Ketogenic Diet in Steatotic Liver Disease: A Metabolic Approach to Hepatic Health
Abstract
1. Introduction
2. Materials and Methods
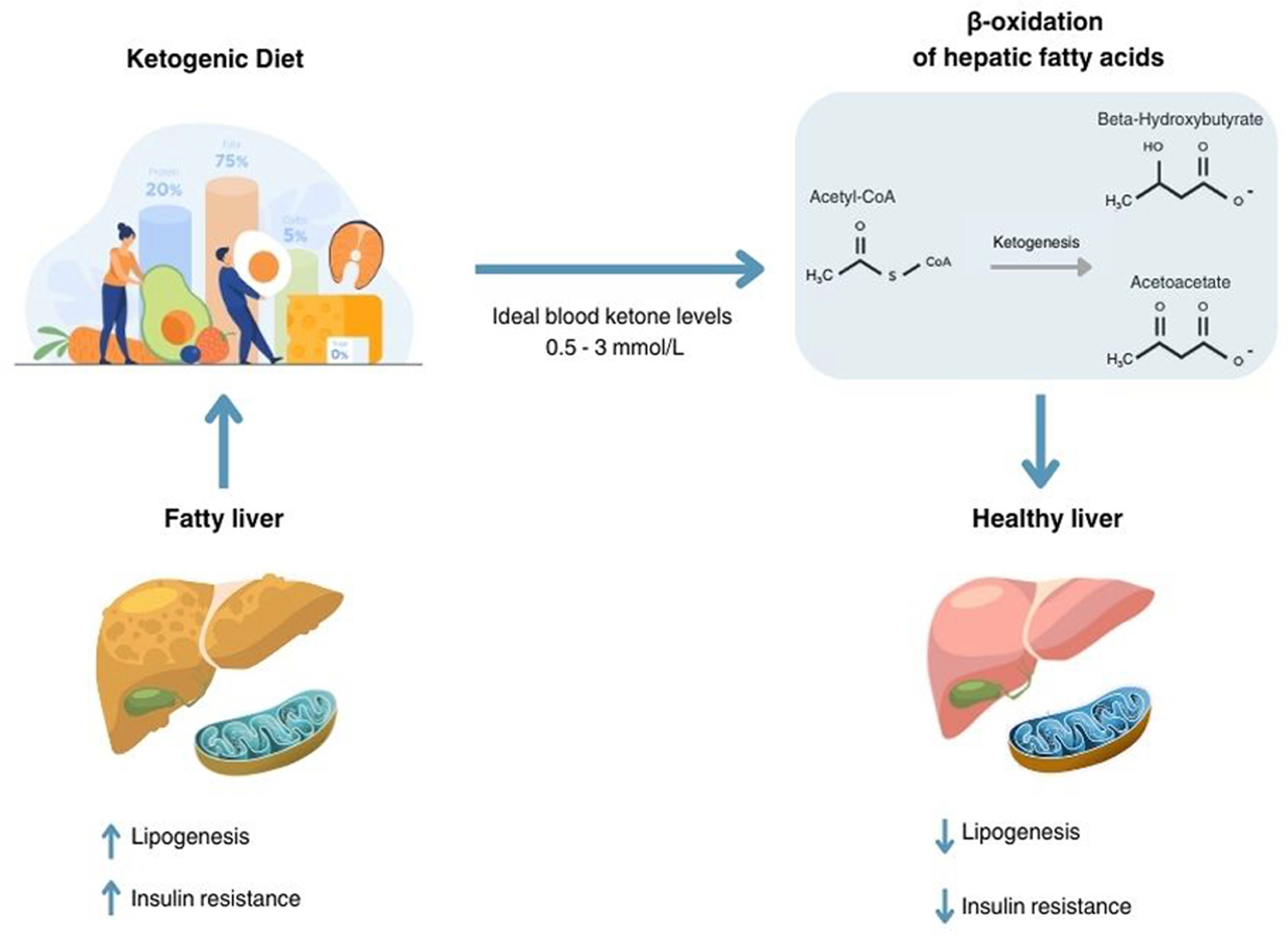
3. Mechanisms of Action and Rationale of the Ketogenic Diet in Steatotic Liver Disease
4. Clinical Studies
4.1. Studies Conducted on Patients Scheduled for Bariatric Surgery
4.2. Studies on Gender Differences
4.3. Clinical Studies with Technological Enhancements
5. Adverse Events
5.1. Ketogenic Flu
5.2. Lipid Metabolism
5.3. Gastrointestinal Side Effects and Electrolyte Balance
5.4. Nephrolithiasis and Bone Health
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, A.; Wong, R.J.; Harrison, S.A. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin. Gastroenterol. Hepatol. 2015, 13, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD Diagnostic Criteria in Real World. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The Prevalence and Incidence of NAFLD Worldwide: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Riazi, K.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. Race and Ethnicity in Non-Alcoholic Fatty Liver Disease (NAFLD): A Narrative Review. Nutrients 2022, 14, 4556. [Google Scholar] [CrossRef]
- Sepulveda-Villegas, M.; Roman, S.; Rivera-Iñiguez, I.; Ojeda-Granados, C.; Gonzalez-Aldaco, K.; Torres-Reyes, L.A.; Jose-Abrego, A.; Panduro, A. High Prevalence of Nonalcoholic Steatohepatitis and Abnormal Liver Stiffness in a Young and Obese Mexican Population. PLoS ONE 2019, 14, e0208926. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-Alcoholic Fatty Liver Disease: Definition and Subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef]
- Tsai, E.; Lee, T.-P. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Noninvasive Biomarkers and Transient Elastography. Clin. Liver Dis. 2018, 22, 73–92. [Google Scholar] [CrossRef]
- Troelstra, M.A.; Witjes, J.J.; Van Dijk, A.; Mak, A.L.; Gurney-Champion, O.; Runge, J.H.; Zwirs, D.; Stols-Gonçalves, D.; Zwinderman, A.H.; Ten Wolde, M.; et al. Assessment of Imaging Modalities Against Liver Biopsy in Nonalcoholic Fatty Liver Disease: The Amsterdam NAFLD-NASH Cohort. Magn. Reson. Imaging 2021, 54, 1937–1949. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Torres-Peña, J.D.; Arenas-de Larriva, A.P.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review. Nutrients 2023, 15, 1483. [Google Scholar] [CrossRef]
- Lazo, M.; Solga, S.F.; Horska, A.; Bonekamp, S.; Diehl, A.M.; Brancati, F.L.; Wagenknecht, L.E.; Pi-Sunyer, F.X.; Kahn, S.E.; Clark, J.M.; et al. Effect of a 12-Month Intensive Lifestyle Intervention on Hepatic Steatosis in Adults with Type 2 Diabetes. Diabetes Care 2010, 33, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial Effects of the Ketogenic Diet on Nonalcoholic Fatty Liver Disease: A Comprehensive Review of the Literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with Diet, Physical Activity and Exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Dyńka, D.; Rodzeń, Ł.; Rodzeń, M.; Łojko, D.; Kraszewski, S.; Ibrahim, A.; Hussey, M.; Deptuła, A.; Grzywacz, Ż.; Ternianov, A.; et al. Beneficial Effects of the Ketogenic Diet on Nonalcoholic Fatty Liver Disease (NAFLD/MAFLD). J. Clin. Med. 2024, 13, 4857. