Breastfeeding: The Multifaceted Impact on Child Development and Maternal Well-Being
Abstract
1. Introduction
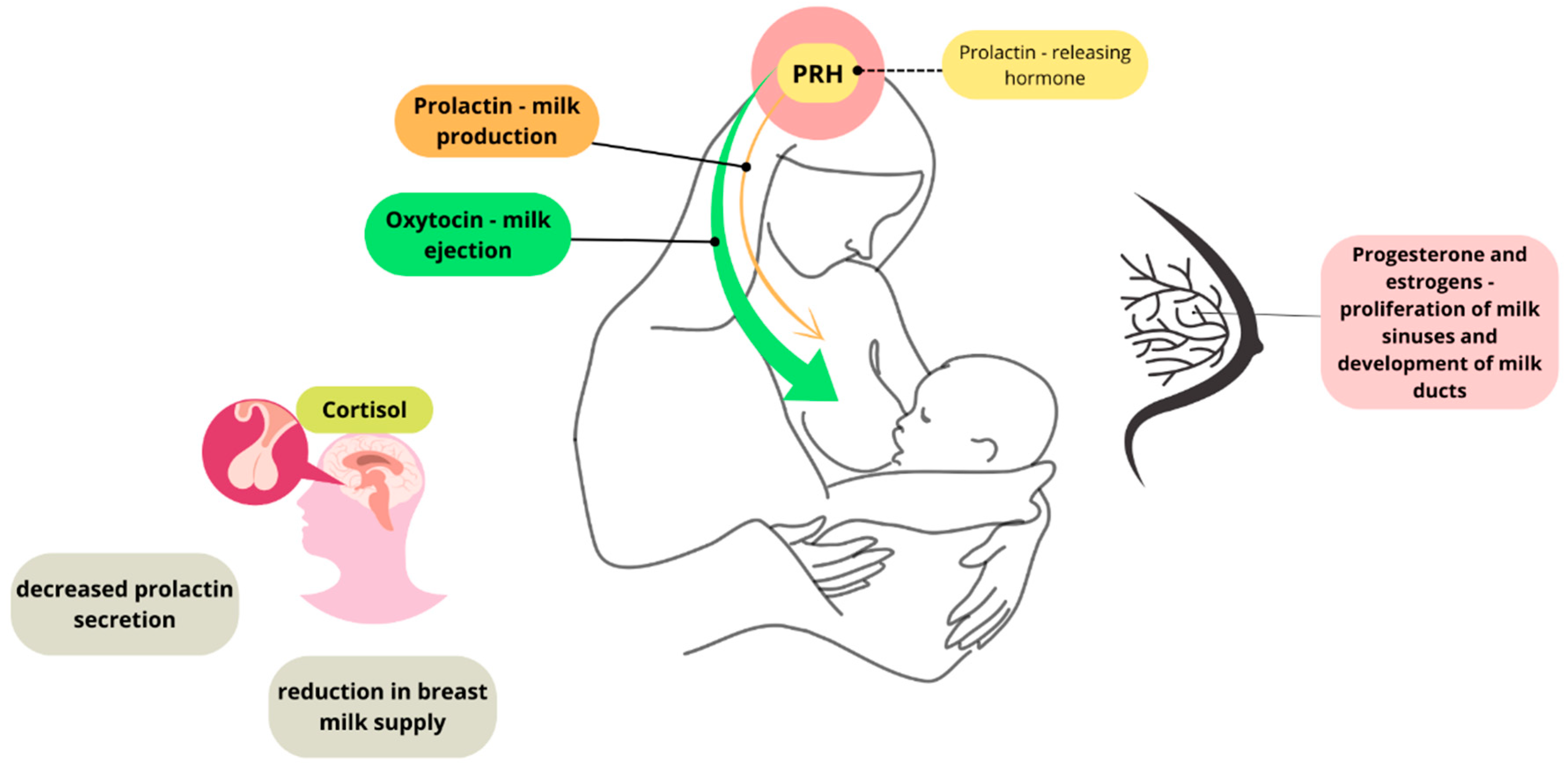
2. Regulation of Lactation—The Effect of Stress on Lactation
3. Nutritional and Immune Function of Breastfeeding
4. The Cognitive Aspect of Breastfeeding: The Impact on a Child and the Social and Emotional Development of a Baby
5. Breastfeeding and the Development of the Child’s Nervous System and Cognitive Functions
5.1. Human Milk Oligosaccharides
5.2. Sialic Acid
5.3. Fatty Acids
5.4. Hormones
5.5. Vitamins and Minerals
6. Effect of Feeding on the Regulation of a Baby’s and Child’s Sleep
7. The Effect of Breastfeeding on Emotions Among Mothers
8. Challenges and Difficulties of Breastfeeding
8.1. Lactation Difficulties
8.2. Social Factors
8.3. Return to Work After Maternity Leave
8.4. Medical Staff Support and Lactation Education
8.5. Cultural Influences
9. Cessation of Breastfeeding: Effect on Mother and Child
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Risch, L.; Hotzy, F.; Vetter, S.; Hiller, S.; Wallimann, K.; Seifritz, E.; Mötteli, S. Assessment of Nutritional Status and Risk of Malnutrition Using Adapted Standard Tools in Patients with Mental Illness and in Need of Intensive Psychiatric Treatment. Int. J. Environ. Res. Public Health 2023, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Romero-Velarde, E.; Delgado-Franco, D.; García-Gutiérrez, M.; Gurrola-Díaz, C.; Larrosa-Haro, A.; Montijo-Barrios, E.; Muskiet, F.A.J.; Vargas-Guerrero, B.; Geurts, J. The Importance of Lactose in the Human Diet: Outcomes of a Mexican Consensus Meeting. Nutrients 2019, 11, 2737. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Tucker, Z.; O’Malley, C. Mental Health Benefits of Breastfeeding: A Literature Review. Cureus 2022, 14, e29199. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Rech, R.S.; Chávez, B.A.; Fernandez, P.B.; Fridman, C.G.; Faustino-Silva, D.D.; Hilgert, J.B.; Hugo, F.N. Factors Associated with the Initiation of Breastfeeding in a Maternity Hospital in Lima, Peru. CoDAS 2021, 33, e20200173. [Google Scholar] [CrossRef]
- Rollins, N.C.; Bhandari, N.; Hajeebhoy, N.; Horton, S.; Lutter, C.K.; Martines, J.C.; Piwoz, E.G.; Richter, L.M.; Victora, C.G.; Lancet Breastfeeding Series Group. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016, 387, 491–504. [Google Scholar] [CrossRef] [PubMed]
- UNICEF; WHO. Breastfeeding and Family-Friendly Policies: An Evidence Brief. Global Breastfeeding Collective 2022. Available online: https://www.globalbreastfeedingcollective.org/media/1921/file (accessed on 3 April 2025).
