Anthocyanin-Rich Fraction from Kum Akha Black Rice Attenuates NLRP3 Inflammasome-Driven Lung Inflammation In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Herb Resources and Extraction Technique
2.3. Determination of Phenolics and Flavonoids
2.4. Determination of Anthocyanins
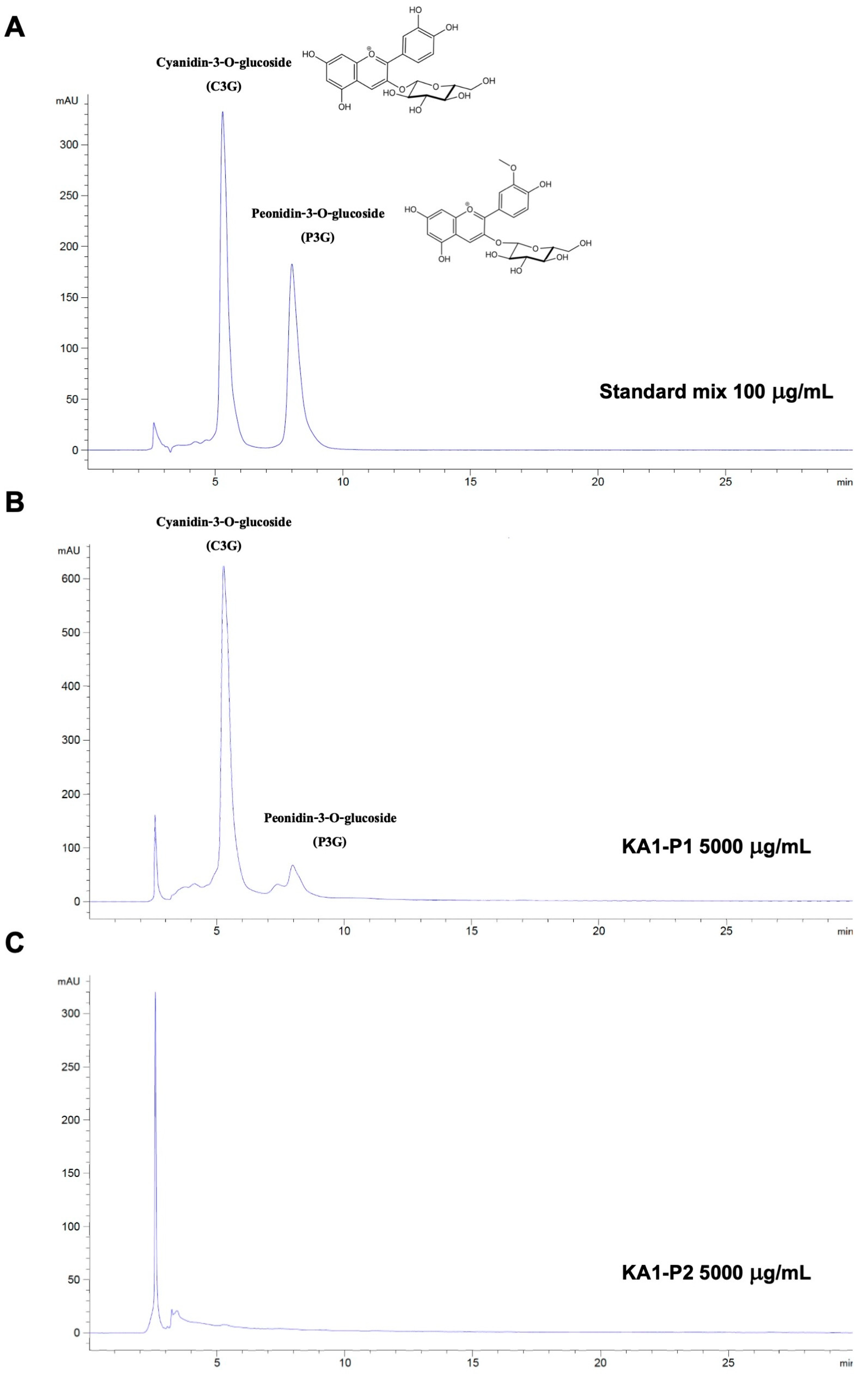
2.5. Identification of Cyanidin-3-O-Glucoside (C3G) and Peonidin-3-O-Glucoside (P3G) Anthocyanin Compounds in KA1 Using High-Performance Liquid Chromatography (HPLC)
2.6. Determination of Antioxidant Properties by ABTS and DPPH Assays
