Impact of a Fish-Based Restrictive Ketogenic Diet on Body Composition and Strength Capacity: A Pre–Post Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Participants
2.3. Eligibility Criteria
2.4. Ethics
2.5. Study Design and Procedures
2.6. Dietary Intervention
2.7. Body Composition
2.8. Skeletal Muscle Mass Analysis
2.9. Isometric Muscle Strength Test
2.10. Ketone Bodies in Blood and Urine
2.11. Somatic Test
2.12. Statistical Analysis
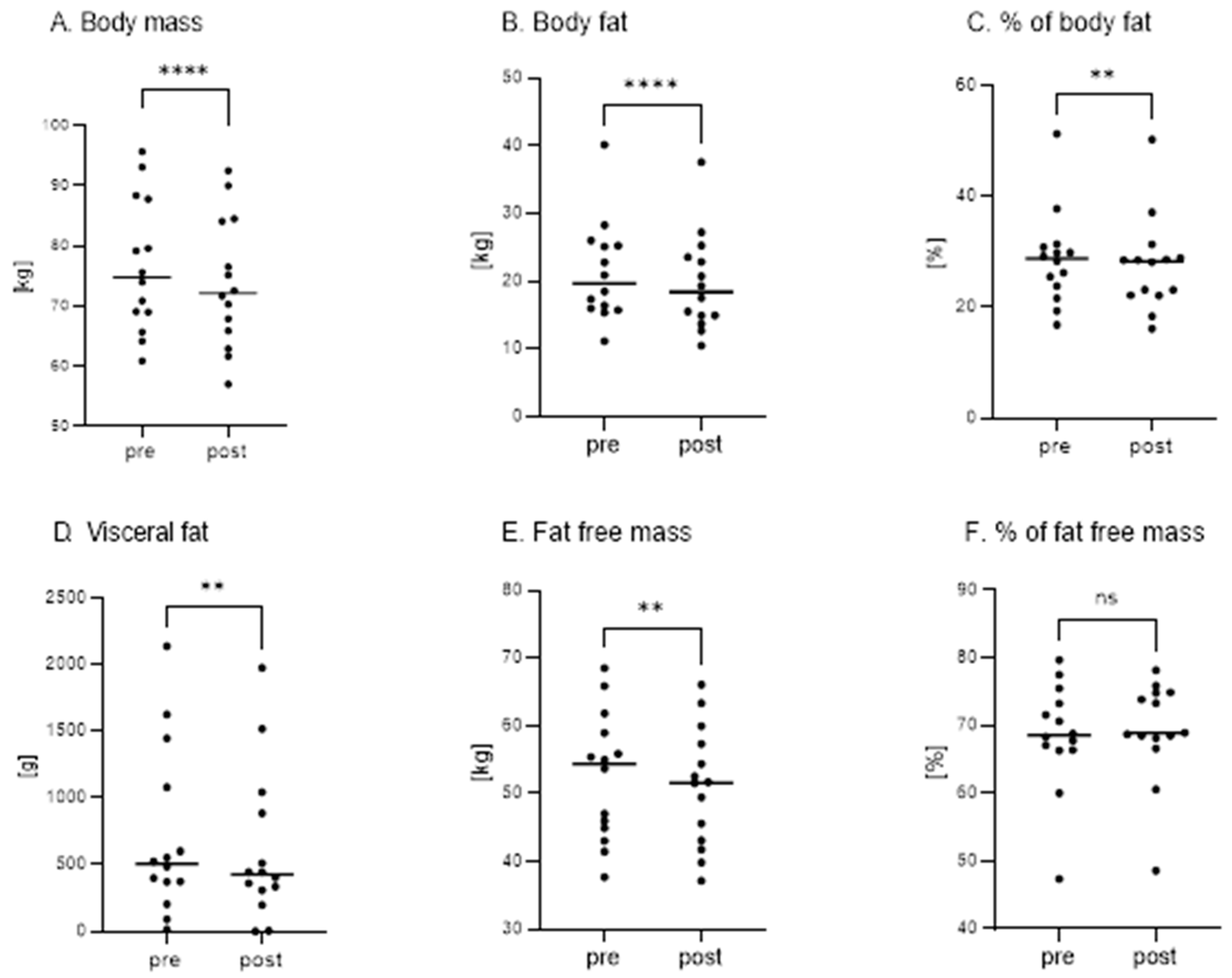
3. Results
4. Discussion
4.1. KD, Rich in Fish and Omega-3 Acids, Induces Ketosis
4.2. KD Significantly Reduces Body Mass and Body Fat
4.3. KD Significantly Reduces Skeletal Muscle Mass but Does Not Change the Relative Isometric Strength of Skeletal Muscles
4.4. The KD Is a Well-Tolerated Dietary Intervention
4.5. KD—Future Research Perspectives
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Energy (kcal) | 2000 | ||
|---|---|---|---|
| Energy (kcal) | 2001.6 | Sodium (mg) | 2105.8 |
| Protein (g) | 101.4 | Potassium (mg) | 2472.0 |
| Fat (g) | 170.2 | Calcium (mg) | 978.0 |
| Carbohydrates Total (g) | 20.5 | Magnesium (mg) | 521.0 |
| Digestible Carbohydrates (g) | 13.1 | Iron (mg) | 11.4 |
| Fiber (g) | 7.4 | Zinc (mg) | 8.8 |
| Plant Protein (g) | 7.4 | Selenium (µg) | 2.5 |
| Animal Protein (g) | 93.9 | Iodine (µg) | 246.5 |
| Sugars (g) | 2.0 | Vitamin A (µg) | 1874.6 |
| SFA Total (g) | 27.5 | Retinol (µg) | 748.3 |
| MUFA Total (g) | 39.6 | Vitamin D (µg) | 19.7 |
| Omega-3 Total (g) | 67.9 | Vitamin E (mg) | 9.9 |
| Omega-6 Total (g) | 20.8 | Vitamin K (mg) | 40.0 |
| PUFA Total (g) | 93.4 | Vitamin B1 (mg) | 0.8 |
| Omega-3:Omega-6 Ratio | 3:1 | Vitamin B2 (mg) | 2.1 |
| GI (Glycemic index) | 27.0 | Vitamin B3 (mg) | 17.5 |
| GL (Glycemic load) | 2.6 | Vitamin B6 (mg) | 2.1 |
| CU (Carbohydrate Unit) | 1.3 | Folates (µg) | 451.5 |
| % Energy (Protein) | 20.2 | Vitamin B12 (µg) | 13.2 |
| % Energy (Fat) | 76.4 | Vitamin C (mg) | 114.7 |
| % Energy (Carbohydrates) | 3.4 | ||
Appendix B
| Mark the degree of severity where | |||||
| 1—I strongly disagree, | |||||
| 2—I rather disagree, | |||||
| 3—I have no opinion, | |||||
| 4—I rather agree, | |||||
| 5—I definitely agree | |||||
| Before start ketogenic diet | |||||
| 1. Drowsiness | 1 | 2 | 3 | 4 | 5 |
