahman, K.M.; Hackshaw, K.V. Nutritional Supplements for the Treatment of Neuropathic Pain. Biomedicines 2021, 9, 674. [Google Scholar] [CrossRef]
- Luo, J.; Bavencoffe, A.; Yang, P.; Feng, J.; Yin, S.; Qian, A.; Yu, W.; Liu, S.; Gong, X.; Cai, T.; et al. Zinc Inhibits TRPV1 to Alleviate Chemotherapy-Induced Neuropathic Pain. J. Neurosci. 2018, 38, 474–483. [Google Scholar] [CrossRef]
- Rondón, L.J.; Privat, A.M.; Daulhac, L.; Davin, N.; Mazur, A.; Fialip, J.; Eschalier, A.; Courteix, C. Magnesium Attenuates Chronic Hypersensitivity and Spinal Cord NMDA Receptor Phosphorylation in a Rat Model of Diabetic Neuropathic Pain. J. Physiol. 2010, 588, 4205–4215. [Google Scholar] [CrossRef]
- Sun, J.; Lin, H.; He, G.; Lin, W.; Yang, J. Magnesium Sulphate Attenuate Remifentanil-Induced Postoperative Hyperalgesia via Regulating Tyrosine Phosphorylation of the NR2B Subunit of the NMDA Receptor in the Spinal Cord. BMC Anesthesiol. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Torlak, M.S.; Bagcaci, S.; Akpinar, E.; Okutan, O.; Nazli, M.S.; Kuccukturk, S. The Effect of Intermittent Diet and/or Physical Therapy in Patients with Chronic Low Back Pain: A Single-Blinded Randomized Controlled Trial. EXPLORE 2022, 18, 76–81. [Google Scholar] [CrossRef]