- Królak-Olejnik, B.; Błasiak, I.; Szczygieł, A. Promotion of breastfeeding in Poland: The current situation. J. Int. Med. Res. 2017, 45, 1976–1984. [Google Scholar] [CrossRef]
- Lechosa-Muñiz, C.; Paz-Zulueta, M.; Cayón-De las Cuevas, J.; Llorca, J.; Cabero-Pérez, M. Declared Reasons for Cessation of Breastfeeding during the First Year of Life: An Analysis Based on a Cohort Study in Northern Spain. Int. J. Environ. Res. Public Health 2021, 18, 8414. [Google Scholar] [CrossRef]
- Gianni, M.L.; Bettinelli, M.E.; Manfra, P.; Sorrentino, G.; Bezze, E.; Plevani, L.; Cavallaro, G.; Raffaeli, G.; Crippa, B.L.; Colombo, L.; et al. Breastfeeding Difficulties and Risk for Early Breastfeeding Cessation. Nutrients 2019, 11, 2266. [Google Scholar] [CrossRef]
- Dos Santos, B.O.; De Lima, L.F. Galactosemia, lactose intolerance and allergy to milk protein: Understanding of phatophysiological mechanisms in early childhood and their respective nutritional prescriptions. Temas Em Educ. E Saúde 2020, 16, 500–512. [Google Scholar] [CrossRef]
- Pier, J.; Järvinen, K.M. Food allergy and breast-feeding. J. Food Allergy 2020, 2, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Elcombe, E.; Pierce, H.; Hugman, S.; Gannon, S. Breastfeeding after return to work: An Australian national workplace survey. Matern. Child. Nutr. 2023, 19, e13516. [Google Scholar] [CrossRef]
- Rimes, K.A.; de Oliveira, M.I.C.; Boccolini, C.S. Maternity leave and exclusive breastfeeding. Rev. De Saúde Pública 2019, 31, 10. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.R.; Buccini, G.D.S.; Venâncio, S.I.; da Costa, T.H.M. Influence of maternity leave on exclusive breastfeeding. J. Pediatr. 2017, 93, 475–481. [Google Scholar] [CrossRef]
- Hastings, G.; Angus, K.; Eadie, D.; Hunt, K. Selling second best: How infant formula marketing works. Global Health 2020, 16, 77. [Google Scholar] [CrossRef]
- Nagel, E.M.; Howland, M.A.; Pando, C.; Stang, J.; Mason, S.M.; Fields, D.A.; Demerath, E.W. Maternal psychological distress and lactation and breastfeeding outcomes: A narrative review. Clin. Ther. 2021, 44, 215–227. [Google Scholar] [CrossRef]
- Pope, C.J.; Mazmanian, D. Breastfeeding and Postpartum Depression: An Overview and Methodological Recommendations for Future Research. Depress. Res. Treat. 2016, 2016, 4765310. [Google Scholar] [CrossRef]
- Krol, K.M.; Rajhans, P.; Missana, M.; Grossmann, T. Duration of Exclusive Breastfeeding Is Associated with Differences in Infants’ Brain Responses to Emotional Body Expressions. Front. Behav. Neurosci. 2015, 8, 459. [Google Scholar] [CrossRef]
- Petersohn, I.; Hellinga, A.H.; Van Lee, L.; Keukens, N.; Bont, L.; Hettinga, K.A.; Feskens, E.J.M.; Brouwer-Brolsma, E.M. Maternal diet and human milk composition: An updated systematic review. Front. Nutr. 2023, 10, 1320560. [Google Scholar] [CrossRef]
- Perrella, S.; Gridneva, Z.; Lai, C.T.; Stinson, L.; George, A.; Bilston-John, S.; Geddes, D. Human milk composition promotes optimal infant growth, development and health. Semin. Perinatol. 2021, 45, 151380. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.Y.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef] [PubMed]
- Truchet, S.; Honvo-Houéto, E. Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 367–384. [Google Scholar] [CrossRef]
- Lawrence, R.A. Physiology of Lactation. In Breastfeeding, 9th ed.; Lawrence, R.A., Lawrence, R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 58–92. [Google Scholar] [CrossRef]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress during Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kelleher, S.L. Biological Underpinnings of Breastfeeding Challenges: The Role of Genetics, Diet, and Environment on Lactation Physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef]
- Ryoo, C.J.; Kang, N.M. Maternal Factors Affecting the Macronutrient Composition of Transitional Human Milk. Int. J. Environ. Res. Public Health 2022, 19, 3308. [Google Scholar] [CrossRef]
- Borski, R.J.; Hyde, G.N.; Fruchtman, S.; Tsai, W.S. Cortisol suppresses prolactin release through a non-genomic mechanism involving interactions with the plasma membrane. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 533–541. [Google Scholar] [CrossRef]
- Binart, N. Prolactin. The Pituitary, 4th ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 129–161. [Google Scholar] [CrossRef]
- Syam, A.; Qasim, M.; Iskandar, I.; Kadir, A. Cortisol, Prolactin, and Breastmilk Volume; A Promising Pattern for Reducing Postpartum Depression. Open Access Maced. J. Med. Sci. 2022, 10, 1399–1405. [Google Scholar] [CrossRef]
- Hinde, K.; Skibiel, A.L.; Foster, A.B.; Rosso, L.D.; Mendoza, S.P.; Capitanio, J.P. Cortisol in Mother’s Milk across Lactation Reflects Maternal Life History and Predicts Infant Temperament. Behav. Ecol. 2015, 26, 269–281. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Babiszewska, M.; Apanasewicz, A.; Piosek, M.; Wychowaniec, P.; Cierniak, A.; Barbarska, O.; Szołtysik, M.; Danel, D.; Wichary, S. Psychosocial Stress and Cortisol Stress Reactivity Predict Breast Milk Composition. Sci. Rep. 2021, 11, 11605. [Google Scholar] [CrossRef]
- Szyller, H.; Antosz, K.; Batko, J.; Mytych, A.; Dziedziak, M.; Wrześniewska, M.; Braksator, J.; Pytrus, T. Bioactive Components of Human Milk and Their Impact on Child’s Health and Development, Literature Review. Nutrients 2024, 16, 1487. [Google Scholar] [CrossRef] [PubMed]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yi, D.Y. Components of Human Breast Milk: From Macronutrient to Microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, G.; Trambusti, I.; Cicco, M.E.; Peroni, D.G. Breast milk: More than just nutrition! Minerva Pediatr. 2021, 73, 111–114. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, K.; Suman, S.; Kumar, P. Cow Milk and Human Health—A Review. Res. Rev. J. Dairy Sci. Technol. 2014, 3, 1–3. [Google Scholar]
- Galan, M.B.S.; De Carvalho, F.G.; Carvalho, S.C.S.; Brandao, C.F.C.; Terrazas, S.I.M.; Abud, G.F.; Meirelles, M.S.S.; Sakagute, S.; Ortiz, G.U.; Marchini, J.S.; et al. Casein and Whey Protein in the Breast Milk Ratio: Could It Promote Protein Metabolism Enhancement in Physically Active Adults? Nutrients 2021, 13, 2153. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zhang, M.; Liu, X.; Gao, Z.; Zhao, J.; Qiao, W.; Chen, L. Human Milk Whey Proteins: Constituents, Influencing Factors, Detection Methods, and Comparative Analysis with Other Sources. Food Chem. X 2025, 25, 102082. [Google Scholar] [CrossRef]