| 2. More energy | 1 | 2 | 3 | 4 | 5 |
| 3. Nausea | 1 | 2 | 3 | 4 | 5 |
| 4. Vomiting | 1 | 2 | 3 | 4 | 5 |
| 5. Concentration disorders | 1 | 2 | 3 | 4 | 5 |
| 6. Sleep disorders | 1 | 2 | 3 | 4 | 5 |
| 7. Diarrhea | 1 | 2 | 3 | 4 | 5 |
| 8. Constipation | 1 | 2 | 3 | 4 | 5 |
| 9. Bad breath | 1 | 2 | 3 | 4 | 5 |
| 10. Increased thirst | 1 | 2 | 3 | 4 | 5 |
| 11. Hunger | 1 | 2 | 3 | 4 | 5 |
| Other? | 1 | 2 | 3 | 4 | 5 |
| After 14 days of ketogenic diet | |||||
| 1. Drowsiness | 1 | 2 | 3 | 4 | 5 |
| 2. More energy | 1 | 2 | 3 | 4 | 5 |
| 3. Nausea | 1 | 2 | 3 | 4 | 5 |
| 4. Vomiting | 1 | 2 | 3 | 4 | 5 |
| 5. Concentration disorders | 1 | 2 | 3 | 4 | 5 |
| 6. Sleep disorders | 1 | 2 | 3 | 4 | 5 |
| 7. Diarrhea | 1 | 2 | 3 | 4 | 5 |
| 8. Constipation | 1 | 2 | 3 | 4 | 5 |
| 9. Bad breath | 1 | 2 | 3 | 4 | 5 |
| 10. Increased thirst | 1 | 2 | 3 | 4 | 5 |
| 11. Hunger | 1 | 2 | 3 | 4 | 5 |
| Other? | 1 | 2 | 3 | 4 | 5 |
References
- Hite, A.H.; Berkowitz, V.G.; Berkowitz, K. Low-Carbohydrate Diet Review. Nutr. Clin. Pract. 2011, 26, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Dilliraj, L.N.; Schiuma, G.; Lara, D.; Strazzabosco, G.; Clement, J.; Giovannini, P.; Trapella, C.; Narducci, M.; Rizzo, R. The Evolution of Ketosis: Potential Impact on Clinical Conditions. Nutrients 2022, 14, 3613. [Google Scholar] [CrossRef]
- Lange, K.H.W. Fat Metabolism in Exercise—With Special Reference to Training and Growth Hormone Administration. Scand. J. Med. Sci. Sports 2004, 14, 74–99. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Moro, T.; Mota, J.F.; Coelho-Ravagnani, C.F. The Effects of Ketogenic Diet on Insulin Sensitivity and Weight Loss, Which Came First: The Chicken or the Egg? Nutrients 2023, 15, 3120. [Google Scholar] [CrossRef]
- Gingras, A.-A.; White, P.J.; Chouinard, P.Y.; Julien, P.; Davis, T.A.; Dombrowski, L.; Couture, Y.; Dubreuil, P.; Myre, A.; Bergeron, K.; et al. Long-Chain Omega-3 Fatty Acids Regulate Bovine Whole-Body Protein Metabolism by Promoting Muscle Insulin Signalling to the Akt-MTOR-S6K1 Pathway and Insulin Sensitivity. J. Physiol. 2007, 579, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic Diets, Physical Activity, and Body Composition: A Review. Br. J. Nutr. 2021, 127, 1898–1920. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Ziolkowski, W.; Vaccaro, P.S.; Ghafourifar, P. Effect of Short-Term Ketogenic Diet on Redox Status of Human Blood. Rejuvenation Res. 2007, 10, 435–440. [Google Scholar] [CrossRef]