- Safari, M.B.; Nozad, A.; Ghaffari, F.; Ghavamzadeh, S.; Alijaniha, F.; Naseri, M. Efficacy of a Short-Term Low-Calorie Diet in Overweight and Obese Patients with Chronic Sciatica: A Randomized Controlled Trial. J. Altern. Complement. Med. 2020, 26, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Pianta, T.J.; Pussell, B.A.; Kirby, A.; O’Brien, K.; Sullivan, K.; Holyday, M.; Cormack, C.; Kiernan, M.C.; Krishnan, A.V. Randomized, Controlled Trial of the Effect of Dietary Potassium Restriction on Nerve Function in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1569–1577. [Google Scholar] [CrossRef]
- Bunner, A.E.; Wells, C.L.; Gonzales, J.; Agarwal, U.; Bayat, E.; Barnard, N.D. A Dietary Intervention for Chronic Diabetic Neuropathy Pain: A Randomized Controlled Pilot Study. Nutr. Diabetes 2015, 5, e158. [Google Scholar] [CrossRef]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The Effects of a Low-Fat, Plant-Based Dietary Intervention on Body Weight, Metabolism, and Insulin Sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian Diets and Blood Pressure Ameta-Analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- Berkow, S.E.; Barnard, N. Vegetarian Diets and Weight Status. Nutr. Rev. 2006, 64, 175–188. [Google Scholar] [CrossRef]