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Pereira Costa, M.; Ferraz Spisso, B.; Conte-Junior, C.A. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef]
- Donovam, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, 16–28. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Singh, P.; Liu, Y.; Medina-Morales, E.; Yakah, W.; Freedman, S.D.; Martin, C.R. Breast Milk Lipids and Fatty Acids in Regulating Neonatal Intestinal Development and Protecting against Intestinal Injury. Nutrients 2020, 12, 534. [Google Scholar] [CrossRef]
- Purkiewicz, A.; Pietrzak-Fiećko, R. Changes in the Fatty Acid Profile of Lactating Women Living in Poland—A Comparison with the Fatty Acid Profile of Selected Infant Formulas. Nutrients 2024, 16, 2411. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, A.; Tounian, P.; Adel-Patient, K.; Thomas, M. Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients 2023, 15, 1231. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Lin, Y.; Li, Y.; Ren, F.; Guo, H. How far is it from infant formula to human milk? A look at the human milk oligosaccharides. Trends Food Sci. Technol. 2021, 118, 374–387. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Samolińska, W.; Kiczorowska, B.; Rusinek-Prystupa, E. The content of magnesium, calcium, sodium and potassium in infant formulas. J. Elem. 2017, 22, 339–347. [Google Scholar] [CrossRef]
- Roshdy El Safy, U.; Fathy, M.M.; Hassan, T.H.; Zakaria, M.; Kader Al Malky, M.A.; Arafa, M.; El Sayed, H.; Al Ghobashy, A.; Zaho, B.; Wahab, A.A.; et al. Effect of breastfeeding versus infant formula on iron status of infants with beta thalassemia major. Int. Breastfeed. J. 2016, 12, 18. [Google Scholar] [CrossRef]
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of Breast Milk. Rev. Assoc. Med. Bras. 2016, 62, 584–593. [Google Scholar] [CrossRef]
- Wedekind, S.I.S.; Shenker, N.S. Antiviral Properties of Human Milk. Microorganisms 2021, 9, 715. [Google Scholar] [CrossRef]
- Sindi, A.S.; Stinson, L.F.; Lai, C.T.; Gridneva, Z.; Leghi, G.E.; Netting, M.J.; Wlodek, M.E.; Muhlhausler, B.S.; Zhou, X.; Payne, M.S.; et al. Human Milk Lactoferrin and Lysozyme Concentrations Vary in Response to a Dietary Intervention. J. Nutr. Biochem. 2025, 135, 109760. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; De Ruvo, E.; Campanelli, M.; Longo, M.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. Difference in the Intestinal Microbiota between Breastfeed Infants and Infants Fed with Artificial Milk: A Systematic Review. Pathogens 2024, 13, 533. [Google Scholar] [CrossRef]
- Newman, L.; Sivaratnam, C.; Komiti, A. Attachment and Early Brain Development—Neuroprotective Interventions in Infant–Caregiver Therapy. Transl. Dev. Psychiatry 2015, 3, 28647. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.; Low, W.Y. The long-term public health benefits of breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef]
- Sameroff, A.J.; Mackenzie, M. A Quarter-Century of the Transactional Model: How Have Things Changed? Zero Three 2003, 24, 14–22. [Google Scholar]
- Tong, P.; Shidong An, I. Review of studies applying Bronfenbrenner’s bioecological theory in international and intercultural education research. Front. Psychol. 2024, 14, 1233925. [Google Scholar] [CrossRef]
- Bronfenbrenner, U.; Morris, P.A.; Belsky, J.; Canfield, R.; Dar-Ling, N.; Elder, G.H.; Hamilton, S.F.; Kohn, M.L.; Lüscher, K.; Moen, P.; et al. The Bioecological Model of Human Development 1995. In Handbook of Child Psychology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Feldman, R. Physiological Measures of Emotion from a Developmental Perspective: State of the Science: Parent–Infant Synchrony: A Biobehavioral Model of Mutual Influences in the Formation of Affiliative Bonds. Monogr. Soc. Res. Child Dev. 2012, 77, 42–51. [Google Scholar] [CrossRef]
- Fitzsimons, E.; Vera-Hernández, M. Breastfeeding and Child Development. Available online: https://www.aeaweb.org/articles?id=10.1257/app.20180385 (accessed on 4 April 2025).
- Quigley, M.A.; Hockley, C.; Carson, C.; Kelly, Y.; Renfrew, M.J.; Sacker, A. Breastfeeding Is Associated with Improved Child Cognitive Development: A Population-Based Cohort Study. J. Pediatr. 2012, 160, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wallenborn, J.T.; Levine, G.A.; dos Santos, A.C.; Grisi, S.; Brentani, A.; Fink, G. Breastfeeding, Physical Growth, and Cognitive Development. Pediatrics 2021, 147, e2020008029. [Google Scholar] [CrossRef]
- Halle, T.G.; Darling-Churchill, K.E. Review of Measures of Social and Emotional Development. J. Appl. Dev. Psychol. 2016, 45, 8–18. [Google Scholar] [CrossRef]
- Chiurazzi, M.; Cozzolino, M.; Reinelt, T.; Nguyen, T.D.; Elke Chie, S.; Natalucci, G.; Miletta, M.C. Human Milk and Brain Development in Infants. Reprod. Med. 2021, 2, 107–117. [Google Scholar] [CrossRef]
- Oliveros, E.; Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Buck, R.; Rueda, R.; Martín, M.J. Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients 2018, 10, 1519. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef]
- Steenhout, P.; Sperisen, P.; Martin, F.-P.; Sprenger, N.; Wernimont, S.; Pecquet, S.; Berger, B. Term infant formula supplemented with human milk oligosaccharides (2′fucosyllactose and lacto-N-neotetraose) shifts stool microbiota and metabolic signatures closer to that of breastfed infants. J. Pediatr. Gastroenterol. Nutr. 2016, 63, S55. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2016, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rawal, P.; Zhao, L. Sialometabolism in Brain Health and Alzheimer’s Disease. Front. Neurosci. 2021, 15, 648617. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Sialylated Oligosaccharides and Glycoconjugates of Human Milk—The Impact on Infant and Newborn Protection, Development and Well-Being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef]
- Hobbs, M.; Jahan, M.; Ghorashi, S.A.; Wang, B. Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns. Foods 2021, 10, 473. [Google Scholar] [CrossRef]