- Armamento-Villareal, R.; Aguirre, L.; Napoli, N.; Shah, K.; Hilton, T.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Changes in Thigh Muscle Volume Predict Bone Mineral Density Response to Lifestyle Therapy in Frail, Obese Older Adults. Osteoporos. Int. 2013, 25, 551–558. [Google Scholar] [CrossRef]
- Janssen, I.; Ross, R. Effects of Sex on the Change in Visceral, Subcutaneous Adipose Tissue and Skeletal Muscle in Response to Weight Loss. Int. J. Obes. 1999, 23, 1035–1046. [Google Scholar] [CrossRef]
- Santanasto, A.J.; Glynn, N.W.; Newman, M.A.; Taylor, C.A.; Brooks, M.M.; Goodpaster, B.H.; Newman, A.B. Impact of Weight Loss on Physical Function with Changes in Strength, Muscle Mass, and Muscle Fat Infiltration in Overweight to Moderately Obese Older Adults: A Randomized Clinical Trial. J. Obes. 2011, 2011, 516576. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; de Jonge, L.; Frisard, M.I.; DeLany, J.P.; Larson-Meyer, D.E.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; Most, M.M.; et al. Effect of 6-Month Calorie Restriction on Biomarkers of Longevity, Metabolic Adaptation, and Oxidative Stress in Overweight Individuals. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Ravussin, E. Caloric Restriction in Humans: Impact on Physiological, Psychological, and Behavioral Outcomes. Antioxid. Redox Signal. 2011, 14, 275–287. [Google Scholar] [CrossRef]
- Usydus, Z.; Szlinder-Richert, J.; Polak-Juszczak, L.; Komar, K.; Adamczyk, M.; Malesa-Ciecwierz, M.; Ruczynska, W. Fish Products Available in Polish Market—Assessment of the Nutritive Value and Human Exposure to Dioxins and Other Contaminants. Chemosphere 2009, 74, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Byrd, K.A.; Thilsted, S.H.; Fiorella, K.J. Fish Nutrient Composition: A Review of Global Data from Poorly Assessed Inland and Marine Species. Public Health Nutr. 2020, 24, 476–486. [Google Scholar] [CrossRef]
- Brinton, E.A.; Mason, R.P. Prescription Omega-3 Fatty Acid Products Containing Highly Purified Eicosapentaenoic Acid (EPA). Lipids Health Dis. 2017, 16, 23. [Google Scholar] [CrossRef]