- Barnard, N.D.; Akhtar, A.; Nicholson, A. Factors That Facilitate Compliance to Lower Fat Intake. Arch. Fam. Med. 1995, 4, 153–158. [Google Scholar] [CrossRef]
- Castañeda-Corral, G.; Velázquez-Salazar, N.B.; Martínez-Martínez, A.; Taboada-Serrano, J.N.; Núñez-Aragón, P.N.; González-Palomares, L.; Acosta-González, R.I.; Petricevich, V.L.; Acevedo-Fernández, J.J.; Montes, S.; et al. Characterization of Mechanical Allodynia and Skin Innervation in a Mouse Model of Type-2 Diabetes Induced by Cafeteria-Style Diet and Low-Doses of Streptozotocin. Front. Pharmacol. 2021, 11, 628438. [Google Scholar] [CrossRef]
- Borgonetti, V.; Galeotti, N. Rosmarinic Acid Reduces Microglia Senescence: A Novel Therapeutic Approach for the Management of Neuropathic Pain Symptoms. Biomedicines 2022, 10, 1468. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Rui, T.; Kang, A.; Li, G.; Fu, T.; Li, J.; Di, L.; Cai, B. Pharmacokinetics of Rosmarinic Acid in Rats by LC-MS/MS: Absolute Bioavailability and Dose Proportionality. RSC Adv. 2017, 7, 9057–9063. [Google Scholar] [CrossRef]
- Antonelli, A.; Bianchi, M.; Fear, E.J.; Giorgi, L.; Rossi, L. Management of Fibromyalgia: Novel Nutraceutical Therapies Beyond Traditional Pharmaceuticals. Nutrients 2025, 17, 530. [Google Scholar] [CrossRef]