- Liu, F.; Simpson, A.B.; D’Costa, E.; Bunn, F.S.; Van Leeuwen, S.S. Sialic acid, the secret gift for the brain. Crit. Rev. Food Sci. Nutr. 2023, 63, 9875–9894. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of Gut Microbiota in Exclusively Breast-Fed and Formula-Fed Babies: A Study of 91 Term Infants. Sci. Rep. 2020, 10, 72635. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal Microbiota Composition of Breast-Fed Infants Is Correlated with Human Milk Oligosaccharides Consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef]
- Wang, B. Molecular Mechanism Underlying Sialic Acid as an Essential Nutrient for Brain Development and Cognition. Adv. Nutr. 2012, 3, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; McVeagh, P.; Petocz, P.; Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 2003, 78, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, C.H.; Choi, S.S.; Baldwin, N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit. Rev. Food Sci. Nutr. 2017, 57, 1017–1038. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Wang, L.; Jin, J.; Mi, L.; Pang, J.; Liu, Z.; Gong, J.; Sun, C.; Li, J.; Wei, W.; et al. Role Medium-Chain Fatty Acids in the Lipid Metabolism of Infants. Front. Nutr. 2022, 9, 804880. [Google Scholar] [CrossRef]
- Boquien, C.Y. Human milk: An ideal food for nutrition of preterm newborn. Front. Pediatr. 2018, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Inam, S.A.K.M. Effects of LC-PUFA (Long Chain Poly Unsaturated Fatty Acids) in Infancy. Adv. Obes. Weight Manag. Control 2017, 7, 00207. [Google Scholar] [CrossRef]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Kuratko, C.N.; Barrett, E.C.; Nelson, E.B.; Salem, N. The Relationship of Docosahexaenoic Acid (DHA) with Learning and Behavior in Healthy Children: A Review. Nutrients 2013, 5, 2777–2810. [Google Scholar] [CrossRef]
- Whitehouse, A.J.; Robinson, M.; Li, J.; Oddy, W.H. Duration of breast feeding and language ability in middle childhood. Paediatr. Perinat. Epidemiol. 2011, 25, 44–52. [Google Scholar] [CrossRef]
- Barros, F.C.; Victora, C.G.; Morris, S.S.; Halpern, R.; Horta, B.L.; Tomasi, E. Breast feeding, pacifier use and infant development at 12 months of age: A birth cohort study in Brazil. Paediatr. Perinat. Epidemiol. 1997, 11, 441–450. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wu, S.Y. The effect of exclusive breastfeeding on development and incidence of infection in infants. J. Hum. Lact. 1996, 12, 27–30. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Q.; Chu, J.; Zeng, W.; Yang, M.; Zhu, S. Effect of n−3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Doaei, S.; Bourbour, F.; Teymoori, Z.; Jafari, F.; Kalantari, N.; Torki, S.A.; Ashoori, N.; Gorgani, S.N.; Gholamalizadeh, M. The effect of omega-3 fatty acids supplementation on social and behavioral disorders of children with autism: A randomized clinical trial. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 12–18. [Google Scholar] [CrossRef]
- Hahn-Holbrook, J.; Fish, A.; Glynn, L.M. Human Milk Omega-3 Fatty Acid Composition Is Associated with Infant Temperament. Nutrients 2019, 11, 2964. [Google Scholar] [CrossRef]
- Liu, J.; Raine, A.; Venables, P.H.; Mednick, S.A. Malnutrition at age 3 years and externalizing behavior problems at ages 8, 11, and 17 years. Am. J. Psychiat. 2004, 161, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, R.; Hoek, H.W.; Susser, E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA 1999, 282, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.U.; Vyas, R.; Gagoski, B.; Vu, C.; Litt, J.; Larsen, R.J.; Kuchan, M.J.; Lasekan, J.B.; Sutton, B.P.; Grant, P.E.; et al. Maternal Dietary Intake of Omega-3 Fatty Acids Correlates Positively with Regional Brain Volumes in 1-Month-Old Term Infants. Cereb. Corex 2019, 30, 2057–2069. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Agrawal, R.; Sharma, S.; Huo, Y.X.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE 2011, 6, e28451. [Google Scholar] [CrossRef]
- Clayton, E.H.; Hanstock, T.L.; Hirneth, S.J.; Kable, C.J.; Garg, M.L.; Hazell, P.L. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids 2008, 43, 1031–1038. [Google Scholar] [CrossRef]
- Su, K.P.; Matsuoka, Y.; Pae, C.U. Omega-3 Polyunsaturated Fatty Acids in Prevention of Mood and Anxiety Disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 129–137. [Google Scholar] [CrossRef]
- Augustine, R.A.; Seymour, A.J.; Campbell, R.E.; Grattan, D.R.; Brown, C.H. Integrative neurohumoural regulation of oxytocin neurone activity in pregnancy and lactation. J. Neuroendocrinol. 2018, 30, 10.1111. [Google Scholar] [CrossRef]
- Carter, C.S. Oxytocin and love: Myths, metaphors and mysteries. Compr. Psychoneuroendocrinol. 2022, 9, 100107. [Google Scholar] [CrossRef] [PubMed]
- Krol, K.M.; Grossmann, T. Psychological Effects of Breastfeeding on Children and Mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018, 61, 977–985. [Google Scholar] [CrossRef]
- Nishizato, M.; Fujisawa, T.X.; Kosaka, H.; Tomoda, A. Developmental changes in social attention and oxytocin levels in infants and children. Sci. Rep. 2017, 7, 2540. [Google Scholar] [CrossRef] [PubMed]
- Scatliffe, N.; Casavant, S.; Vittner, D.; Cong, X. Oxytocin and Early Parent-Infant Interactions: A Systematic Review. Int. J. Nurs. Sci. 2019, 6, 445–453. [Google Scholar] [CrossRef]
- Festante, F.; Rayson, H.; Paukner, A.; Kaburu, S.S.K.; Toschi, G.; Fox, N.A.; Ferrari, P.F. Oxytocin Promotes Prosocial Behavior and Related Neural Responses in Infant Macaques at Risk for Compromised Social Development. Dev. Cogn. Neurosci. 2021, 48, 100950. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Overview of nutrients in human milk. Adv. Nutr. 2018, 9, 278–294. [Google Scholar] [CrossRef]
- Grilo, E.C.; Lima, M.S.; Cunha, L.R.; Gurgel, C.S.; Clemente, H.A.; Dimenstein, R. Effect of maternal vitamin a supplementation on retinol concentration in colostrum. J. Pediatr. 2015, 91, 81–86. [Google Scholar] [CrossRef]
- Grilo, E.C.; Medeiros, W.F.; Silva, A.G.; Gurgel, C.S.; Ramalho, H.M.; Dimenstein, R. Maternal supplementation with a megadose of vitamin a reduces colostrum level of alpha-tocopherol: A randomised controlled trial. J. Hum. Nutr. Diet. 2016, 29, 652–661. [Google Scholar] [CrossRef]
