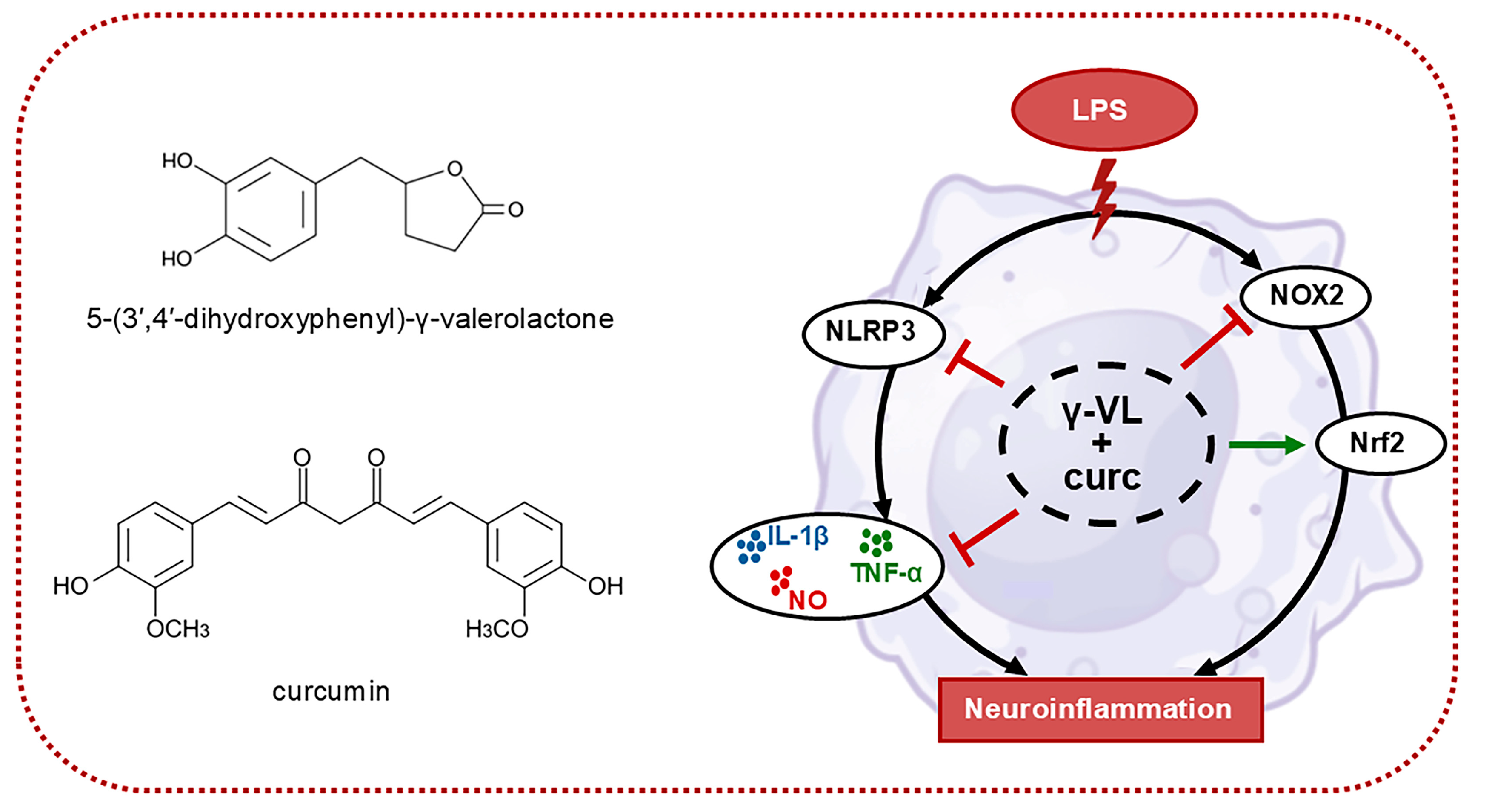
A Combination of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone and Curcumin Synergistically Reduces Neuroinflammation in Cortical Microglia by Targeting the NLRP3 Inflammasome and the NOX2/Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Cultures
2.3. Cell Treatment
2.4. Cell Viability Assay
2.5. Cytokine Determination
2.6. Nitric Oxide Quantification Assay
2.7. Real-Time Polymerase Chain Reaction (Real-Time PCR)
2.8. Synergistic Effect Analysis
2.9. Statistical Analysis
3. Results
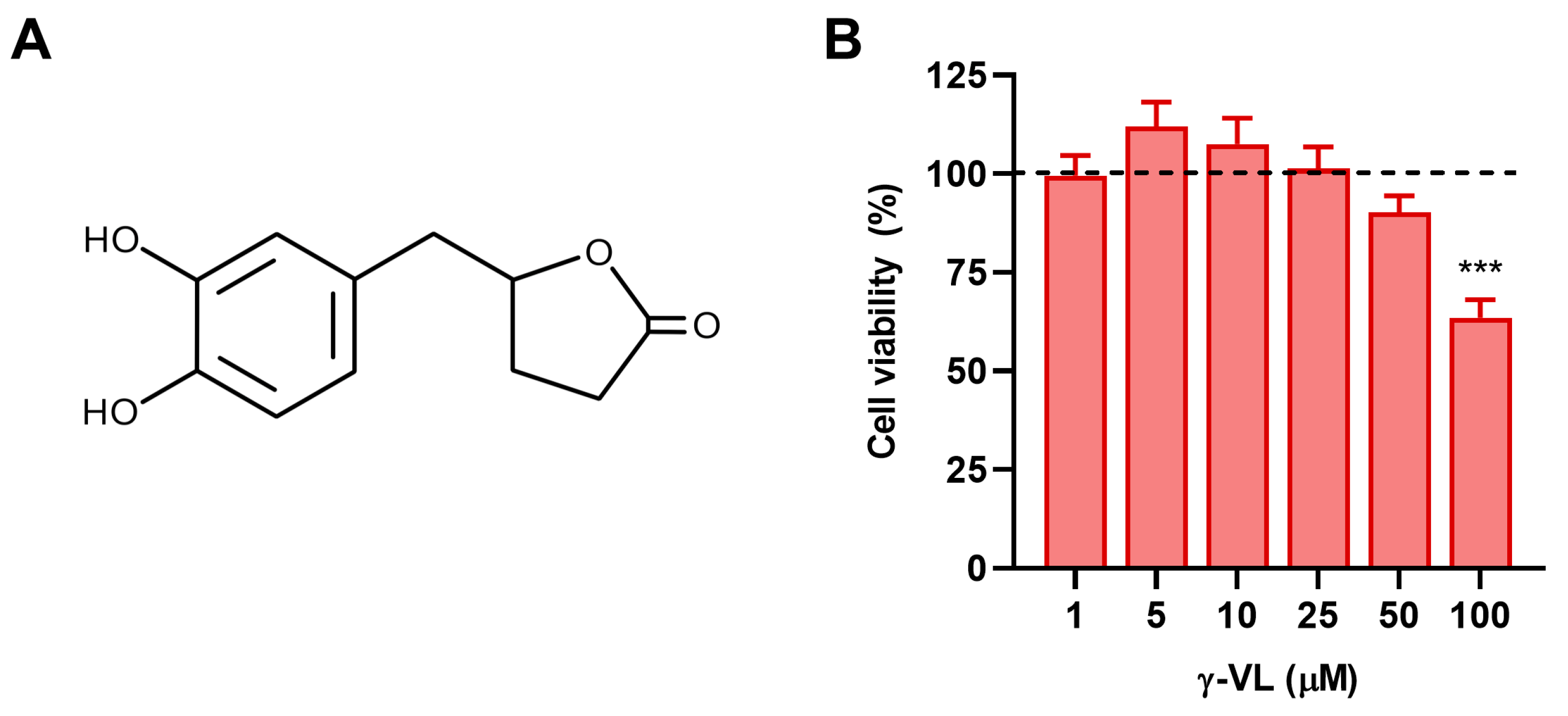
3.1. Evaluation of Non-Cytotoxic Concentrations of γ-VL in Microglial Cells
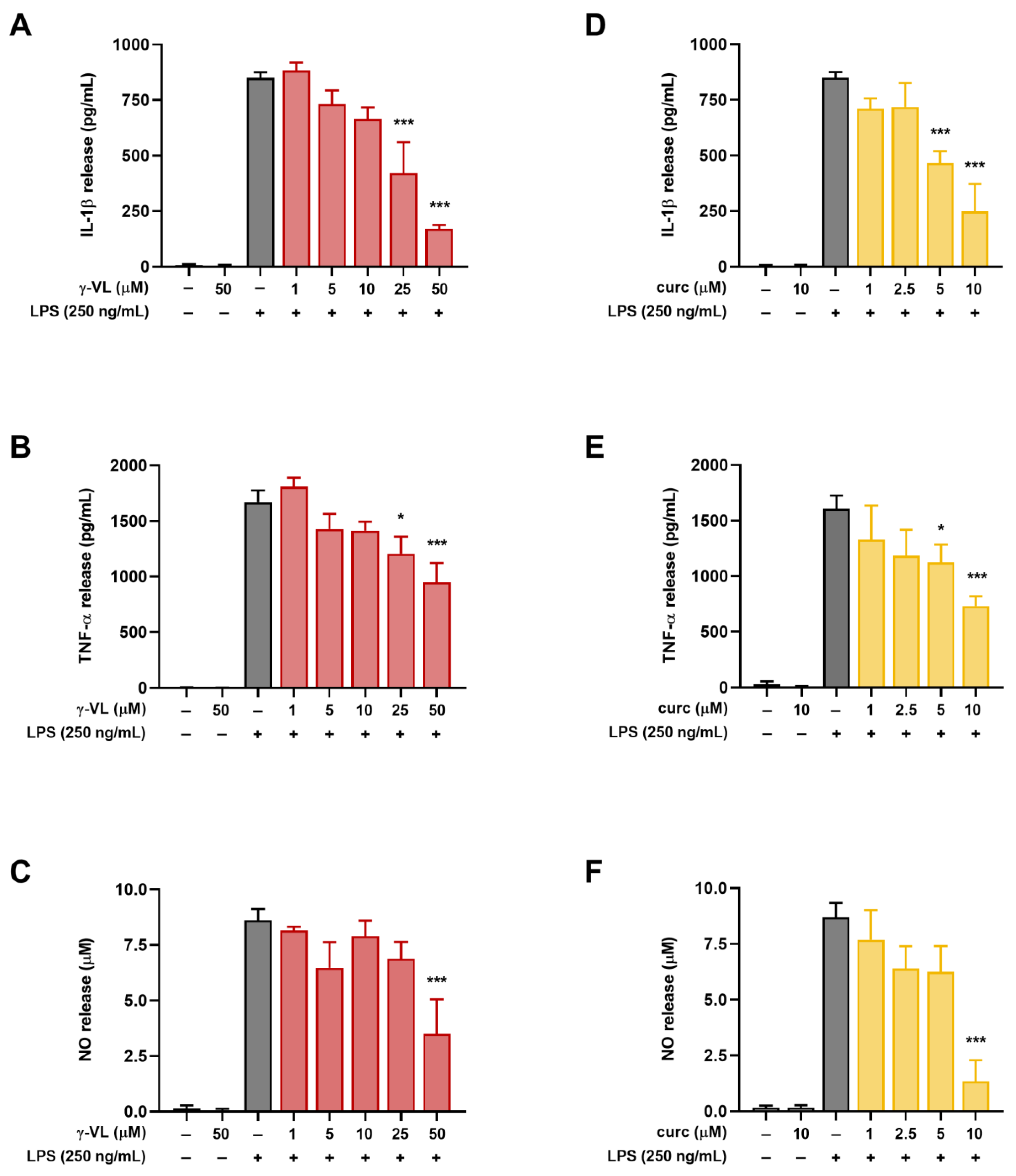
3.2. γ-VL and Curcumin Reduced the Release of Pro-Inflammatory Factors from LPS-Stimulated Microglial Cells
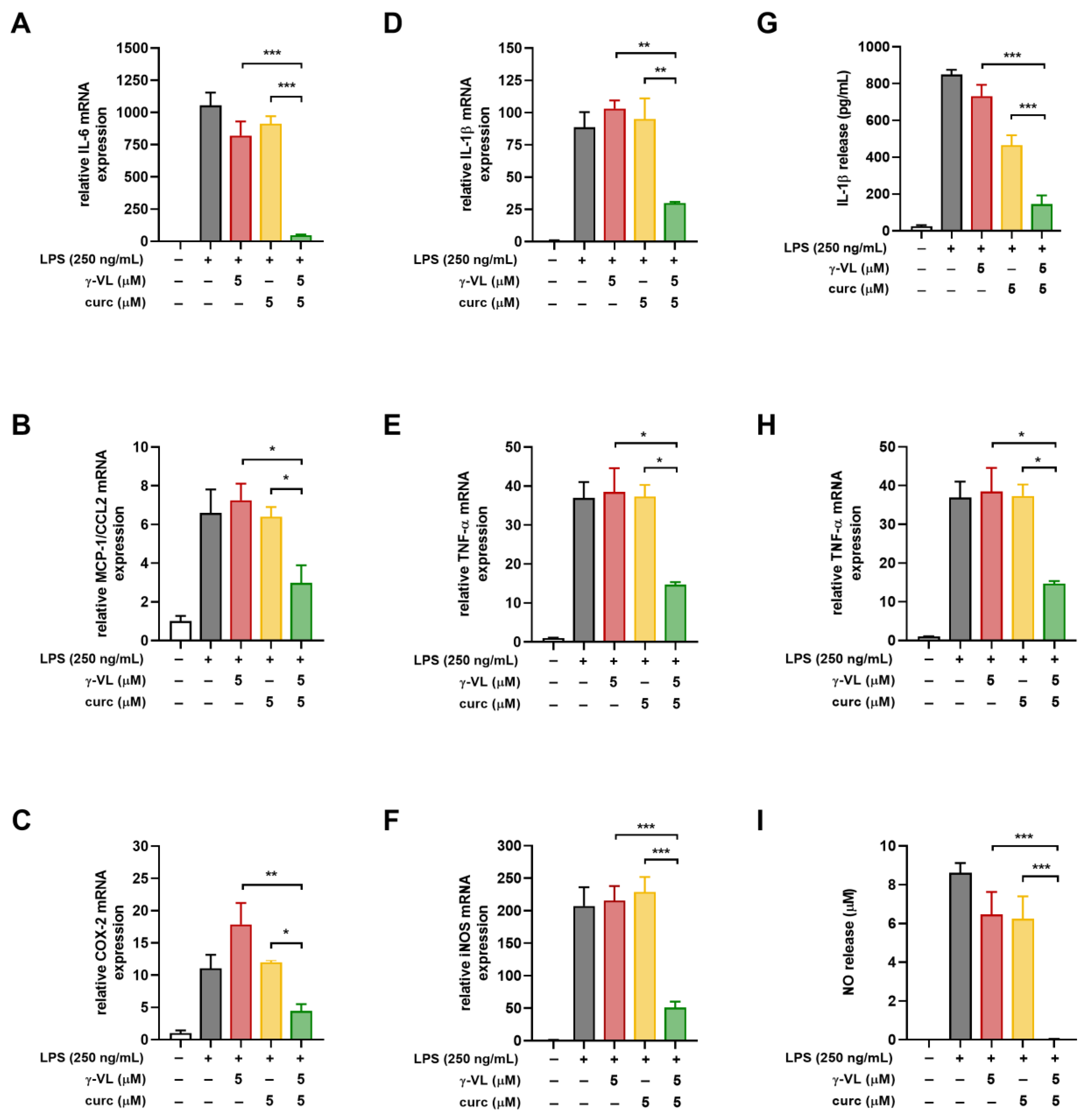
3.3. The Combined Treatment with γ-VL and Curcumin Exhibited a Synergistic Inhibition of the Pro-Inflammatory Response in Microglia
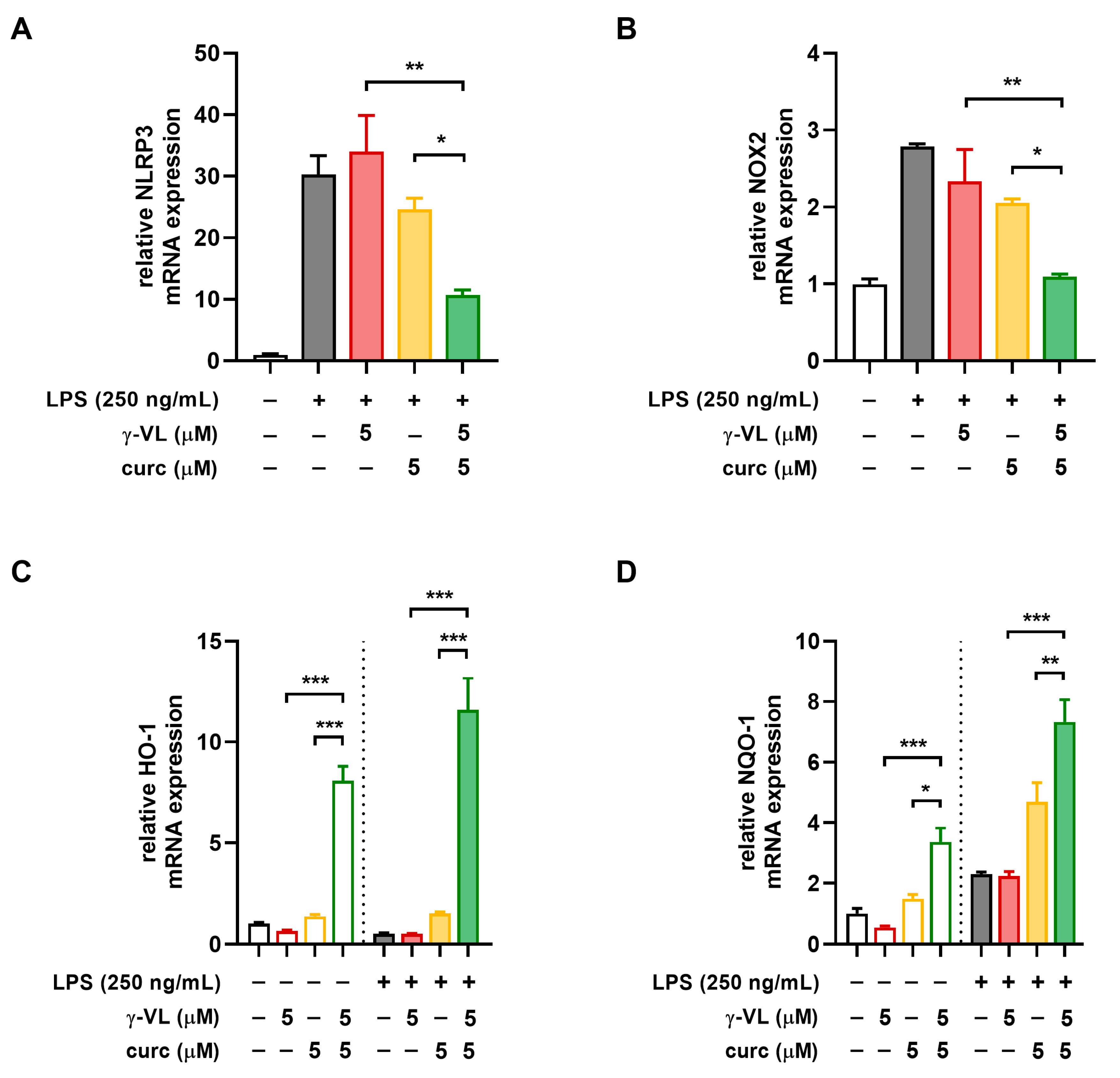
3.4. The Combination of γ-VL and Curcumin Synergistically Reduced NLRP3 Expression and Restored the NOX2/Nrf2 Balance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Combination Index |
| CNS | Central nervous system |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| HO-1 | Heme-oxygenase 1 |
| HSA | Highest single agent |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NO | Nitric oxide |
| NQO-1 | NAD(P)H quinone dehydrogenase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PCAs | Proanthocyanidins |
| ROS | Reactive oxygen species |
| SEM | Standard error of the mean |
| TNF | Tumor necrosis factor |
| ZIP | Zero interaction potency |
| γ-VL | 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone |
References