- Lang-Illievich, K.; Klivinyi, C.; Rumpold-Seitlinger, G.; Dorn, C.; Bornemann-Cimenti, H. The Effect of Palmitoylethanolamide on Pain Intensity, Central and Peripheral Sensitization, and Pain Modulation in Healthy Volunteers-A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 4084. [Google Scholar] [CrossRef]
- Tasleem, F.; Azhar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and Anti-Inflammatory Activities of Piper nigrum L. Asian Pac. J. Trop. Med. 2014, 7S1, S461–S468. [Google Scholar] [CrossRef]
- Nagakura, Y. Therapeutic Approaches to Nociplastic Pain Based on Findings in the Reserpine-Induced Fibromyalgia-Like Animal Model. J. Pharmacol. Exp. Ther. 2022, 381, 106–119. [Google Scholar] [CrossRef]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano Curcumin Supplementation Reduced the Severity of Diabetic Sensorimotor Polyneuropathy in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo- Controlled Clinical Trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Bagdas, D.; Cinkilic, N.; Ozboluk, H.Y.; Ozyigit, M.O.; Gurun, M.S. Antihyperalgesic Activity of Chlorogenic Acid in Experimental Neuropathic Pain. J. Nat. Med. 2013, 67, 698–704. [Google Scholar] [CrossRef]