- Nagayama, J.; Noda, K.; Uchikawa, T.; Maruyama, I.; Shimomura, H.; Miyahara, M. Effect of maternal Chlorella supplementation on carotenoid concentration in breast milk at early lactation. Int. J. Food Sci. Nutr. 2014, 65, 573–576. [Google Scholar] [CrossRef]
- Ketha, H.; Thacher, T.D.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Kumar, R. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio. Bone 2018, 110, 321–325. [Google Scholar] [CrossRef]
- Oberhelman, S.S.; Meekins, M.E.; Fischer, P.R.; Lee, B.R.; Singh, R.J.; Cha, S.S.; Gardner, B.M.; Pettifor, J.M.; Croghan, I.T.; Thacher, T.D. Maternal vitamin D supplementation to improve the vitamin D status of breast-fed infants: A randomized controlled trial. Mayo Clin. Proc. 2013, 88, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.R.; Stewart, A.W.; Camargo, C.A.; Scragg, R.; Mitchell, E.A.; Ekeroma, A.; Crane, J.; Milne, T.; Rowden, J.; Horst, R.; et al. Vitamin D activity of breast milk in women randomly assigned to vitamin D3 supplementation during pregnancy. Am. J. Clin. Nutr. 2016, 103, 382–388. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Congdon, E.L.; Westerlund, A.; Algarin, C.R.; Peirano, P.D.; Gregas, M.; Lozoff, B.; Nelson, C.A. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J. Pediatr. 2012, 160, 1027–1033. [Google Scholar] [CrossRef]
- Lukowski, A.F.; Koss, M.; Burden, M.J.; Jonides, J.; Nelson, C.A.; Kaciroti, N.; Jimenez, E.; Lozoff, B. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr. Neurosci. 2010, 13, 54–70. [Google Scholar] [CrossRef]
- Hurtado, E.K.; Claussen, A.H.; Scott, K.G. Early childhood anemia and mild or moderate mental retardation. Am. J. Clin. Nutr. 1999, 69, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Navarro, B.; Matute, E.; Vásquez-Garibay, E.; Zarabozo, D. Effect of chronic iron deficiency on neuropsychological domains in infants. J. Child. Neurol. 2012, 27, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The Role of Nutrition in Children’s Neurocognitive Development, from Pregnancy through Childhood. In Prenatal and Childhood Nutrition: Evaluating the Neurocognitive Connections; Croft, C., Ed.; Apple Academic Press: Palm Bay, FL, USA, 2015; pp. 35–77. [Google Scholar] [CrossRef]
- Farias, P.M.; Marcelino, G.; Santana, L.F.; de Almeida, E.B.; Guimarães, R.d.C.A.; Pott, A.; Hiane, P.A.; Freitas, K.D.C. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules 2020, 25, 5630. [Google Scholar] [CrossRef]
- Rosen, L.A. Infant Sleep and Feeding. J. Obstet. Gynecol. Neonatal Nurs. 2008, 37, 706–714. [Google Scholar] [CrossRef]
- Abdul Jafar, N.K.; Tham, E.K.H.; Pang, W.W.; Fok, D.; Chua, M.C.; Teoh, O.H.; Goh, D.Y.T.; Shek, L.P.C.; Yap, F.; Tan, K.H.; et al. Association between breastfeeding and sleep patterns in infants and preschool children. Am. J. Clin. Nutr. 2021, 114, 1986–1996. [Google Scholar] [CrossRef]
- Brown, A.; Harries, V. Infant Sleep and Night Feeding Patterns during Later Infancy: Association with Breastfeeding Frequency, Daytime Complementary Food Intake, and Infant Weight. Breastfeed. Med. 2015, 10, 246–252. [Google Scholar] [CrossRef]
- Modak, A.; Ronghe, V.; Gomase, K.P. The Psychological Benefits of Breastfeeding: Fostering Maternal Well-Being and Child Development. Cureus 2023, 15, e46730. [Google Scholar] [CrossRef]
- Zanetti, N.; D’Souza, L.; Tchernegovski, P.; Blunden, S. Parents’ Perceptions of the Quality of Infant Sleep Behaviours and Practices: A Qualitative Systematic Review. Infant. Child. Dev. 2023, 32, e2369. [Google Scholar] [CrossRef]
- Barry, E.S. Co-sleeping as a proximal context for infant development: The importance of physical touch. Infant. Behav. Dev. 2019, 57, 101385. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.S. What Is “Normal” Infant Sleep? Why We Still Do Not Know. Psychol. Rep. 2021, 124, 651–692. [Google Scholar] [CrossRef]
- Smith, J.P.; Forrester, R.I. Association between Breastfeeding and New Mothers’ Sleep: A Unique Australian Time Use Study. Int. Breastfeed. J. 2021, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, D.A.; O’Brien, L.M.; Pitts, D.S.; Sagong, C.; Arnett, L.K.; Harb, N.C.; Cheng, P.; Drake, C.L. Mother-to-Infant Bonding Is Associated with Maternal Insomnia, Snoring, Cognitive Arousal, and Infant Sleep Problems and Colic. Behav. Sleep Med. 2022, 20, 393–409. [Google Scholar] [CrossRef]
- Hong, Y.R.; Park, J.S. Impact of Attachment, Temperament and Parenting on Human Development. Korean J. Pediatr. 2012, 55, 449–454. [Google Scholar] [CrossRef]
- Schneider, N.; Mutungi, G.; Cubero, J. Diet and Nutrients in the Modulation of Infant Sleep: A Review of the Literature. Nutr. Neurosci. 2018, 21, 151–161. [Google Scholar] [CrossRef]
- Koura, H. Myths about Breastfeeding. Al-Azhar Assiut Med. J. 2019, 17, 109. [Google Scholar] [CrossRef]
- Purkiewicz, A.; Stasiewicz, M.; Nowakowski, J.J.; Pietrzak-Fiećko, R. The Influence of the Lactation Period and the Type of Milk on the Content of Amino Acids and Minerals in Human Milk and Infant Formulas. Foods 2023, 12, 3674. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2021, 22, 10181. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Xu, Y.; Zhang, W.; Zhu, M.; Wang, X.; Huang, J.; Zhuang, Y.; Lan, H.; Chen, X.; Guo, D. Concentration and Distribution of Sialic Acid in Human Milk and Its Correlation with Dietary Intake. Front. Nutr. 2022, 9, 929661. [Google Scholar] [CrossRef]
- Gombert, M.; Codoñer-Franch, P. Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System. Int. J. Mol. Sci. 2021, 22, 6809. [Google Scholar] [CrossRef]
- Doan, T.; Gay, C.L.; Kennedy, H.P.; Newman, J.; Lee, K.A. Nighttime breastfeeding behavior is associated with more nocturnal sleep among first-time mothers at one month postpartum. J. Clin. Sleep. Med. 2014, 10, 313–319. [Google Scholar] [CrossRef]
- Yate, Z.M. A Qualitative Study on Negative Emotions Triggered by Breastfeeding; Describing the Phenomenon of Breastfeeding/Nursing Aversion and Agitation in Breastfeeding Mothers. Iran. J. Nurs. Midwifery Res. 2017, 22, 449–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chęcińska-Maciejewska, Z.; Ciborek, A.; Krauss, H.; Gibas-Dorna, M. Advantages of breastfeeding for the mother-infant dyad. J. Health Inequalities 2024, 10, 64–71. [Google Scholar] [CrossRef]