- Cova, I.; Markova, A.; Campini, I.; Grande, G.; Mariani, C.; Pomati, S. Worldwide trends in the prevalence of dementia. J. Neurol. Sci. 2017, 379, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Prata, C.; Freschi, M.; Barbalace, M.C.; Hrelia, S. Mechanisms underlying neurodegenerative disorders and potential neuroprotective activity of agrifood by-products. Antioxidants 2022, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as dynamic cellular mediators of brain function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and neuroinflammation: Crucial pathological mechanisms in traumatic brain injury-induced neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Mele, L.; Carobbio, S.; Brindani, N.; Curti, C.; Rodriguez-Cuenca, S.; Bidault, G.; Mena, P.; Zanotti, I.; Vacca, M.; Vidal-Puig, A.; et al. Phenyl-γ-valerolactones, flavan-3-ol colonic metabolites, protect brown adipocytes from oxidative stress without affecting their differentiation or function. Mol. Nutr. Food Res. 2017, 61, 1700074. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Aura, A.M.; Hollman, P.C.H.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, L.; Husain, I.; Wang, Y.H.; Kothapalli, H.B.; Gado, F.; Baron, G.; Manzi, S.; Morazzoni, P.; Aldini, G.; Khan, I.A. Pre-ADMET studies of 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone, the bioactive intestinal metabolite of proanthocyanidins. Arch. Pharm. (Weinheim) 2024, 358, e2400575. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andrés-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267. [Google Scholar] [CrossRef]