- Bagdas, D.; Ozboluk, H.Y.; Cinkilic, N.; Gurun, M.S. Antinociceptive Effect of Chlorogenic Acid in Rats with Painful Diabetic Neuropathy. J. Med. Food 2014, 17, 730–732. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Mizisin, L.M.; Nelson, A.; Cunha, J.M.; Ramos, K.M.; Bonke, D.; Calcutt, N.A. B Vitamins Alleviate Indices of Neuropathic Pain in Diabetic Rats. Eur. J. Pharmacol. 2009, 612, 41–47. [Google Scholar] [CrossRef]
- Long, W.; Fatehi, M.; Soni, S.; Panigrahi, R.; Kelly, R.G.; Philippaert, K.; Barr, A.J.; Held, M.; Yu, Y.; Campbell, S.A.; et al. 258-LB: Vitamin D Directly Regulates TRPV1 Channel Activity and May Play an Important Role in the Autoimmune Disease of Type 1 Diabetes. Diabetes 2019, 68, 258-LB. [Google Scholar] [CrossRef]
- Huang, W.; Shah, S.; Long, Q.; Crankshaw, A.K.; Tangpricha, V. Improvement of Pain, Sleep, and Quality of Life in Chronic Pain Patients with Vitamin D Supplementation. Clin. J. Pain 2013, 29, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kopruszinski, C.M.; Reis, R.C.; Chichorro, J.G. B Vitamins Relieve Neuropathic Pain Behaviors Induced by Infraorbital Nerve Constriction in Rats. Life Sci. 2012, 91, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Maladkar, M.; Tekchandani, C.; Dave, U.; Maladkar, M.; Tekchandani, C.; Dave, U. Post-Marketing Surveillance of Fixed Dose Combination of Methylcobalamin, Alpha Lipoic Acid, Folic Acid, Biotin, Benfotiamine & Vitamin B6-Nutripathy for the Management of Peripheral Neuropathy. J. Diabetes Mellit. 