- Bengouh, T.; Dawson, S.; Cheng, H.-L.; McFadden, A.l.; Gavine, A.; Rees, R.; Sacks, E.; Hannes, K. Factors that influence women’s engagement with breastfeeding support: A qualitative evidence synthesis. Matern. Child. Nutr. 2022, 18, e13405. [Google Scholar] [CrossRef]
- Lee, E.N. Effects of Parity and Breastfeeding Duration on Bone Density in Postmenopausal Women. Asian Nurs. Res. 2019, 13, 161–167. [Google Scholar] [CrossRef]
- Bjørnerem, Å.; Ahmed, L.A.; Jørgensen, L.; Størmer, J.; Joakimsen, R.M. Breastfeeding protects against hip fracture in postmenopausal women: The Tromsø study. J. Bone Miner. Res. 2021, 26, 2843–2850. [Google Scholar] [CrossRef]
- Schwarz, E.B.; Ray, R.M.; Stuebe, A.M.; Allison, M.A.; Ness, R.B.; Freiberg, M.S.; Cauley, J.A. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet. Gynecol. 2009, 113, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Leurer, D.; Misskey, E. The Psychosocial and Emotional Experience of Breastfeeding: Reflections of Mothers. Glob. Qual. Nurs. Res. 2015, 2, 2333393615611654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Sehmawy, A.A.; Elaziz, S.Y.A.; Elwahed, R.M.A.; Elsheikh, A.A. Skin-to-skin contact and its effect on mothers’ post-partum psychological distress and their full-term neonate in Egypt. J. Tropo Pediatr. 2023, 69, fmad020. [Google Scholar] [CrossRef] [PubMed]
- Mangesi, L.; Zakarija-Grkovic, I. Treatments for Breast Engorgement during Lactation. Cochrane Database Syst. Rev. 2016, 2016, CD006946. [Google Scholar] [CrossRef] [PubMed]
- Zakarija-Grkovic, I.; Stewart, F. Treatments for Breast Engorgement during Lactation. Cochrane Database Syst. Rev. 2020, 2020, CD006946. [Google Scholar] [CrossRef]
- Gresh, A.; Robinson, K.; Thornton, C.P.; Plesko, C.M. Caring for Women Experiencing Breast Engorgement: A Case Report. J. Midwifery Women's Health 2019, 64, 763–768. [Google Scholar] [CrossRef]
- Cox, K.N.; Giglia, R.C.; Binns, C.W. The influence of infant feeding attitudes on breastfeeding duration: Evidence from a cohort study in rural Western Australia. Int. Breastfeed. J. 2015, 10, 25. [Google Scholar] [CrossRef]
- Olejnik, A.; Adamczewska, A.; Kossakowska, K. Factors Affecting Attitude towards Breastfeeding in Public: A Cross-Sectional Web-Based Study on Polish Women. J. Public Health 2022, 30, 263–272. [Google Scholar] [CrossRef]
- Zaikman, Y.; Houlihan, A.E. It’s Just a Breast: An Examination of the Effects of Sexualization, Sexism, and Breastfeeding Familiarity on Evaluations of Public Breastfeeding. BMC Pregnancy Childbirth 2022, 22, 122. [Google Scholar] [CrossRef]
- Carlin, R.F.; Mathews, A.; Oden, R.; Moon, R.Y. The Influence of Social Networks and Norms on Breastfeeding in African American and Caucasian Mothers: A Qualitative Study. Breastfeed. Med. 2019, 14, 640–647. [Google Scholar] [CrossRef]
- Kent, G. Comparing Breastfeeding and Feeding with Infant Formula. World Nutr. 2019, 10, 100–118. [Google Scholar] [CrossRef]
- Franzoi, I.G.; Sauta, M.D.; De Luca, A.; Granieri, A. Returning to Work after Maternity Leave: A Systematic Literature Review. Arch. Womens Ment. Health 2024, 27, 737–749. [Google Scholar] [CrossRef]
- Chen, J.; Xin, T.; Gaoshan, J.; Li, Q.; Zoi, K.; Tan, S.; Cheng, Y.; Liu, Y.; Chen, J.; Wang, H.; et al. The association between work related factors and breastfeeding practices among Chinese working mothers: A mixed-method approach. Int. Breastfeed. J. 2019, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.C.; Taylor, Y.J.; Basquin, C.; Venkitsubramanian, K. Impact of Key Workplace Breastfeeding Support Characteristics on Job Satisfaction, Breastfeeding Duration, and Exclusive Breastfeeding among Health Care Employees. Breastfeed. Med. 2019, 14, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Surówka, J.; Humaj-Grysztar, M.; Madetko, R. The Midwife’s Role in Lactation Care in Poland. Nurs. Probl. 2021, 29, 26–30. [Google Scholar] [CrossRef]
- Wdowiak, A.; Bakalczuk, G.; Dadej, E.; Wdowiak, A.; Filip, M.; Wdowiak, E.; Lewicka, M.; Sulima, M. Birthing School—Patient’s Assessment on the Influence of Classes on Parturition. Pielęgniarstwo XXI Wieku 2016, 15, 12–17. [Google Scholar] [CrossRef]
- Kehinde, J.; O’Donnell, C.; Grealish, A. The Effectiveness of Prenatal Breastfeeding Education on Breastfeeding Uptake Postpartum: A Systematic Review. Midwifery 2023, 118, 103579. [Google Scholar] [CrossRef]
- De Villepin, B.P.; Barasinski, C.; Rigourd, V. Initiating and Supporting Breastfeeding: Guidelines for Interventions during the Perinatal Period from the French National College of Midwives. J. Midwifery Womens Health 2022, 67, S56–S73. [Google Scholar] [CrossRef]
- Fallon, V.M.; Harrold, J.A.; Chisholm, A. The Impact of the UK Baby Friendly Initiative on Maternal and Infant Health Outcomes: A Mixed-Methods Systematic Review. Matern. Child. Nutr. 2019, 15, e12778. [Google Scholar] [CrossRef]
- McLelland, G.; Hall, H.; Gilmour, C.; Cant, R. Support Needs of Breast-Feeding Women: Views of Australian Midwives and Health Nurses. Midwifery 2015, 31, 1–6. [Google Scholar] [CrossRef]
- Reinsma, K.; Bolima, N.; Fonteh, F.; Okwen, P.; Yota, D.; Motgomery, S. Incorporating cultural beliefs in promoting exclusive breastfeeding. Afr. J. Midwifery Womens Health 2016, 6, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sosseh, S.A.L.; Barrow, A.; Lu, Z.J. Cultural beliefs, attitudes and perceptions of lactating mothers on exclusive breastfeeding in The Gambia: An ethnographic study. BMC Womens Health 2023, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Wallenborn, J.T.; Valera, C.B.; Kounnavong, S.; Sayasone, S.; Odermatt, P.; Fink, G. Urban-Rural Gaps in Breastfeeding Practices: Evidence From Lao People’s Democratic Republic. Int. J. Public Health 2021, 66, 1604062. [Google Scholar] [CrossRef]
- Grandahl, M.; Stern, J.; Funkquist, E.-L. Longer shared parental leave is associated with longer duration of breastfeeding: A cross-sectional study among Swedish mothers and their partners. BMC Pediatr. 2020, 20, 159. [Google Scholar] [CrossRef]
- Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s Call to Action to Support Breastfeeding. Office of the Surgeon General. 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK52682/ (accessed on 4 April 2025).