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Skow, S.L.; Jha, R.K. A Ketogenic Diet Is Effective in Improving Insulin Sensitivity in Individualswith Type 2 Diabetes. Curr. Diabetes Rev. 2023, 19, e250422203985. [Google Scholar] [CrossRef]
- Guarnotta, V.; Emanuele, F.; Amodei, R.; Giordano, C. Very Low-Calorie Ketogenic Diet: A Potential Application in the Treatment of Hypercortisolism Comorbidities. Nutrients 2022, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Guarnotta, V.; Amodei, R.; Di Gaudio, F.; Giordano, C. Nutritional Intervention in Cushing’s Disease: The Ketogenic Diet’s Effects on Metabolic Comorbidities and Adrenal Steroids. Nutrients 2023, 15, 4647. [Google Scholar] [CrossRef]
- Shalabi, H.; Alotaibi, A.; Alqahtani, A.; Alattas, H.; Alghamdi, Z. Ketogenic Diets: Side Effects, Attitude, and Quality of Life. Cureus 2021, 13, e20390. [Google Scholar] [CrossRef] [PubMed]
- Alkhorayef, N.; Almutery, F.T.; Rasheed, Z.; Althwab, S.A.; Aljohani, A.S.M.; Alhawday, Y.A.N.; Salem, T.; Alharbi, A.M.; Wahaq, A.A.A.B.; Alharbi, F.S.; et al. Regulatory Effects of Ketogenic Diet on the Inflammatory Response in Obese Saudi Women. J. Taibah Univ. Med. Sci. 2023, 18, 1101–1107. [Google Scholar] [CrossRef]
- Srivastava, A.; Gailer, R.; Tanwar, S.; Trembling, P.; Parkes, J.; Rodger, A.; Suri, D.; Thorburn, D.; Sennett, K.; Morgan, S.; et al. Prospective Evaluation of a Primary Care Referral Pathway for Patients with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2019, 71, 371–378. [Google Scholar] [CrossRef]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kwon, S.; Jeyaratnam, R.; Kim, K.-H. Targeting Ketone Body Metabolism to Treat Fatty Liver Disease. J. Pharm. Pharm. Sci. 2024, 27, 13375. [Google Scholar] [CrossRef]
- Cantrell, C.B.; Mohiuddin, S.S. Biochemistry, Ketone Metabolism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and Control of Ketone Body Metabolism: On the Fringe of Lipid Biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Calimag, A.P.P.; Chlebek, S.; Lerma, E.V.; Chaiban, J.T. Diabetic Ketoacidosis. Dis. Mon. 2023, 69, 101418. [Google Scholar] [CrossRef]
- Elendu, C.; David, J.A.; Udoyen, A.-O.; Egbunu, E.O.; Ogbuiyi-Chima, I.C.; Unakalamba, L.O.; Temitope, A.I.; Ibhiedu, J.O.; Ibhiedu, A.O.; Nwosu, P.U.; et al. Comprehensive Review of Diabetic Ketoacidosis: An Update. Ann. Med. Surg. 2023, 85, 2802–2807. [Google Scholar] [CrossRef]
- Gomez-Arbelaez, D.; Crujeiras, A.B.; Castro, A.I.; Goday, A.; Mas-Lorenzo, A.; Bellon, A.; Tejera, C.; Bellido, D.; Galban, C.; Sajoux, I.; et al. Acid–Base Safety during the Course of a Very Low-Calorie-Ketogenic Diet. Endocrine 2017, 58, 81–90. [Google Scholar] [CrossRef]