- Cecarini, V.; Cuccioloni, M.; Zheng, Y.; Bonfili, L.; Gong, C.; Angeletti, M.; Mena, P.; Del Rio, D.; Eleuteri, A.M. Flavan-3-ol microbial metabolites modulate proteolysis in neuronal cells reducing amyloid-beta (1-42) levels. Mol. Nutr. Food Res. 2021, 65, e2100380. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular antioxidant effect of an Aronia extract and its polyphenolic fractions enriched in proanthocyanidins, phenolic acids, and anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Della Vedova, L.; Gado, F.; Quagliano, O.; Casati, S.; Tosi, N.; Bresciani, L.; Del Rio, D.; Roda, G.; et al. Unraveling the parahormetic mechanism underlying the health-protecting effects of grapeseed procyanidins. Redox Biol. 2024, 69, 102981. [Google Scholar]
- Mercanti, G.; Ragazzi, E.; Toffano, G.; Giusti, P.; Zusso, M. Phosphatidylserine and curcumin act synergistically to down-regulate release of interleukin-1β from lipopolysaccharide-stimulated cortical primary microglial cells. CNS Neurol. Disord. Drug Targets 2014, 13, 792–800. [Google Scholar] [CrossRef]
- Zusso, M.; Mercanti, G.; Belluti, F.; Di Martino, R.M.C.; Pagetta, A.; Marinelli, C.; Brun, P.; Ragazzi, E.; Lo, R.; Stifani, S.; et al. Phenolic 1,3-diketones attenuate lipopolysaccharide-induced inflammatory response by an alternative magnesium-mediated mechanism. Br. J. Pharmacol. 2017, 174, 1090–1103. [Google Scholar] [CrossRef]
- Sorrenti, V.; Contarini, G.; Sut, S.; Dall’Acqua, S.; Confortin, F.; Pagetta, A.; Giusti, P.; Zusso, M. Curcumin prevents acute neuroinflammation and long-term memory impairment induced by systemic lipopolysaccharide in mice. Front. Pharmacol. 2018, 9, 183. [Google Scholar]
- Bisceglia, F.; Seghetti, F.; Serra, M.; Zusso, M.; Gervasoni, S.; Verga, L.; Vistoli, G.; Lanni, C.; Catanzaro, M.; De Lorenzi, E.; et al. Prenylated curcumin analogues as multipotent tools to tackle Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 1420–1433. [Google Scholar] [CrossRef]