- Oscarsson, J.; Hurt-Camejo, E. Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Their Mechanisms of Action on Apolipoprotein B-Containing Lipoproteins in Humans: A Review. Lipids Health Dis. 2017, 16, 149. [Google Scholar] [CrossRef]
- Egalini, F.; Guardamagna, O.; Gaggero, G.; Varaldo, E.; Giannone, B.; Beccuti, G.; Benso, A.; Broglio, F. The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review. Nutrients 2023, 15, 2672. [Google Scholar] [CrossRef]
- Colica, C.; Merra, G.; Gasbarrini, A.; De Lorenzo, A.; Cioccoloni, G.; Gualtieri, P.; Perrone, M.A.; Bernardini, S.; Bernardo, V.; Di Renzo, L.; et al. Efficacy and Safety of Very-Low-Calorie Ketogenic Diet: A Double Blind Randomized Crossover Study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2274–2289. [Google Scholar] [PubMed]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a Ketogenic Diet in Overweight Women with Polycystic Ovary Syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef]
- Almassri, H.F.; Abdul Kadir, A.; Srour, M.; Foo, L.H. The Effects of Omega-3 Fatty Acids and Vitamin D Supplementation on the Quality of Life and Blood Inflammation Markers in Newly Diagnosed Breast Cancer Women: An Open-Labelled Randomised Controlled Trial. Clin. Nutr. ESPEN 2024, 65, 64–75. [Google Scholar] [CrossRef]
- Mancin, S.; Sguanci, M.; Ferrara, G.; Caccialanza, R.; Cereda, E.; Lo Cascio, A.; Parozzi, M.; Petrelli, F.; Cangelosi, G.; Morales Palomares, S. Effect of Omega-3 in Patients Undergoing Bone Marrow Transplantation: A Narrative Review. Hemato 2025, 6, 5. [Google Scholar] [CrossRef]
- You, J.-S.; Park, M.-N.; Song, W.; Lee, Y.-S. Dietary Fish Oil Alleviates Soleus Atrophy during Immobilization in Association with Akt Signaling to P70s6k and E3 Ubiquitin Ligases in Rats. Appl. Physiol. Nutr. Metab. 2010, 35, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Caprio, M.; Watanabe, M.; Cammarata, G.; Feraco, A.; Muscogiuri, G.; Verde, L.; Colao, A.; Savastano, S. Could Very Low-Calorie Ketogenic Diets Turn off Low Grade Inflammation in Obesity? Emerging evidence. Crit. Rev. Food Sci. Nutr. 2022, 63, 8320–8336. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.C. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493179/ (accessed on 6 April 2025).