- Nogueira, J.P.; Cusi, K. Role of Insulin Resistance in the Development of Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: From Bench to Patient Care. Diabetes Spectr. 2024, 37, 20–28. [Google Scholar] [CrossRef]
- Badman, M.K.; Kennedy, A.R.; Adams, A.C.; Pissios, P.; Maratos-Flier, E. A Very Low Carbohydrate Ketogenic Diet Improves Glucose Tolerance in Ob/Ob Mice Independently of Weight Loss. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1197–E1204. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q. Control of Blood Glucose in Type 2 Diabetes without Weight Loss by Modification of Diet Composition. Nutr. Metab. 2006, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Sripongpun, P.; Churuangsuk, C.; Bunchorntavakul, C. Current Evidence Concerning Effects of Ketogenic Diet and Intermittent Fasting in Patients with Nonalcoholic Fatty Liver. J. Clin. Transl. Hepatol. 2022, 10, 730–739. [Google Scholar] [CrossRef]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Moro, T.; Mota, J.F.; Coelho-Ravagnani, C.D.F. The Effects of Ketogenic Diet on Insulin Sensitivity and Weight Loss, Which Came First: The Chicken or the Egg? Nutrients 2023, 15, 3120. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, J.; Liu, C.; Le, T.N.; Lu, Y.; Feng, F.; Zhao, M. High-Fat Diet-Induced Decreased Circulating Bile Acids Contribute to Obesity Associated with Gut Microbiota in Mice. Foods 2024, 13, 699. [Google Scholar] [CrossRef]
- Song, W.-Q.; Zhong, W.-F.; Gao, J.; Li, Z.-H.; Ren, J.-J.; Shen, D.; Wang, X.-M.; Shen, Q.-Q.; You, F.-F.; Fu, Q.; et al. Metabolic Obesity Phenotypes and All-Cause Mortality among the Chinese Oldest-Old Population: A Prospective Cohort Study. Int. J. Obes. 2024, 48, 1438–1446. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-Low-Calorie Ketogenic Diet (VLCKD) in the Management of Metabolic Diseases: Systematic Review and Consensus Statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Moreno, B.; Bellido, D.; Sajoux, I.; Goday, A.; Saavedra, D.; Crujeiras, A.B.; Casanueva, F.F. Comparison of a Very Low-Calorie-Ketogenic Diet with a Standard Low-Calorie Diet in the Treatment of Obesity. Endocrine 2014, 47, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.L.; Dixon, J.B.; Marks, P.; Strauss, B.J.; O’Brien, P.E. Preoperative Weight Loss with a Very-Low-Energy Diet: Quantitation of Changes in Liver and Abdominal Fat by Serial Imaging. Am. J. Clin. Nutr. 2006, 84, 304–311. [Google Scholar] [CrossRef]
- Tendler, D.; Lin, S.; Yancy, W.S.; Mavropoulos, J.; Sylvestre, P.; Rockey, D.C.; Westman, E.C. The Effect of a Low-Carbohydrate, Ketogenic Diet on Nonalcoholic Fatty Liver Disease: A Pilot Study. Dig. Dis. Sci. 2007, 52, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guisado, J.; Muñoz-Serrano, A. The Effect of the Spanish Ketogenic Mediterranean Diet on Nonalcoholic Fatty Liver Disease: A Pilot Study. J. Med. Food 2011, 14, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, F.; Campanile, F.C.; Coccia, F.; Capoccia, D.; Alessandroni, L.; Puzziello, A.; Coluzzi, I.; Silecchia, G. Very Low-Carbohydrate Ketogenic Diet Before Bariatric Surgery: Prospective Evaluation of a Sequential Diet. Obes. Surg. 2015, 25, 64–71. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef]