2014, 4, 124–132. [Google Scholar] [CrossRef]
- Nozaki, C.; Vergnano, A.M.; Filliol, D.; Ouagazzal, A.M.; Le Goff, A.; Carvalho, S.; Reiss, D.; Gaveriaux-Ruff, C.; Neyton, J.; Paoletti, P.; et al. Zinc Alleviates Pain through High-Affinity Binding to the NMDA Receptor NR2A Subunit. Nat. Neurosci. 2011, 14, 1017–1022. [Google Scholar] [CrossRef]
- Mauro, G.L.; Martorana, U.; Cataldo, P.; Brancato, G.; Letizia, G. Vitamin B12 in Low Back Pain: A Randomised, Double-Blind, Placebo-Controlled Study. Eur. Rev. Med. Pharmacol. Sci. 2000, 4, 53–58. [Google Scholar]
- Bujalska-Zadrożny, M.; Tatarkiewicz, J.; Kulik, K.; Filip, M.; Naruszewicz, M. Magnesium Enhances Opioid-Induced Analgesia—What We Have Learnt in the Past Decades? Eur. J. Pharm. Sci. 2017, 99, 113–127. [Google Scholar] [CrossRef]
- Na, H.-S.; Ryu, J.-H.; Do, S.-H. The Role of Magnesium in Pain; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 9780987073051. [Google Scholar]