- De Lorenzi, E.; Franceschini, D.; Contardi, C.; Di Martino, R.M.C.; Seghetti, F.; Serra, M.; Bisceglia, F.; Pagetta, A.; Zusso, M.; Belluti, F. Modulation of amyloid β-induced microglia activation and neuronal cell death by curcumin and analogues. Int. J. Mol. Sci. 2022, 23, 4381. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, E.; Seghetti, F.; Tarozzi, A.; Pruccoli, L.; Contardi, C.; Serra, M.; Bisi, A.; Gobbi, S.; Vistoli, G.; Gervasoni, S.; et al. Targeting the multifaceted neurotoxicity of Alzheimer’s disease by tailored functionalisation of the curcumin scaffold. Eur. J. Med. Chem. 2023, 252, 115297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Wu, C.; Du, Y.; Li, P.; Wang, M.; Wang, X.; Wang, Y.; Hao, Y.; Wang, T.; et al. Network pharmacology based research on the combination mechanism between escin and low dose glucocorticoids in anti-rheumatoid arthritis. Front. Pharmacol. 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Skaper, S.D.; Facci, L. Culture of neonatal rodent microglia, astrocytes, and oligodendrocytes from the cortex, spinal cord, and cerebellum. Methods Mol. Biol. 2018, 1727, 49–61. [Google Scholar]
- Marinelli, C.; Di Liddo, R.; Facci, L.; Bertalot, T.; Conconi, M.T.; Zusso, M.; Skaper, S.D.; Giusti, P. Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes. J. Neuroinflammation 2015, 12, 244. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and citotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mor, M.; Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Silva, C.; Vacondio, F.; Rivara, M.; Plazzi, P.V.; Franceschini, D.; et al. Antioxidant and cytoprotective activity of indole derivatives related to melatonin. Adv. Exp. Med. Biol. 2003, 527, 