- Moscatelli, F.; Valenzano, A.; Polito, R.; Francesco, S.; Montana, A.; Salerno, M.; Messina, A.; Monda, M.; Cibelli, G.; Monda, V.; et al. Ketogenic Diet and Sport Performance. Sport Mont 2020, 18, 91–94. [Google Scholar]
- Zambrano, A.K.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Frias-Toral, E.; Ruiz-Pozo, V.A.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Chapela, S.; Montalván, M.; Sarno, G.; et al. The Impact of a Very-Low-Calorie Ketogenic Diet in the Gut Microbiota Composition in Obesity. Nutrients 2023, 15, 2728. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a Four-Week Ketogenic Diet on Exercise Metabolism in CrossFit-Trained Athletes. J. Int. Soc. Sports Nutr. 2019, 16, 16. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Główka, N.; Ziobrowska, A.; Podgórski, T. Is a Four-Week Ketogenic Diet an Effective Nutritional Strategy in CrossFit-Trained Female and Male Athletes? Nutrients 2021, 13, 864. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Kim, J.; Wang, Z.; Heymsfield, S.B.; Baumgartner, R.N.; Gallagher, D. Total-Body Skeletal Muscle Mass: Estimation by a New Dual-Energy X-Ray Absorptiometry Method. Am. J. Clin. Nutr. 2002, 76, 378–383. [Google Scholar] [CrossRef]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, Ketogenic Diet and Food Intake Control: A Complex Relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef]
- Veech, R.L. The Therapeutic Implications of Ketone Bodies: The Effects of Ketone Bodies in Pathological Conditions: Ketosis, Ketogenic Diet, Redox States, Insulin Resistance, and Mitochondrial Metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Niepmann, M. Importance of Michaelis Constants for Cancer Cell Redox Balance and Lactate Secretion—Revisiting the Warburg Effect. Cancers 2024, 16, 2290. [Google Scholar] [CrossRef] [PubMed]
- Leino, R.L.; Gerhart, D.Z.; Duelli, R.; Enerson, B.E.; Drewes, L.R. Diet-Induced Ketosis Increases Monocarboxylate Transporter (MCT1) Levels in Rat Brain. Neurochem. Int. 2001, 38, 519–527. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic Diet for Obesity: Friend or Foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and Control of Ketone Body Metabolism: On the Fringe of Lipid Biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.; Lodi, A.; Bosco, G. Long Term Successful Weight Loss with a Combination Biphasic Ketogenic Mediterranean Diet and Mediterranean Diet Maintenance Protocol. Nutrients 2013, 5, 5205–5217. [Google Scholar] [CrossRef]
- Neeland, I.J.; Marso, S.P.; Ayers, C.R.; Lewis, B.; Oslica, R.; Francis, W.; Rodder, S.; Pandey, A.; Joshi, P.H. Effects of Liraglutide on Visceral and Ectopic Fat in Adults with Overweight and Obesity at High Cardiovascular Risk: A Randomised, Double-Blind, Placebo-Controlled, Clinical Trial. Lancet Diabetes Endocrinol. 2021, 9, 595–605. [Google Scholar] [CrossRef]
- Salman, H.B.; Salman, M.A.; Yildiz, E.A. The Effect of Omega-3 Fatty Acid Supplementation on Weight Loss and Cognitive Function in Overweight or Obese Individuals on Weight-Loss Diet. Nutr. Hosp. 2022, 39, 803–813. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in Consumption of Omega-3 and Omega-6 Fatty Acids in the United States during the 20th Century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Meyer, B.J. Are We Consuming Enough Long Chain Omega-3 Polyunsaturated Fatty Acids for Optimal Health? Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Couet, C.; Delarue, J.; Ritz, P.; Antoine, J.M.; Lamisse, F. Effect of Dietary Fish Oil on Body Fat Mass and Basal Fat Oxidation in Healthy Adults. Int. J. Obes. 1997, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Thorsdottir, I.; Tomasson, H.; Gunnarsdottir, I.; Gisladottir, E.; Kiely, M.; Parra, M.D.; Bandarra, N.M.; Schaafsma, G.; Martinéz, J.A. Randomized Trial of Weight-Loss-Diets for Young Adults Varying in Fish and Fish Oil Content. Int. J. Obes. 2007, 31, 1560–1566. [Google Scholar] [CrossRef]
- Slee, A.; Reid, J. Exercise and Nutrition Interventions for Renal Cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 219. [Google Scholar] [CrossRef] [PubMed]
- Chung, N. Impact of the Ketogenic Diet on Body Fat, Muscle Mass, and Exercise Performance: A Review. Phys. Act. Nutr. 2023, 27, 1–7. [Google Scholar] [CrossRef]
- Cipryan, L.; Dostal, T.; Litschmannova, M.; Hofmann, P.; Maffetone, P.B.; Laursen, P.B. Effects of a Very Low-Carbohydrate High-Fat Diet and High-Intensity Interval Training on Visceral Fat Deposition and Cardiorespiratory Fitness in Overfat Individuals: A Randomized Controlled Clinical Trial. Front. Nutr. 2021, 8, 785694. [Google Scholar] [CrossRef]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Weight without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benítez-Porres, J. Efficacy of ketogenic diet on body composition during resistance training in trained men: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. Available online: https://pubmed.ncbi.nlm.nih.gov/29986720/ (accessed on 6 April 2025).