- Ministrini, S.; Calzini, L.; Nulli Migliola, E.; Ricci, M.A.; Roscini, A.R.; Siepi, D.; Tozzi, G.; Daviddi, G.; Martorelli, E.-E.; Paganelli, M.T.; et al. Lysosomal Acid Lipase as a Molecular Target of the Very Low Carbohydrate Ketogenic Diet in Morbidly Obese Patients: The Potential Effects on Liver Steatosis and Cardiovascular Risk Factors. J. Clin. Med. 2019, 8, 621. [Google Scholar] [CrossRef]
- Watanabe, M.; Risi, R.; Camajani, E.; Contini, S.; Persichetti, A.; Tuccinardi, D.; Ernesti, I.; Mariani, S.; Lubrano, C.; Genco, A.; et al. Baseline HOMA IR and Circulating FGF21 Levels Predict NAFLD Improvement in Patients Undergoing a Low Carbohydrate Dietary Intervention for Weight Loss: A Prospective Observational Pilot Study. Nutrients 2020, 12, 2141. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.-M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a Ketogenic Diet on Hepatic Steatosis and Hepatic Mitochondrial Metabolism in Nonalcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef]
- Crabtree, C.; Kackley, M.; Buga, A.; Fell, B.; LaFountain, R.; Hyde, P.; Sapper, T.; Kraemer, W.; Scandling, D.; Simonetti, O.; et al. Comparison of Ketogenic Diets with and without Ketone Salts versus a Low-Fat Diet: Liver Fat Responses in Overweight Adults. Nutrients 2021, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- De Nucci, S.; Bonfiglio, C.; Donvito, R.; Di Chito, M.; Cerabino, N.; Rinaldi, R.; Sila, A.; Shahini, E.; Giannuzzi, V.; Pesole, P.L.; et al. Effects of an Eight Week Very Low-Calorie Ketogenic Diet (VLCKD) on White Blood Cell and Platelet Counts in Relation to Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Subjects with Overweight and Obesity. Nutrients 2023, 15, 4468. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; De Nucci, S.; Castellana, F.; Di Chito, M.; Giannuzzi, V.; Shahini, E.; Zupo, R.; Lampignano, L.; Piazzolla, G.; Triggiani, V.; et al. The Effects of Eight Weeks’ Very Low-Calorie Ketogenic Diet (VLCKD) on Liver Health in Subjects Affected by Overweight and Obesity. Nutrients 2023, 15, 825. [Google Scholar] [CrossRef]
- Rinaldi, R.; De Nucci, S.; Donghia, R.; Donvito, R.; Cerabino, N.; Di Chito, M.; Penza, A.; Mongelli, F.P.; Shahini, E.; Zappimbulso, M.; et al. Gender Differences in Liver Steatosis and Fibrosis in Overweight and Obese Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease before and after 8 Weeks of Very Low-Calorie Ketogenic Diet. Nutrients 2024, 16, 1408. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; De Nucci, S.; Bonfiglio, C.; Di Stasi, V.; Cerabino, N.; Di Chito, M.; Rinaldi, R.; Cantalice, P.; Shahini, E.; Giannuzzi, V.; et al. Higher-Level Steatosis Is Associated with a Greater Decrease in Metabolic Dysfunction-Associated Steatoic Liver Disease after Eight Weeks of a Very Low-Calorie Ketogenic Diet (VLCKD) in Subjects Affected by Overweight and Obesity. Nutrients 2024, 16, 874. [Google Scholar] [CrossRef]