- Prostran, M.; Vujovic, K.; Kosutic, J.; Milovanovic, A.; Vuckovic, S.; Srebro, D. Magnesium in Pain Research: State of the Art. Curr. Med. Chem. 2017, 24, 424–434. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Velázquez-González, C.; De la O-Arciniega, M.; Castañeda-Ovando, A.; Betanzos-Cabrera, G.; Bautista, M. Pomegranate as a Potential Alternative of Pain Management: A Review. Plants 2020, 9, 419. [Google Scholar] [CrossRef]
- Hoti, G.; Matencio, A.; Pedrazzo, A.R.; Cecone, C.; Appleton, S.L.; Monfared, Y.K.; Caldera, F.; Trotta, F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4102. [Google Scholar] [CrossRef]
- Ilari, S.; Proietti, S.; Russo, P.; Malafoglia, V.; Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; Fini, M.; et al. A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants 2022, 11, 2361. [Google Scholar] [CrossRef]
- Boutin, J.A.; Kennaway, D.J.; Jockers, R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules 2023, 13, 943. [Google Scholar] [CrossRef]
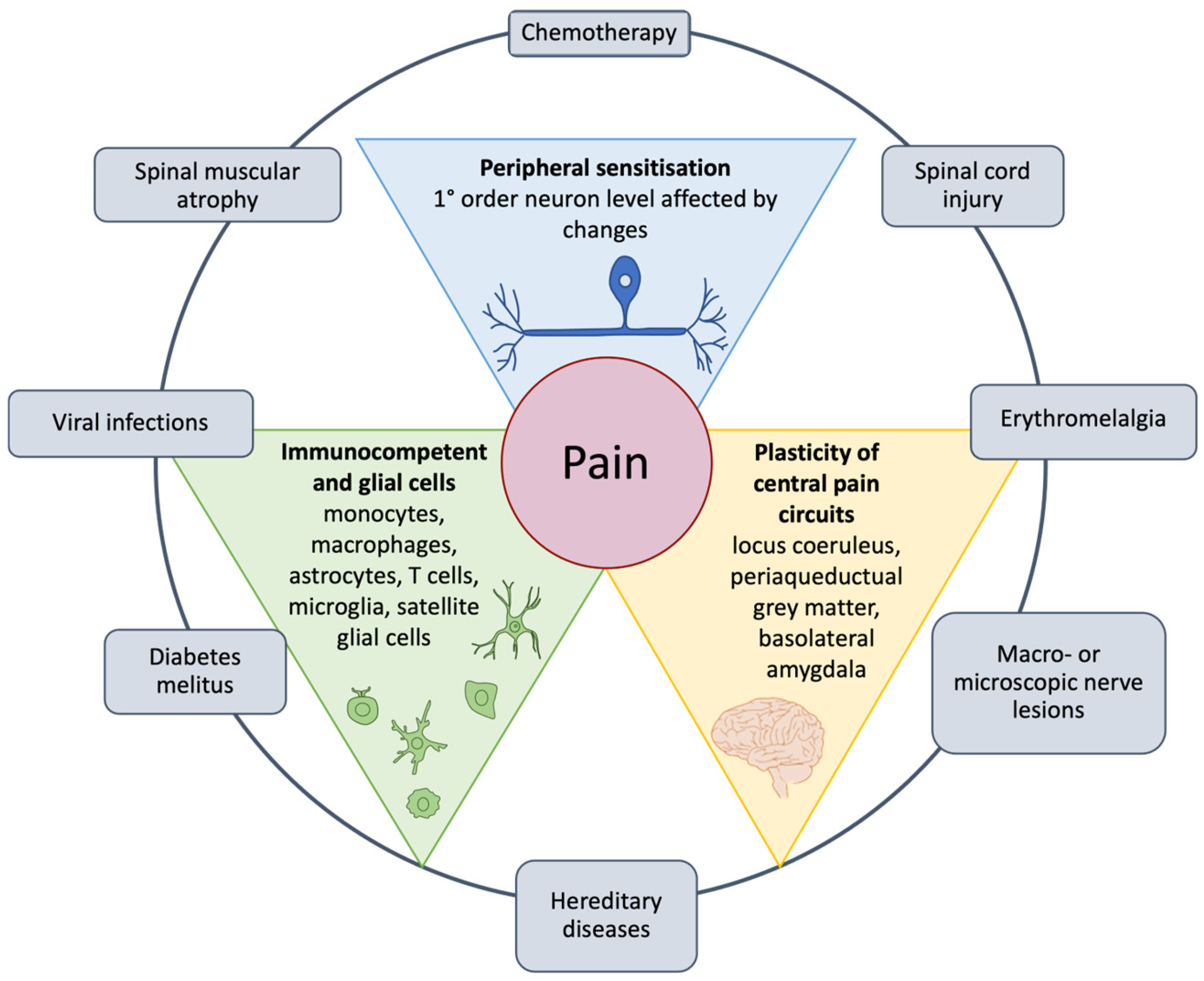
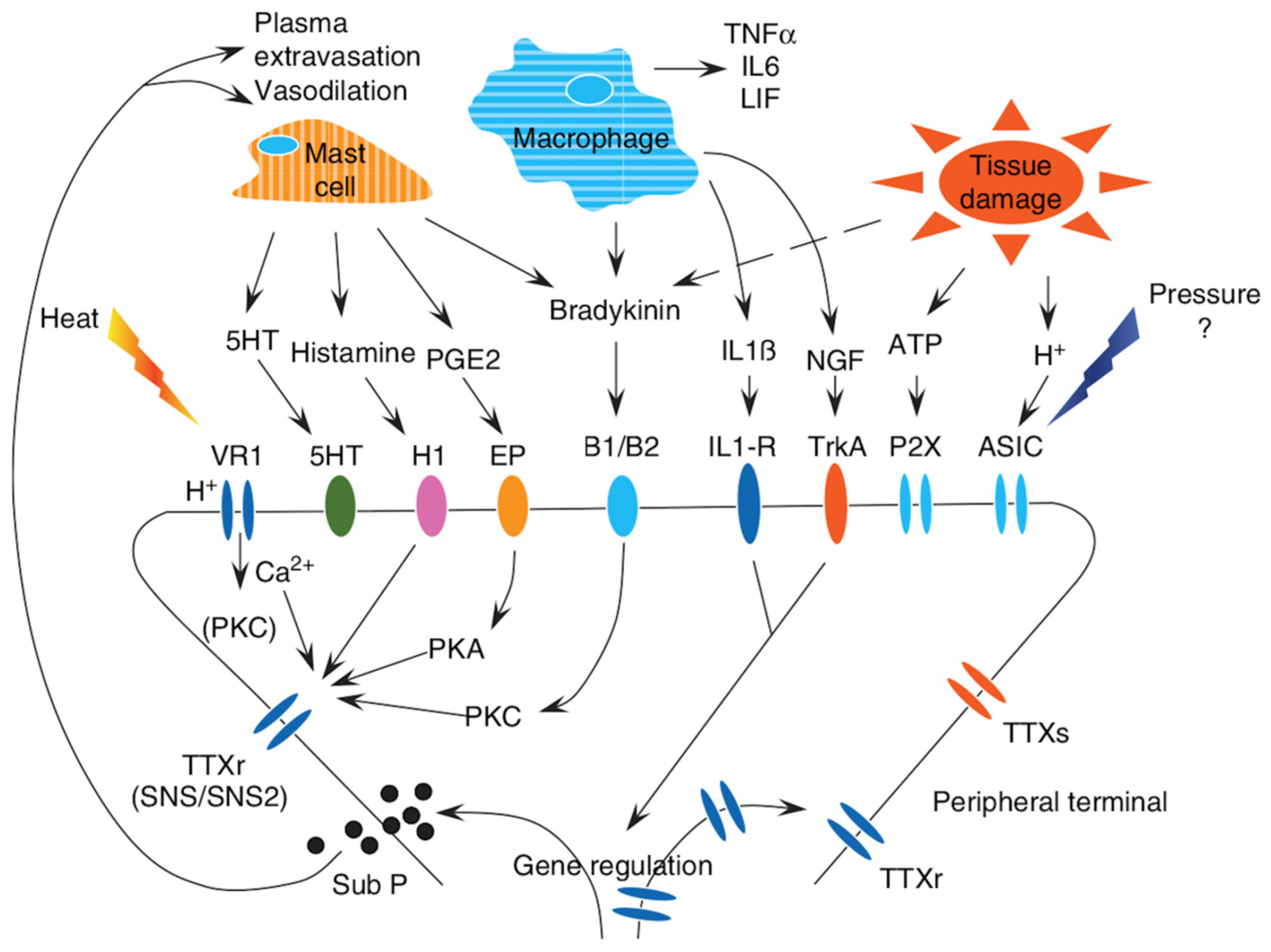
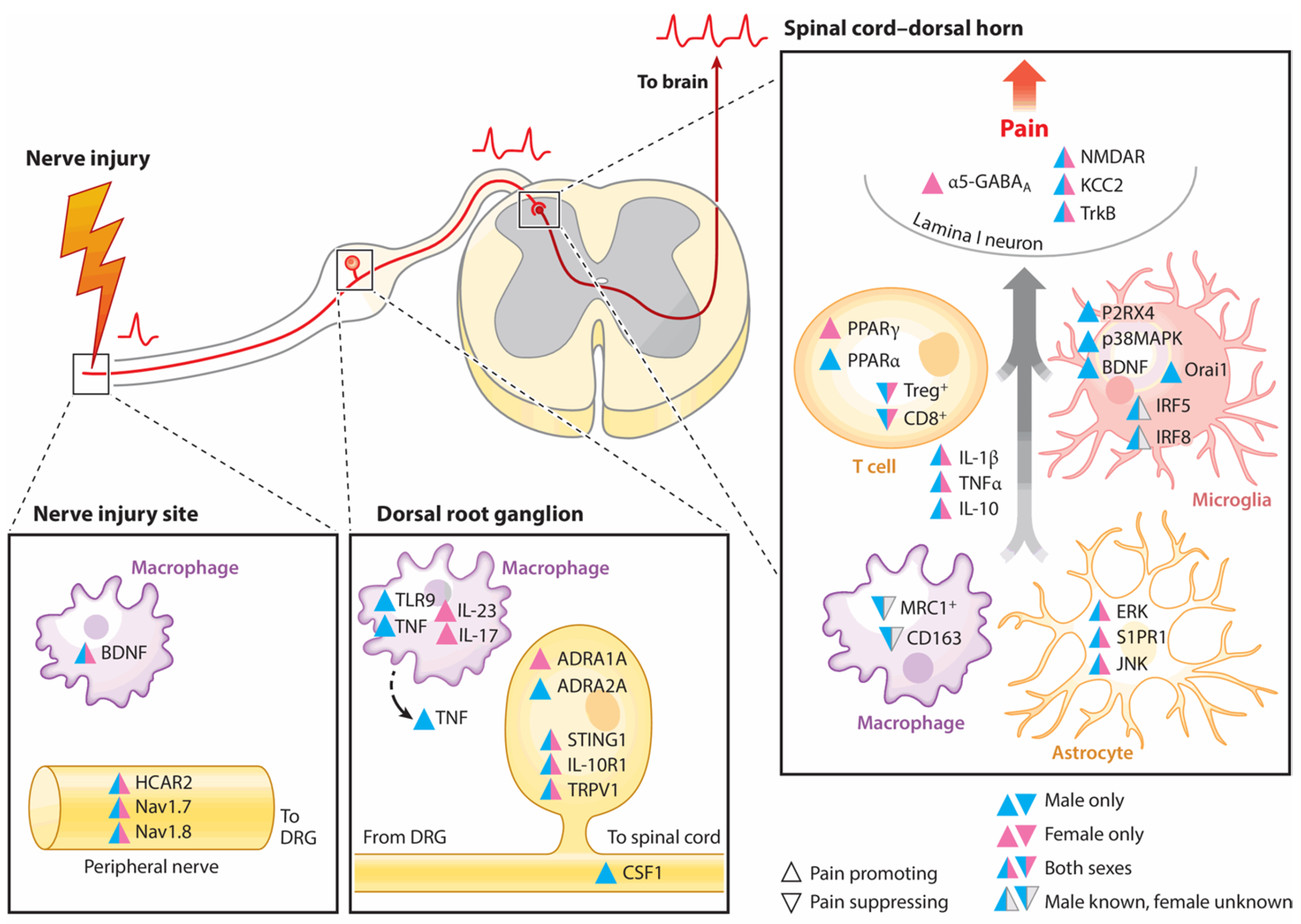
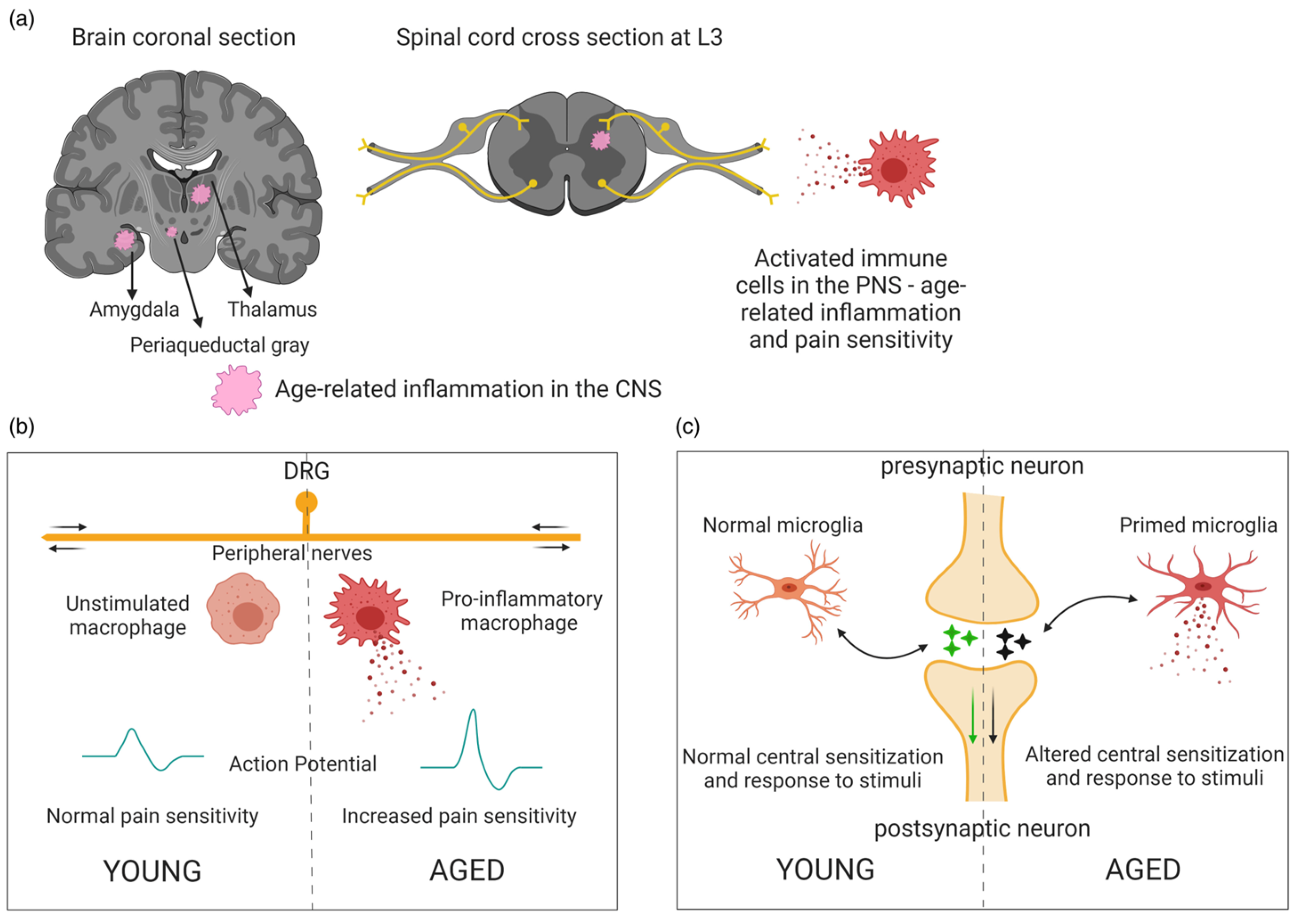

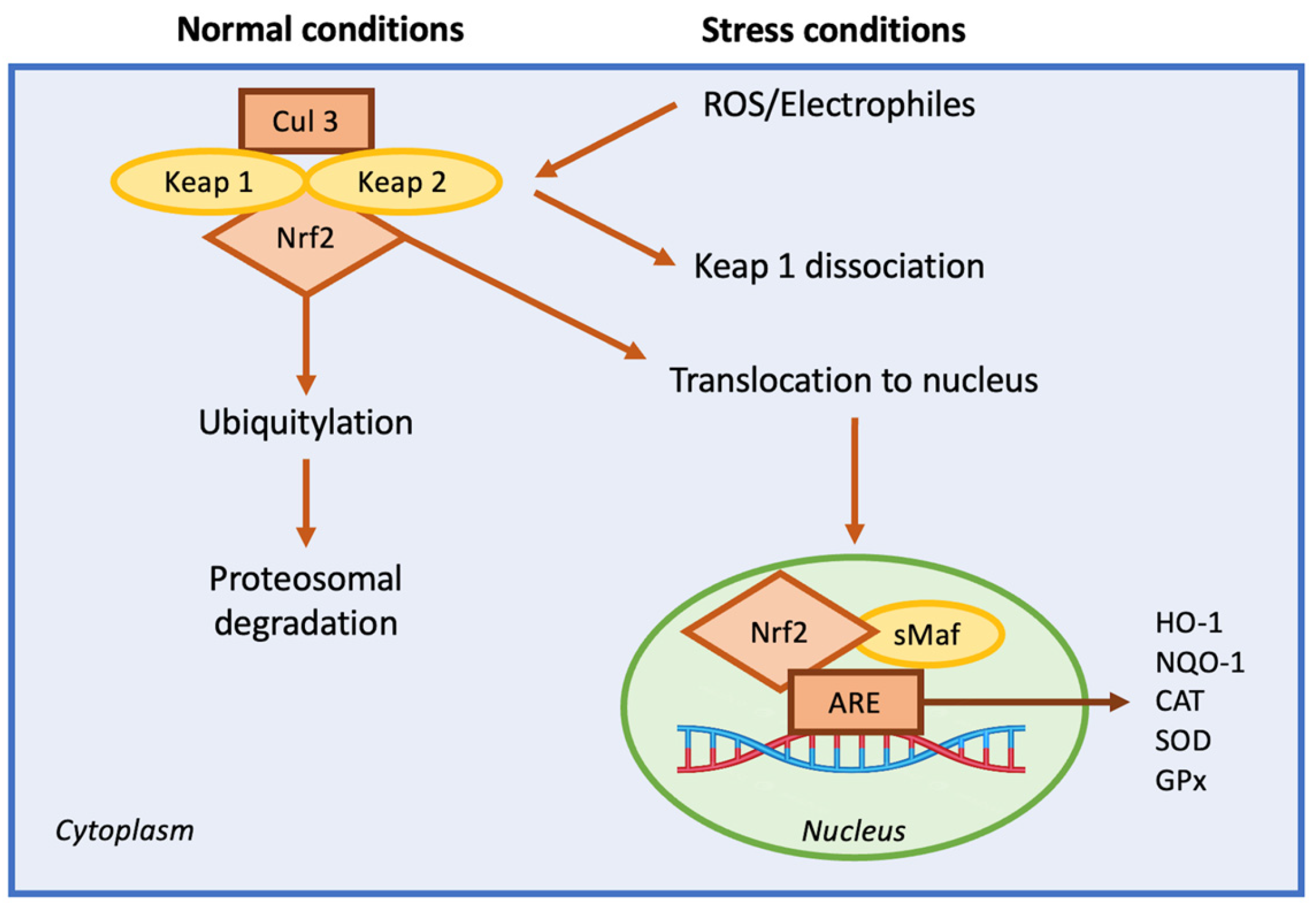
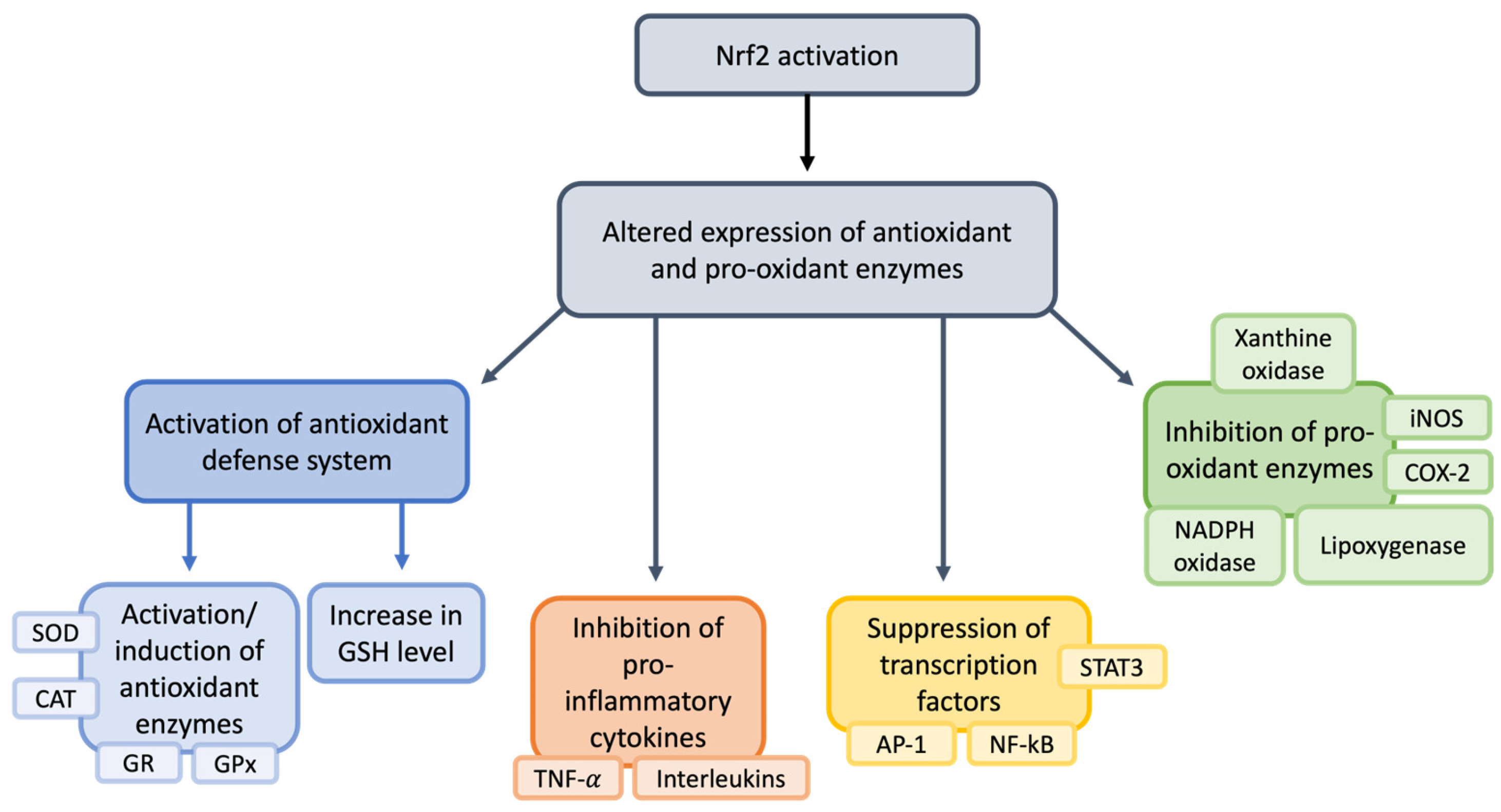
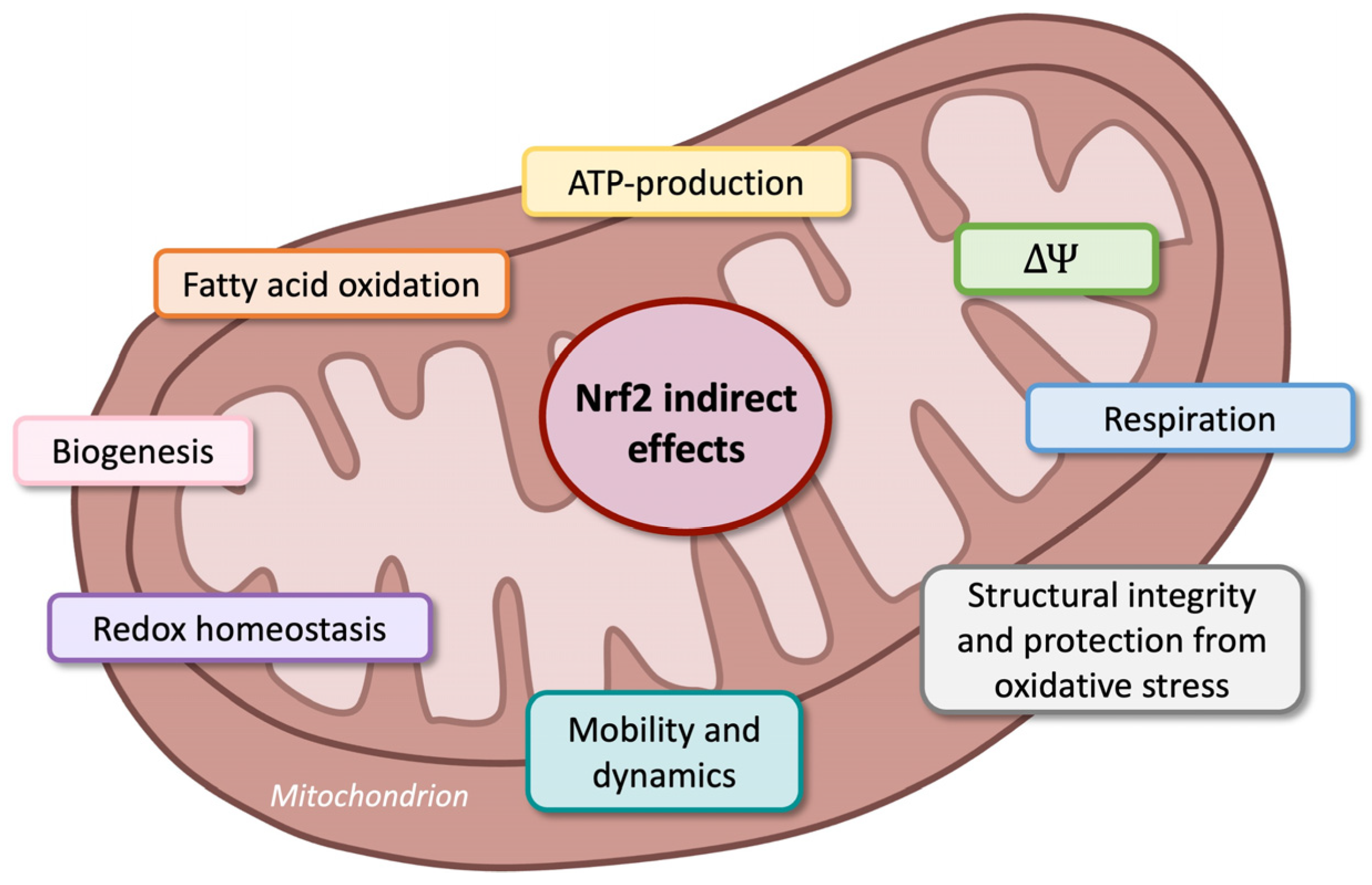
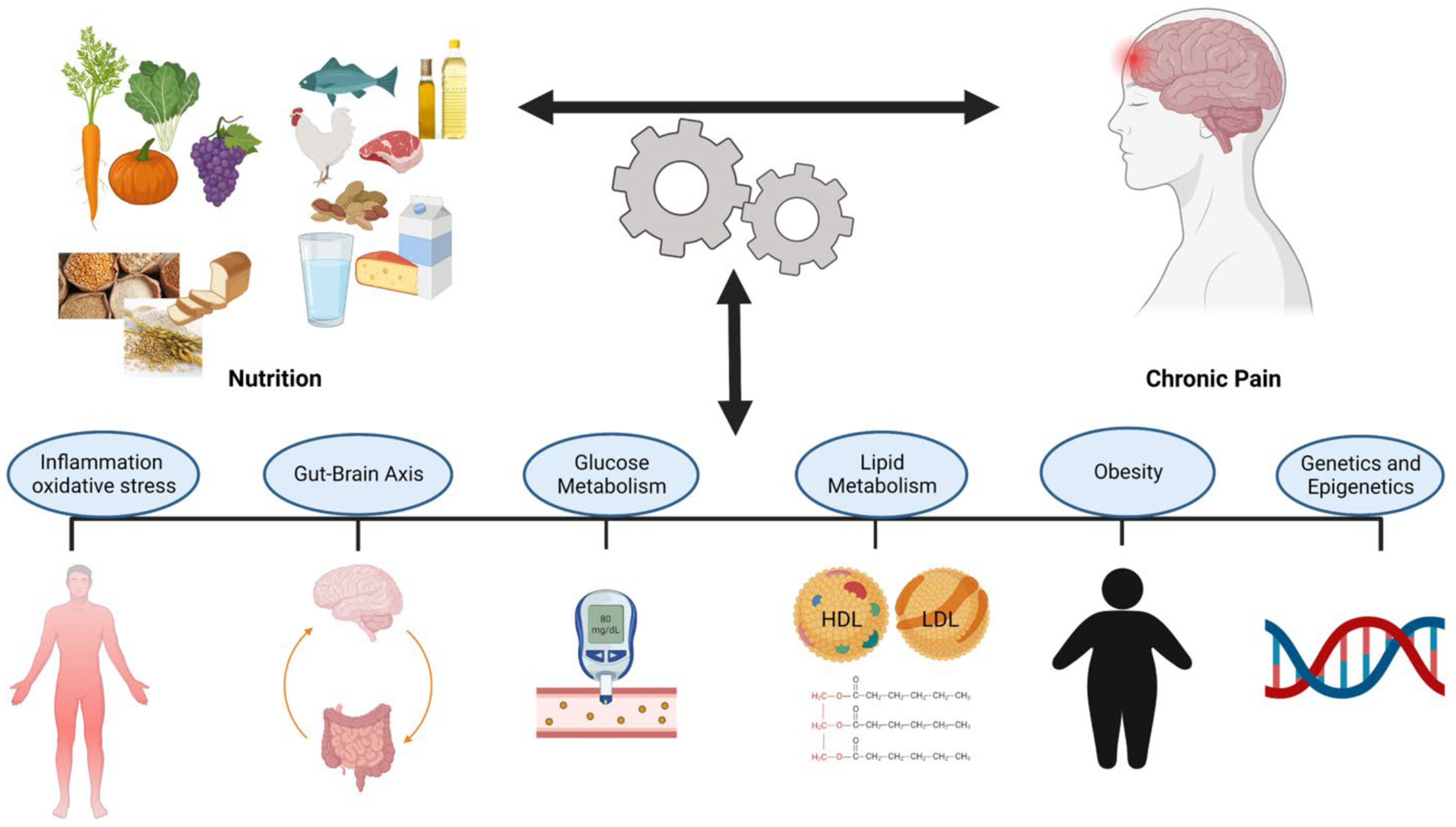
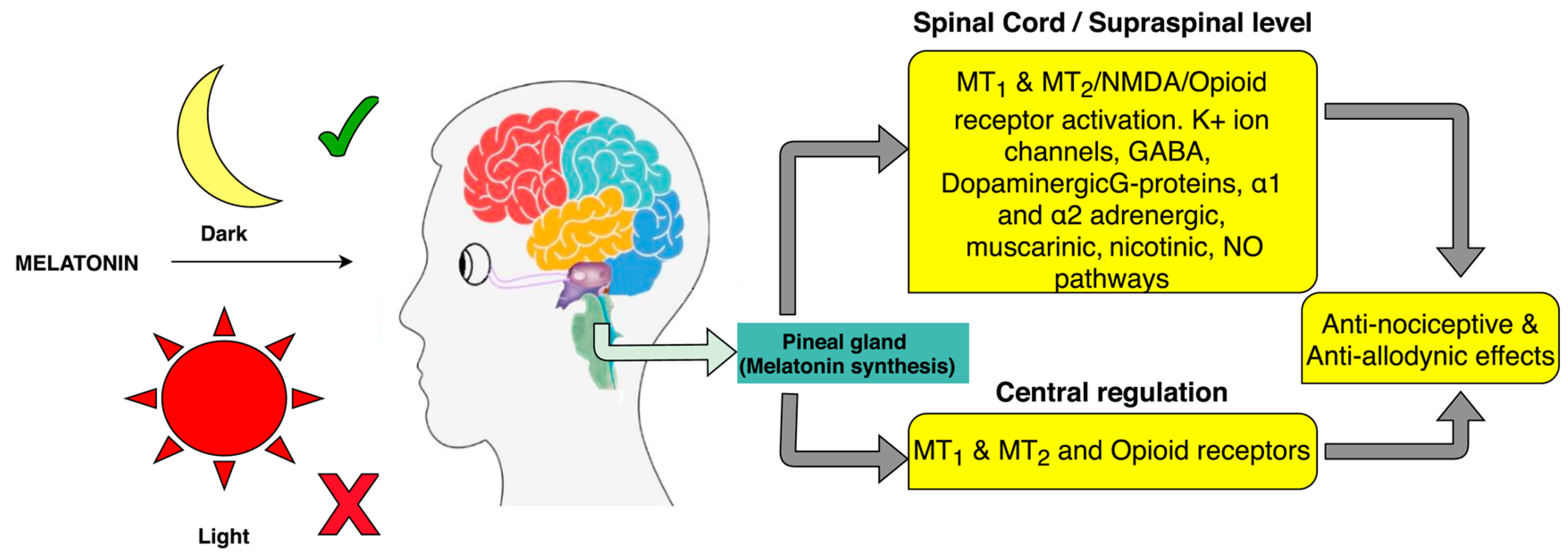
| Intervention | Outcome | Study |