567–575. [Google Scholar]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward better interpretation and annotation of drug combination screening datasets. Genomics Proteomics Bioinformatics 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100110. [Google Scholar] [CrossRef]
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for drug synergy in complex dose-response landscapes using an interaction potency model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Revisiting the isobole and related quantitative methods for assessing drug synergism. J. Pharmacol. Exp. Ther. 2012, 342, 2–8. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Si, H. Synergistic anti-inflammatory effects and mechanisms of the combination of resveratrol and curcumin in human vascular endothelial cells and rodent aorta. J. Nutr. Biochem. 2022, 108, 109083. [Google Scholar] [CrossRef]
- Maroni, L.; Agostinelli, L.; Saccomanno, S.; Pinto, C.; Giordano, D.M.; Rychlicki, C.; De Minicis, S.; Trozzi, L.; Banales, J.M.; Melum, E.; et al. Nlrp3 activation induces IL-18 synthesis and affects the epithelial barrier function in reactive cholangiocytes. Am. J. Pathol. 2017, 187, 366–376. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef]
- Chandran, R.; Kim, T.; Mehta, S.L.; Udho, E.; Chanana, V.; Cengiz, P.; Kim, H.; Kim, C.; Vemuganti, R. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J. Cereb. Blood Flow Metab. 2018, 38, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Capra, A.P.; Repici, A.; Lanza, M.; Bova, V.; Palermo, N.; Paterniti, I.; Esposito, E. Rebalancing NOX2/Nrf2 to limit inflammation and oxidative stress across gut-brain axis in migraine. Free Radic. Biol. Med. 2024, 213, 65–78. [Google Scholar] [CrossRef]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins should be a candidate in the treatment of cancer, cardiovascular diseases and lipid metabolic disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef]