- Johnston, C.; Sears, B.; Perry, M.; Knurick, J. Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial. Nutrients 2017, 9, 1182. [Google Scholar] [CrossRef]
- Kruszewski, M.; Kruszewski, A.; Tabęcki, R.; Stec, K.; Ambroży, T.; Aksenov, M.O.; Merchelski, M.; Danielik, T. Effectiveness of High-Fat and High-Carbohydrate Diets on Body Composition and Maximal Strength After 15 Weeks of Resistance Training. Adv. Med. Sci. 2024, 69, 139–146. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic Diet Does Not Affect Strength Performance in Elite Artistic Gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef]
- Sjödin, A.; Hellström, F.; Sehlstedt, E.; Svensson, M.; Burén, J. Effects of a Ketogenic Diet on Muscle Fatigue in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2020, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Kephart, W.; Pledge, C.; Roberson, P.; Mumford, P.; Romero, M.; Mobley, C.; Martin, J.; Young, K.; Lowery, R.; Wilson, J.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef]
- Sawyer, J.C.; Wood, R.J.; Davidson, P.W.; Collins, S.M.; Matthews, T.D.; Gregory, S.M.; Paolone, V.J. Effects of a Short-Term Carbohydrate-Restricted Diet on Strength and Power Performance. J. Strength Cond. Res. 2013, 27, 2255–2262. [Google Scholar] [CrossRef]
- Lum, D.; Haff, G.G.; Barbosa, T.M. The Relationship between Isometric Force-Time Characteristics and Dynamic Performance: A Systematic Review. Sports 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Horswill, C.A.; Hickner, R.C.; Scott, J.R.; Costill, D.L.; Gould, D. Weight Loss, Dietary Carbohydrate Modifications, and High Intensity, Physical Performance. Med. Sci. Sports Exerc. 1990, 22, 470–476. [Google Scholar] [CrossRef]
- Iacovides, S.; Goble, D.; Paterson, B.; Meiring, R.M. Three Consecutive Weeks of Nutritional Ketosis Has No Effect on Cognitive Function, Sleep, and Mood Compared with a High-Carbohydrate, Low-Fat Diet in Healthy Individuals: A Randomized, Crossover, Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Mehrabani, S.; Moradi, S.; Amani, R. The Association between Diet and Mood: A Systematic Review of Current Literature. Psychiatry Res. 2019, 271, 428–437. [Google Scholar] [CrossRef]
- Dietch, D.M.; Kerr-Gaffney, J.; Hockey, M.; Marx, W.; Ruusunen, A.; Young, A.H.; Berk, M.; Mondelli, V. Efficacy of Low Carbohydrate and Ketogenic Diets in Treating Mood and Anxiety Disorders: Systematic Review and Implications for Clinical Practice. BJPsych Open 2023, 9, e70. [Google Scholar] [CrossRef]
- Kang, J.; Ratamess, N.A.; Faigenbaum, A.D.; Bush, J.A. Ergogenic Properties of Ketogenic Diets in Normal-Weight Individuals: A Systematic Review. J. Am. Coll. Nutr. 2020, 39, 665–675. [Google Scholar] [CrossRef]