|---|---|---|
| Nutritional changes (dietary patterns, nutrients, meal frequency) | Relief comparable to antidepressants, improvement in musculoskeletal conditions | [81,315,316] |
| Mediterranean diet | Alleviates pain, reduces stiffness, and addresses biomarkers in osteoarthritis | [317] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cominelli, G.; Sulas, F.; Pinto, D.; Rinaldi, F.; Favero, G.; Rezzani, R. Neuro-Nutritional Approach to Neuropathic Pain Management: A Critical Review. Nutrients 2025, 17, 1502. https://doi.org/10.3390/nu17091502
Cominelli G, Sulas F, Pinto D, Rinaldi F, Favero G, Rezzani R. Neuro-Nutritional Approach to Neuropathic Pain Management: A Critical Review. Nutrients. 2025; 17(9):1502. https://doi.org/10.3390/nu17091502
Chicago/Turabian StyleCominelli, Giorgia, Francesca Sulas, Daniela Pinto, Fabio Rinaldi, Gaia Favero, and Rita Rezzani. 2025. "Neuro-Nutritional Approach to Neuropathic Pain Management: A Critical Review" Nutrients 17, no. 9: 1502. https://doi.org/10.3390/nu17091502
APA StyleCominelli, G., Sulas, F., Pinto, D., Rinaldi, F., Favero, G., & Rezzani, R. (2025). Neuro-Nutritional Approach to Neuropathic Pain Management: A Critical Review. Nutrients, 17(9), 1502. https://doi.org/10.3390/nu17091502