- Chirapongsathorn, S.; Rintaravitoon, W.; Tangjaturonrasme, B.; Chotsriluecha, S.; Pumsutas, Y.; Kanchanapradith, A.; Treeprasertsuk, S. Effect of a Ketogenic Diet on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Progression: A Randomized Controlled Trial. JGH Open 2025, 9, e70099. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; D’Amore, A.; Giovannini, C.; Gessani, S.; Masella, R. Gender-Related Differences in Lifestyle May Affect Health Status. Ann. Ist. Super. Sanita 2016, 52, 158–166. [Google Scholar] [CrossRef]
- Guy, J.; Peters, M.G. Liver Disease in Women: The Influence of Gender on Epidemiology, Natural History, and Patient Outcomes. Gastroenterol. Hepatol. 2013, 9, 633–639. [Google Scholar]
- Falkenhain, K.; Locke, S.R.; Lowe, D.A.; Reitsma, N.J.; Lee, T.; Singer, J.; Weiss, E.J.; Little, J.P. Keyto App and Device versus WW App on Weight Loss and Metabolic Risk in Adults with Overweight or Obesity: A Randomized Trial. Obesity 2021, 29, 1606–1614. [Google Scholar] [CrossRef]
- Wells, J.; Swaminathan, A.; Paseka, J.; Hanson, C. Efficacy and Safety of a Ketogenic Diet in Children and Adolescents with Refractory Epilepsy—A Review. Nutrients 2020, 12, 1809. [Google Scholar] [CrossRef]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated with the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef]
- O’Neill, B.; Raggi, P. The Ketogenic Diet: Pros and Cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Naveh, N.; Avidan, Y.; Zafrir, B. Extreme Hypercholesterolemia Following a Ketogenic Diet: Exaggerated Response to an Increasingly Popular Diet. Cureus 2023, 15, e43683. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.-E.; Maki, K.C. Review of Current Evidence and Clinical Recommendations on the Effects of Low-Carbohydrate and Very-Low-Carbohydrate (Including Ketogenic) Diets for the Management of Body Weight and Other Cardiometabolic Risk Factors: A Scientific Statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef]
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional Recommendations for Cardiovascular Disease Prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Mindrum, M.R.; Giral, P.; Kontush, A.; Soto-Mota, A.; Wood, T.R.; D’Agostino, D.P.; Manubolu, V.S.; Budoff, M.; Krauss, R.M. Elevated LDL-Cholesterol Levels among Lean Mass Hyper-Responders on Low-Carbohydrate Ketogenic Diets Deserve Urgent Clinical Attention and Further Research. J. Clin. Lipidol. 2022, 16, 765–768. [Google Scholar] [CrossRef]
- Kang, H.C.; Chung, D.E.; Kim, D.W.; Kim, H.D. Early- and Late-onset Complications of the Ketogenic Diet for Intractable Epilepsy. Epilepsia 2004, 45, 1116–1123. [Google Scholar] [CrossRef]
- Acharya, P.; Acharya, C.; Thongprayoon, C.; Hansrivijit, P.; Kanduri, S.R.; Kovvuru, K.; Medaura, J.; Vaitla, P.; Garcia Anton, D.F.; Mekraksakit, P.; et al. Incidence and Characteristics of Kidney Stones in Patients on Ketogenic Diet: A Systematic Review and Meta-Analysis. Diseases 2021, 9, 39. [Google Scholar] [CrossRef]