- Xie, Y.; Gu, Y.; Li, Z.; He, B.; Zhang, L. Effects of Different Exercises Combined with Different Dietary Interventions on Body Composition: A Systematic Review and Network Meta-Analysis. Nutrients 2024, 16, 3007. [Google Scholar] [CrossRef] [PubMed]



| PRE | POST | p | |
|---|---|---|---|
| Flexor peak (Nm/kg BM) | 1.27 ± 0.34 | 1.28 ± 0.35 | 0.652 |
| Flexor average (Nm/kg BM) | 1.19 ± 0.30 | 1.20 ± 0.33 | 0.603 |
| Extensor peak (Nm/kg BM) | 3.08 ± 0.70 | 3.01 ± 0.57 | 0.753 |
| Extensor average (Nm/kg BM) | 2.98 ± 0.67 | 3.00 ± 0.64 | 0.553 |
| PRE | POST | p | |
|---|---|---|---|
| Flexor peak (Nm/kg SMM) | 3.46 ± 0.73 | 3.43 ± 0.72 | 0.647 |
| Flexor average (Nm/kg SMM) | 3.25 ± 0.66 | 3.23 ± 0.68 | 0.756 |
| Extensor peak (Nm/kg SMM) | 8.42 ± 1.30 | 8.13 ± 1.08 | 0.274 |
| Extensor average (Nm/kg SMM) | 8.14 ± 1.29 | 8.08 ± 1.16 | 0.661 |
| Somatic Disorders | PRE | POST | Improvement | Deterioration |
|---|---|---|---|---|
| Drowsiness | 2.36 ± 1.50 | 1.57 ± 1.16 | 5/14 | 0/14 |
| Energy | 3.14 ± 1.29 | 3.50 ± 1.56 | 8/14 | 2/14 |
| Nausea | 1.64 ± 1.15 | 1.64 ± 1.34 | 3/14 | 2/14 |
| Vomiting | 1.00 ± 0.00 | 1.00 ± 0.00 | 0/14 | 0/14 |
| Concentration disorders | 2.57 ± 1.55 | 1.79 ± 1.25 | 5/14 | 0/14 |
| Sleeping disorders | 1.29 ± 0.61 | 1.50 ± 1.16 | 2/14 | 2/14 |
| Diarrhea | 1.00 ± 0.00 | 1.00 ± 0.00 | 0/14 | 0/14 |
| Constipation | 3.07 ± 1.64 | 2.64 ± 1.86 | 4/14 | 1/14 |
| Bad breath | 2.29 ± 1.38 | 2.43 ± 1.60 | 1/14 | 2/14 |
| Increased thirst | 3.57 ± 1.65 | 4.14 ± 1.17 | 1/14 | 4/14 |
| Hunger | 2.50 ± 1.61 | 2.21 ± 1.53 | 4/14 | 2/14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siedzik, K.; Góral, K.; Rodziewicz-Flis, E.; Olek, R.A.; Ziółkowski, W. Impact of a Fish-Based Restrictive Ketogenic Diet on Body Composition and Strength Capacity: A Pre–Post Study. Nutrients 2025, 17, 1297. https://doi.org/10.3390/nu17081297
Siedzik K, Góral K, Rodziewicz-Flis E, Olek RA, Ziółkowski W. Impact of a Fish-Based Restrictive Ketogenic Diet on Body Composition and Strength Capacity: A Pre–Post Study. Nutrients. 2025; 17(8):1297. https://doi.org/10.3390/nu17081297
Chicago/Turabian StyleSiedzik, Katarzyna, Kamil Góral, Ewa Rodziewicz-Flis, Robert A. Olek, and Wiesław Ziółkowski. 2025. "Impact of a Fish-Based Restrictive Ketogenic Diet on Body Composition and Strength Capacity: A Pre–Post Study" Nutrients 17, no. 8: 1297. https://doi.org/10.3390/nu17081297
APA StyleSiedzik, K., Góral, K., Rodziewicz-Flis, E., Olek, R. A., & Ziółkowski, W. (2025). Impact of a Fish-Based Restrictive Ketogenic Diet on Body Composition and Strength Capacity: A Pre–Post Study. Nutrients, 17(8), 1297. https://doi.org/10.3390/nu17081297