- Mancini, M.; Cerny, M.E.V.; Cardoso, N.S.; Verissimo, G.; Maluf, S.W. Grape seed components as protectors of inflammation, DNA damage, and cancer. Curr. Nutr. Rep. 2023, 12, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Oteiza, P.I. Proanthocyanidins at the gastrointestinal tract: Mechanisms involved in their capacity to mitigate obesity-associated metabolic disorders. Crit. Rev. Food Sci. Nutr. 2024, 64, 220–240. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Ruotolo, R.; Minato, I.; La Vitola, P.; Artioli, L.; Curti, C.; Franceschi, V.; Brindani, N.; Amidani, D.; Colombo, L.; Salmona, M.; et al. Flavonoid-derived human phenyl-γ-valerolactone metabolites selectively detoxify amyloid-β oligomers and prevent memory impairment in a mouse model of Alzheimer’s disease. Mol. Nutr. Food Res. 2020, 64, e1900890. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination therapy using polyphenols: An efficient way to improve antitumoral activity and reduce resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Geary, N. Understanding synergy. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E237–E253. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, A.; Jiang, A.; Qi, C.; Liu, Z.; Cheng, Q.; Yuan, S.; Luo, P. Computational frameworks transform antagonism to synergy in optimizing combination therapies. NPJ Digit Med. 2025, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J.; Porreca, F.; Cowan, A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989, 45, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Page, C.; Matera, M.G.; Cazzola, M.; Rogliani, P. Drug-drug interactions and synergy: From pharmacological models to clinical application. Pharmacol. Rev. 2024, 76, 1159–1220. [Google Scholar] [CrossRef]