- Morris, C.; De La Fuente, G.A.Z.; Williams, C.E.T.; Hirst, C. UK Views toward Breastfeeding in Public: An Analysis of the Public’s Response to the Claridge’s Incident. J. Hum. Lact. 2016, 32, 472–480. [Google Scholar] [CrossRef]
- Amzat, J.; Aminu, K.; Matankari, B.; Ismail, A.; Almu, B.; Kanmodi, K.K. Sociocultural context of exclusive breastfeeding in Africa: A narrative review. Health Sci. Rep. 2024, 7, e2115. [Google Scholar] [CrossRef]
- Mokhtar, N.; Sulaiman, F.S.; Kusumawardani, A.; Hidayat, T. Islamic Perspective on Importance of Breastfeeding: Two Case Reports. IIUM Med. J. Malays. 2018, 17, 67–70. [Google Scholar] [CrossRef]
- Valappil, H.C.; Jayalakshmi, R.; Sewor, C. Intersectional inequalities in exclusive breastfeeding practices in India: Analysis of national family health survey-4. Int. Breastfeed. J. 2023, 18, 44. [Google Scholar] [CrossRef]
- Farhadi, R. Spiritual Aspects of Breastfeeding: A Narrative Review. J. Pediatr. Rev. 2020, 8, 229–236. [Google Scholar] [CrossRef]
- Tarabeih, M.; Sabbah, M.; Yahya, O.; Bisharat, S.; Awawdi, K. Factors Contributing to Breastfeeding Cessation Among Arab Women in Israel. Nutrients 2025, 17, 735. [Google Scholar] [CrossRef]
- Steurer, L.M. Maternity Leave Length and Workplace Policies’ Impact on the Sustainment of Breastfeeding: Global Perspectives. Public Health Nurs. 2017, 34, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Narayan, A.; Zhang, S.; Wang, L.; Zhu, Y.; Yang, W.; Cheng, Y.; Zeng, L.; Chang, S. How the marketing practices of commercial milk formula companies impact infant breastfeeding practices in China. BMJ Glob. Health 2023, 8, e012803. [Google Scholar] [CrossRef]
- Chang, P.C.; Li, S.F.; Yang, H.Y.; Wang, L.C.; Weng, C.Y.; Chen, K.F.; Chen, W.; Fan, S.Y. Factors associated with cessation of exclusive breastfeeding at 1 and 2 months postpartum in Taiwan. Int. Breastfeed. J. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.; Palmér, L. Cessation of Breastfeeding in Mothers of Preterm Infants—A Mixed Method Study. PLoS ONE 2020, 15, e023318. [Google Scholar] [CrossRef]
- Lewallen, L.P.; Dick, M.J.; Flowers, J.; Powell, W.; Zickefoose, K.T.; Wall, Y.G.; Price, Z.M. Breastfeeding Support and Early Cessation. J. Obstet. Gynecol. Neonatal Nurs. 2006, 35, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chen, M.; Yin, Y.; Wu, L.; Gao, L. Why Chinese Mothers Stop Breastfeeding: Mothers’ Self-Reported Reasons for Stopping during the First Six Months. J. Child Health Care 2017, 21, 353–363. [Google Scholar] [CrossRef]
- Mcfadden, A.; Gavine, A.; Renfrew, M.J.; Wade, A.; Buchanan, P.; Taylor, J.L.; Veitch, E.; Rennie, A.M.; Crowther, S.A.; Neiman, S.; et al. Support for Healthy Breastfeeding Mothers with Healthy Term Babies. Cochrane Database Syst. Rev. 2017, 2, CD001141. [Google Scholar] [CrossRef]
- Nnebe-Agumadu, U.H.; Racine, E.F.; Laditka, S.B.; Coffman, M.J. Associations between Perceived Value of Exclusive Breastfeeding among Pregnant Women in the United States and Exclusive Breastfeeding to Three and Six Months Postpartum: A Prospective Study. Int. Breastfeed. J. 2016, 11, 8. [Google Scholar] [CrossRef][Green Version]
- Zitkute, V.; Snieckuviene, V.; Zakareviciene, J.; Pestenyte, A.; Jakaite, V.; Ramasauskaite, D. Reasons for Breastfeeding Cessation in the First Year after Childbirth in Lithuania: A Prospective Cohort Study. Medicina 2020, 56, 226. [Google Scholar] [CrossRef]
- Girard, L.C.; Côté, S.M.; De Lauzon-Guillain, B.; Dubois, L.; Falissard, B.; Forhan, A.; Doyle, O.; Bernard, J.Y.; Heude, B.; Saurel-Cubizolles, M.J.; et al. Factors Associated with Breastfeeding Initiation: A Comparison between France and French-Speaking Canada. PLoS ONE 2016, 11, e0166946. [Google Scholar] [CrossRef]
- Nakao, Y.; Moji, K.; Honda, S.; Oishi, K. Initiation of Breastfeeding within 120 Minutes after Birth Is Associated with Breastfeeding at Four Months among Japanese Women: A Self-Administered Questionnaire Survey. Int. Breastfeed. J. 2008, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Hofvander, Y. Breastfeeding and the Baby Friendly Hospitals Initiative (BFHI): Organization, Response and Outcome in Sweden and Other Countries. Acta Paediatr. Int. J. Paediatr. 2005, 94, 1012–1016. [Google Scholar] [CrossRef]