- Garofalo, V.; Barbagallo, F.; Cannarella, R.; Calogero, A.E.; La Vignera, S.; Condorelli, R.A. Effects of the Ketogenic Diet on Bone Health: A Systematic Review. Front. Endocrinol. 2023, 14, 1042744. [Google Scholar] [CrossRef]
- Lian, C.-Y.; Zhai, Z.-Z.; Li, Z.-F.; Wang, L. High Fat Diet-Triggered Non-Alcoholic Fatty Liver Disease: A Review of Proposed Mechanisms. Chem.-Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Parry, S.A.; Hodson, L. Influence of Dietary Macronutrients on Liver Fat Accumulation and Metabolism. J. Investig. Med. 2017, 65, 1102–1115. [Google Scholar] [CrossRef]
- Winters-van Eekelen, E.; Verkouter, I.; Peters, H.P.F.; Alssema, M.; De Roos, B.G.; Schrauwen-Hinderling, V.B.; Roumans, K.H.M.; Schoones, J.W.; Zock, P.L.; Schrauwen, P.; et al. Effects of Dietary Macronutrients on Liver Fat Content in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Nutr. 2021, 75, 588–601. [Google Scholar] [CrossRef]
- Salvador, A.C.; Huda, M.N.; Arends, D.; Elsaadi, A.M.; Gacasan, C.A.; Brockmann, G.A.; Valdar, W.; Bennett, B.J.; Threadgill, D.W. Analysis of Strain, Sex, and Diet-Dependent Modulation of Gut Microbiota Reveals Candidate Keystone Organisms Driving Microbial Diversity in Response to American and Ketogenic Diets. Microbiome 2023, 11, 220. [Google Scholar] [CrossRef]
| Author, Year | Type of Study | Diet Protocol | N° Patients Included | Inclusion Criteria | Duration of the Study | Liver Outcomes |
|---|---|---|---|---|---|---|
| Colles et al., 2006 [45] | Prospective, observational | VLCKD | 32 | Age 18–60 years BMI ≥ 50 kg/m2 (women) BMI ≥ 40 kg/m2 (men) | 12 weeks | Improvement in ALP, GPT, GGT, bilirubin, mean visceral adipose tissue. |
| Tendler et al., 2007 [46] | Prospective, single arm | LCKD | 5 | Age 18–65 years BMI ≥ 30 kg/m2 (but not >40 kg/m2) aminotransferase levels (>1.5 ULN in at least 2 separate occasions) | 6 months | Improvement in liver histology |
| Perez-Guisado et al., 2011 [47] | Prospective | Spanish Ketogenic Mediterranean Diet | 14 | Transaminase levels > 3ULN Plasma creatinine ≤ 1.3 mg/dL and urea ≤ 40 mg/dL No previous gout or high uric acid No alcoholic, and smoking habits, pregnancy or lactating, BMI ≥ 30 kg/m2 Age 18–65 years | 12 weeks | Improvement in GOT, GPT, steatosis degree by ultrasonography |
| Leonetti et al., 2015 [48] | Prospective | VLCKD for 10 days LCKD for 10 days LCD for 10 days | 48 | Age 18–67 years BMI > 40 kg/m2 ASA status I to III Pre bariatric surgery | 1 month | Improvement of the steatosis pattern and a mean 30% reduction in liver volume, evaluated by ultrasonography |
| Schiavo et al., 2018 [49] | Prospective | LCKD | 27 | Obese patients scheduled for bariatric surgery | 1 month | Improvement in GOT, GPT, GGT, left hepatic lobe volume evaluated by ultrasonography |