- Menu, P.; Vince, J.E. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Geng, L.; Fan, L.M.; Liu, F.; Smith, C.; Li, J. Nox2 dependent redox-regulation of microglial response to amyloid-β stimulation and microgliosis in aging. Sci. Rep. 2020, 10, 1582. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef]
- Kim, J.S.; Oh, J.M.; Choi, H.; Kim, S.W.; Kim, S.W.; Kim, B.G.; Cho, J.H.; Lee, J.; Lee, D.C. Activation of the Nrf2/HO-1 pathway by curcumin inhibits oxidative stress in human nasal fibroblasts exposed to urban particulate matter. BMC Comp. Med. Ther. 2020, 20, 101. [Google Scholar] [CrossRef]
- Martin, K.R.; Barrett, J.C. Reactive oxygen species as double-edged swords in cellular processes: Low-dose cell signaling versus high-dose toxicity. Hum. Exp. Toxicol. 2002, 21, 71–75. [Google Scholar] [CrossRef]
- Yeh, S.L.; Wang, H.M.; Chen, P.Y.; Wu, T.C. Interactions of beta-carotene and flavonoids on the secretion of pro-inflammatory mediators in an in vitro system. Chem. Biol. Interact. 2009, 179, 386–393. [Google Scholar] [CrossRef]

| Gene Target | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | GATCAGCAAGCAGGAGTACGATGA | GGTGTAAAACGCAGCTCAGTAACA |
| COX-2 | TTTCAATGTGCAAGACCCGC | ACAGCTCAGTTGAACGCCTT |
| HO-1 | GTTTCCTGTTGGCGACCGTG | GCCAGGCAAGATTCTCCCCT |
| IL-1β | CGTCCTCTGTGACTCGTGGG | ATGGGTCAGACAGCACGAGG |
| IL-6 | AGAGTCACAGAAGGAGTGGCTA | CTTAGGCATAGCACACTAGGT |
| iNOS | GGGAACACCTGGGGATTTTC | CACAGTTTGGTCTGGCGAAG |
| MCP-1/CCL2 | GAGATCTGTGCTGACCCCAA | TGAAGTCCTTAGGGTTGATGCA |
| NLRP3 | TGATGCATGCACGTCTAATCTC | CAAATCGAGATGCGGGAGAG |