- Newby, R.M.; Davies, P.S.W. Why do women stop breast-feeding? Results from a contemporary prospective study in a cohort of Australian women. Eur. J. Clin. Nutr. 2016, 70, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Lee, M. An exploration of experiences of mothers following a baby-led weaning style: Developmental readiness for complementary foods. Matern. Child Nutr. 2013, 9, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Utami, A.F.; Wanda, D.; Hayati, H.; Fowler, C. Becoming an Independent Feeder: Infant’s Transition in Solid Food Introduction through Baby-Led Weaning. BMC Proc. 2020, 14, 18. [Google Scholar] [CrossRef]
- Pearce, J.; Rundle, R. Baby-Led Weaning: A Thematic Analysis of Comments Made by Parents Using Online Parenting Forums. J. Hum. Nutr. Diet. 2023, 36, 772–786. [Google Scholar] [CrossRef]
- Liu, J.; Leung, P.; Yang, A. Breastfeeding and Active Bonding Protects against Children’s Internalizing Behavior Problems. Nutrients 2013, 6, 76–89. [Google Scholar] [CrossRef]
- Funk, L.; Scheidecker, G.; Chapin, B.L.; Schmidt, W.J.; Ouardani, C.E.; Chaudhary, N. Feeding. Bonding, and the Formation of Social Relationships. In Feeding, Bonding, and the Formation of Social Relationships; Keith, K., Ed.; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Gavine, A.; Shinwell, S.C.; Buchanan, P.; Farre, A.; Wade, A.; Lynn, F.; Marshall, J.; Cumming, S.E.; Dare, S.; McFadden, A. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst. Rev. 2022, 2022, CD001141. [Google Scholar] [CrossRef]
- Kelkay, B.; Kindalem, E.; Tagele, A.; Moges, Y. Cessation of Exclusive Breastfeeding and Determining Factors at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Int. J. Pediatr. 2020, 2020, 8431953. [Google Scholar] [CrossRef]
- Rinker, B.; Veneracion, M.; Walsh, C.P. The Effect of Breastfeeding on Breast Aesthetics. Aesthet. Surg. J. 2008, 28, 534–537. [Google Scholar] [CrossRef]
- Broers, B.; Wawrzyniak, J.; Kubiec, W. Women’s body image and breastfeeding. Med. Sci. Pulse 2017, 11, 31–34. [Google Scholar] [CrossRef]
- Sheehan, A.; Gribble, K.; Schmied, V. It’s okay to breastfeed in public but…. Int. Breastfeed. J. 2019, 14, 24. [Google Scholar] [CrossRef] [PubMed]
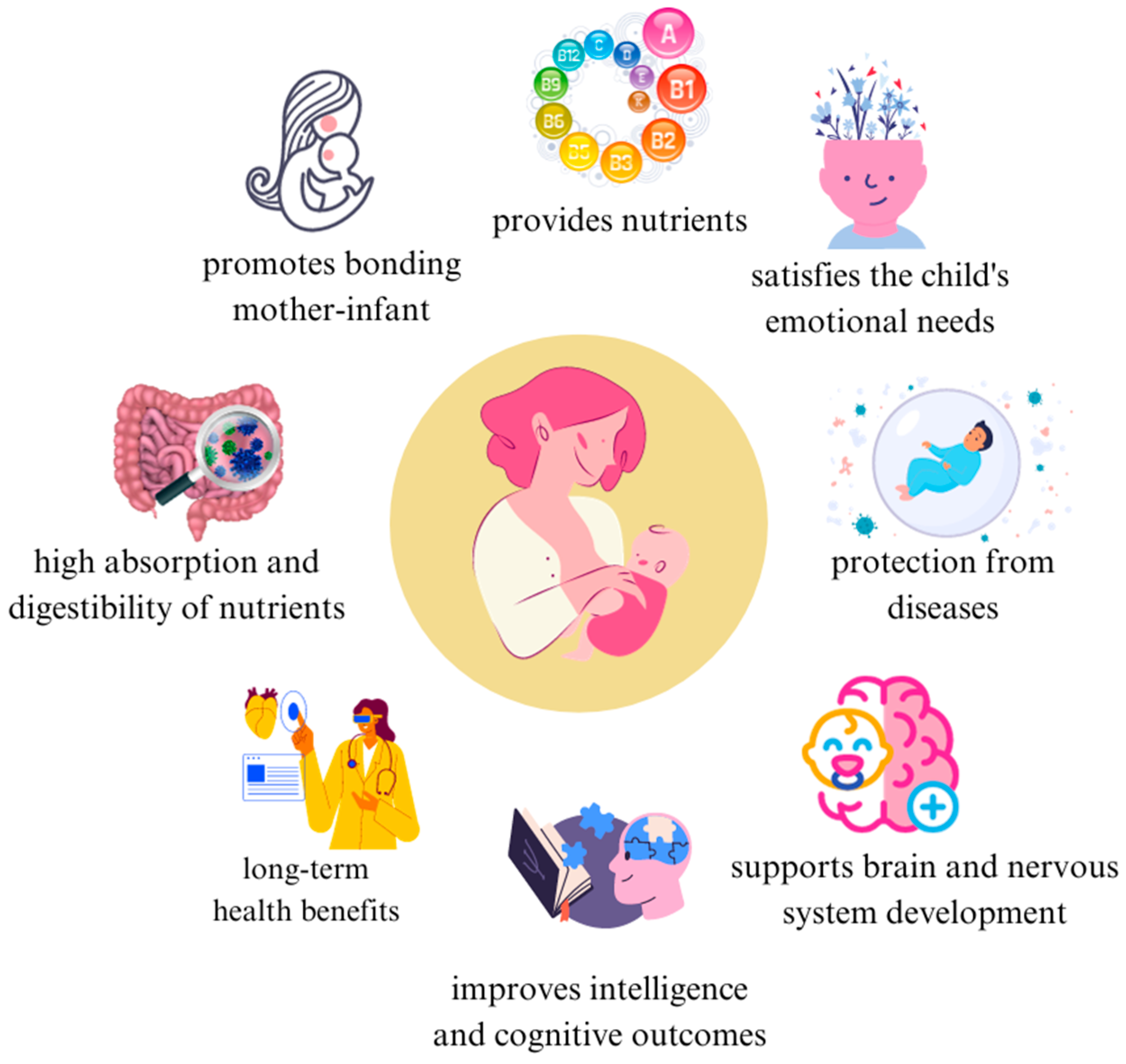

| Functions | Benefits |
|---|---|
| Nutritional | Gold standard in infant nutrition (unique composition) |
| High digestibility and bioavailability of nutrients | |
| Abundance of bioactive ingredients | |
| Cognitive | Promotes brain and nervous system development |
| Promotes concentration and learning processes | |
| Strengthens neural connections, which promotes better development of language and social skills | |
| Emotional | Forming and strengthening the mother-child bond |
| Strengthening the baby’s sense of security | |
| Baby’s sleep regulation | |
| Secreted oxytocin reduces stress in the baby | |
| Immunological | Strengthens the immune system |
| Reduced risk of allergies and infections | |
| Shaping the baby’s microbiome | |
| Increased resistance to disease in later life | |
| Mental | Lowering the risk of postpartum depression |
| Shaping the mother-child relationship from the first moments of life | |
| More efficient return of mothers to hormonal balance after childbirth | |
| Facilitated return to pre-pregnancy body shape increasing women’s acceptance and self-confidence | |
| Motor | Development of baby’s motor skills-sucking, grasping |
| Development of baby’s motor coordination | |
| Social | Development of the child’s social skills |
| Promoting family ties | |
| Building the child’s ability to cooperate and communicate | |
| Economic | Eliminate costs associated with purchasing infant formulas and feeding accessories |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purkiewicz, A.; Regin, K.J.; Mumtaz, W.; Pietrzak-Fiećko, R. Breastfeeding: The Multifaceted Impact on Child Development and Maternal Well-Being. Nutrients 2025, 17, 1326. https://doi.org/10.3390/nu17081326
Purkiewicz A, Regin KJ, Mumtaz W, Pietrzak-Fiećko R. Breastfeeding: The Multifaceted Impact on Child Development and Maternal Well-Being. Nutrients. 2025; 17(8):1326. https://doi.org/10.3390/nu17081326
Chicago/Turabian StylePurkiewicz, Aleksandra, Kamila J. Regin, Wajeeha Mumtaz, and Renata Pietrzak-Fiećko. 2025. "Breastfeeding: The Multifaceted Impact on Child Development and Maternal Well-Being" Nutrients 17, no. 8: 1326. https://doi.org/10.3390/nu17081326
APA StylePurkiewicz, A., Regin, K. J., Mumtaz, W., & Pietrzak-Fiećko, R. (2025). Breastfeeding: The Multifaceted Impact on Child Development and Maternal Well-Being. Nutrients, 17(8), 1326. https://doi.org/10.3390/nu17081326