| Ministrini et al., 2019 [50] | Prospective | VLCKD | 52 | Patients scheduled for bariatric surgery Age 18–65 years BMI > 40 kg/m2 BMI > 35 kg/m2 and obesity related comorbidities | 25 days | Improvement in GOT, GPT, GGT, visceral fat and liver steatosis grade |
| Cunha et al., 2020 [38] | Randomized, prospective | VLCKD LCD | 24 VLCKD 24 LCD | Age > 18 years BMI > 30 kg/m2 | 2 months | Improvement in MR liver fat fraction, liver stiffness |
| Watanabe et al., 2020 [51] | Observational, prospective | VLCKD for 45 days followed by LCD for a further 45 days | 45 | Age 18–60 years BMI > 30 kg/m2 HIS > 36 | 3 months | Improvement in visceral adipose tissue mass, GOT, GPT, HSI |
| Luukkonen et al., 2020 [52] | Prospective | LCKD | 10 | Age 18–70 years Alcohol consumption < 20 g/d for women and <30 g/d for men | 6 days | Improvement in ALP, GGT, insulin sensitivity and intrahepatic triglycerides content |
| Crabtree et al., 2021 [53] | Randomized, prospective | KD + ketone supplementation KD + placebo | 28 | Age 21–65 years BMI 25–34.9 kg/m2 | 6 weeks | Improvement in liver fat, ALP, HSI |
| De Nucci et al., 2023 [54] | Real-life prospective | VLCKD | 87 | Age 18–64 years BMI > 25 kg/m2 | 8 weeks | Improvement in GOT, GPT, GGT, liver fat content by FibroScan, liver stiffness |
| Rinaldi et al., 2023 [55] | Real-life prospective | VLCKD | 33 | Age > 18 years BMI > 25 kg/m2 | 8 weeks | Improvement in FLI, hepatic fat by FibroScan, liver stiffness by FibroScan, GPT, GGT |
| Rinaldi et al., 2024 [56] | Real-life prospective | VLCKD | 112 | Age 18–65 BMI > 25 kg/m2 | 8 weeks | Improvement in liver steatosis and stiffness by FibroScan |
| Sila et al., 2024 [57] | Real-life prospective | VLCKD | 111 | Age 18–64 BMI > 25 kg/m2 | 8 weeks | Decrease in GGT, GPT, controlled attenuation parameter, lipids and fat mass |
| Chirapongsathorn et al., 2025 [58] | Randomized controlled study | 12 ketogenic diet 12 DASH diet | 24 | MASLD diagnosis by ultrasound, CT, or MRI OR FibroScan (CAP > 200 dB/m) | 8 weeks | Decrease in AST, triglyceride, and HDL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emanuele, F.; Biondo, M.; Tomasello, L.; Arnaldi, G.; Guarnotta, V. Ketogenic Diet in Steatotic Liver Disease: A Metabolic Approach to Hepatic Health. Nutrients 2025, 17, 1269. https://doi.org/10.3390/nu17071269
Emanuele F, Biondo M, Tomasello L, Arnaldi G, Guarnotta V. Ketogenic Diet in Steatotic Liver Disease: A Metabolic Approach to Hepatic Health. Nutrients. 2025; 17(7):1269. https://doi.org/10.3390/nu17071269
Chicago/Turabian StyleEmanuele, Fabrizio, Mattia Biondo, Laura Tomasello, Giorgio Arnaldi, and Valentina Guarnotta. 2025. "Ketogenic Diet in Steatotic Liver Disease: A Metabolic Approach to Hepatic Health" Nutrients 17, no. 7: 1269. https://doi.org/10.3390/nu17071269
APA StyleEmanuele, F., Biondo, M., Tomasello, L., Arnaldi, G., & Guarnotta, V. (2025). Ketogenic Diet in Steatotic Liver Disease: A Metabolic Approach to Hepatic Health. Nutrients, 17(7), 1269. https://doi.org/10.3390/nu17071269