| NOX2 | ATCACATCCTCCACCAAAACCATT | GCAAGGCCGATGAAGAAGATCA |
| NQO-1 | AACGAGGTCAGATTAGGGGC | AGAGTATTTTCCCCGCTCGC |
| TNF-α | GCAGGTTCCGTCCCTCTCAT | TGCCAGTTCCACATCTCGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcolin, E.; Chemello, C.; Piovan, A.; Barbierato, M.; Morazzoni, P.; Ragazzi, E.; Zusso, M. A Combination of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone and Curcumin Synergistically Reduces Neuroinflammation in Cortical Microglia by Targeting the NLRP3 Inflammasome and the NOX2/Nrf2 Signaling Pathway. Nutrients 2025, 17, 1316. https://doi.org/10.3390/nu17081316
Marcolin E, Chemello C, Piovan A, Barbierato M, Morazzoni P, Ragazzi E, Zusso M. A Combination of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone and Curcumin Synergistically Reduces Neuroinflammation in Cortical Microglia by Targeting the NLRP3 Inflammasome and the NOX2/Nrf2 Signaling Pathway. Nutrients. 2025; 17(8):1316. https://doi.org/10.3390/nu17081316
Chicago/Turabian StyleMarcolin, Emma, Chiara Chemello, Anna Piovan, Massimo Barbierato, Paolo Morazzoni, Eugenio Ragazzi, and Morena Zusso. 2025. "A Combination of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone and Curcumin Synergistically Reduces Neuroinflammation in Cortical Microglia by Targeting the NLRP3 Inflammasome and the NOX2/Nrf2 Signaling Pathway" Nutrients 17, no. 8: 1316. https://doi.org/10.3390/nu17081316
APA StyleMarcolin, E., Chemello, C., Piovan, A., Barbierato, M., Morazzoni, P., Ragazzi, E., & Zusso, M. (2025). A Combination of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone and Curcumin Synergistically Reduces Neuroinflammation in Cortical Microglia by Targeting the NLRP3 Inflammasome and the NOX2/Nrf2 Signaling Pathway. Nutrients, 17(8), 1316. https://doi.org/10.3390/nu17081316