2.7. Cell Cultures
2.8. Determination of Cytotoxicity of KA1 Extracts
2.9. Determination of the Inhibitory Effects of KA Extract on Inflammatory Cytokine Secretions
2.10. Determination of the Inhibitory Effects of KA Extract on Inflammatory Gene Expressions
2.11. Determination of the Inhibitory Effects of KA1 Extract on NLRP3 Inflammasome-Associated Proteins Expressions
2.12. Animal Model
2.13. In Vivo Study of the Anti-Inflammatory Effects of KA1-P1 on LPS-Induced Respiratory Inflammation
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Characteristics of Kum Akha 1 (KA1) Black Rice Germ and Bran Extracts
3.2. Antioxidant Capacity of KA1 Black Rice Germ and Bran Extracts
3.3. Cell Viability Effects of KA1-P1 on A549 Lung Epithelial Cells
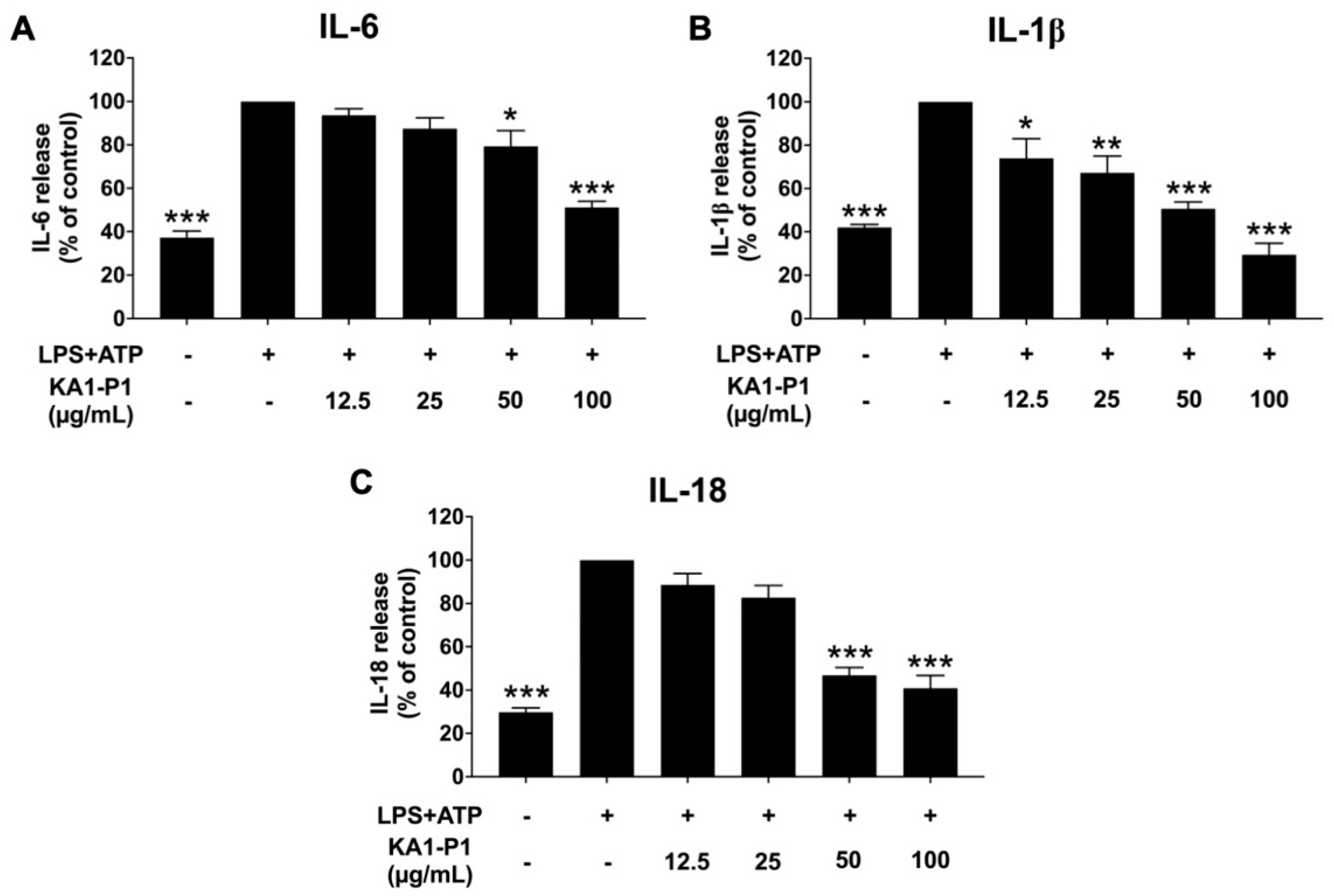
3.4. Inhibitory Effects of KA1-P1 on Inflammatory Cytokine Secretions in LPS and ATP-Induced A549 Lung Cells
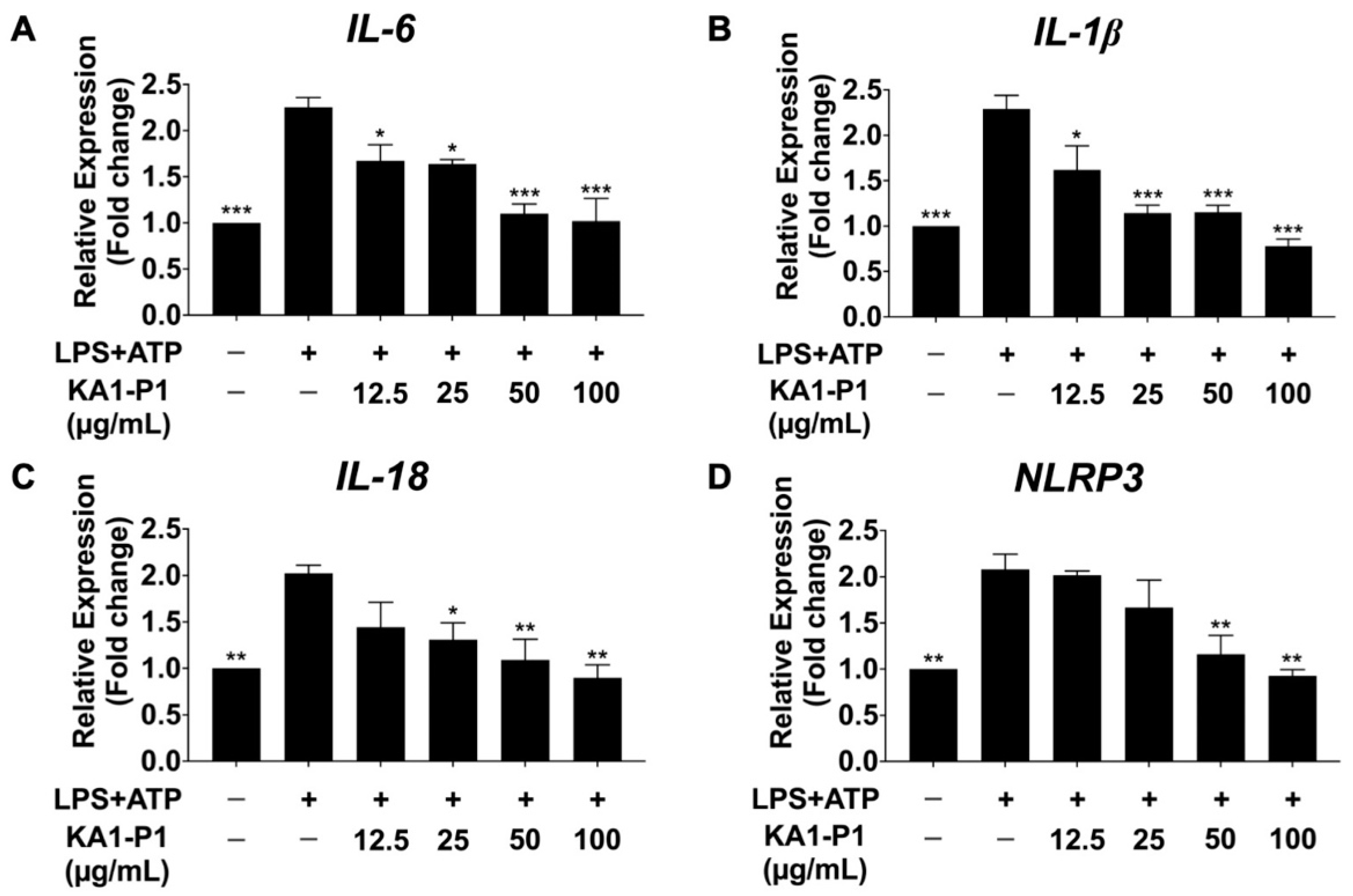
3.5. Effect of KA1-P1 on the Inhibition of IL-6, IL-1β, IL-18, and NLRP3 Gene Expressions in LPS + ATP-Exposed A549 Cells
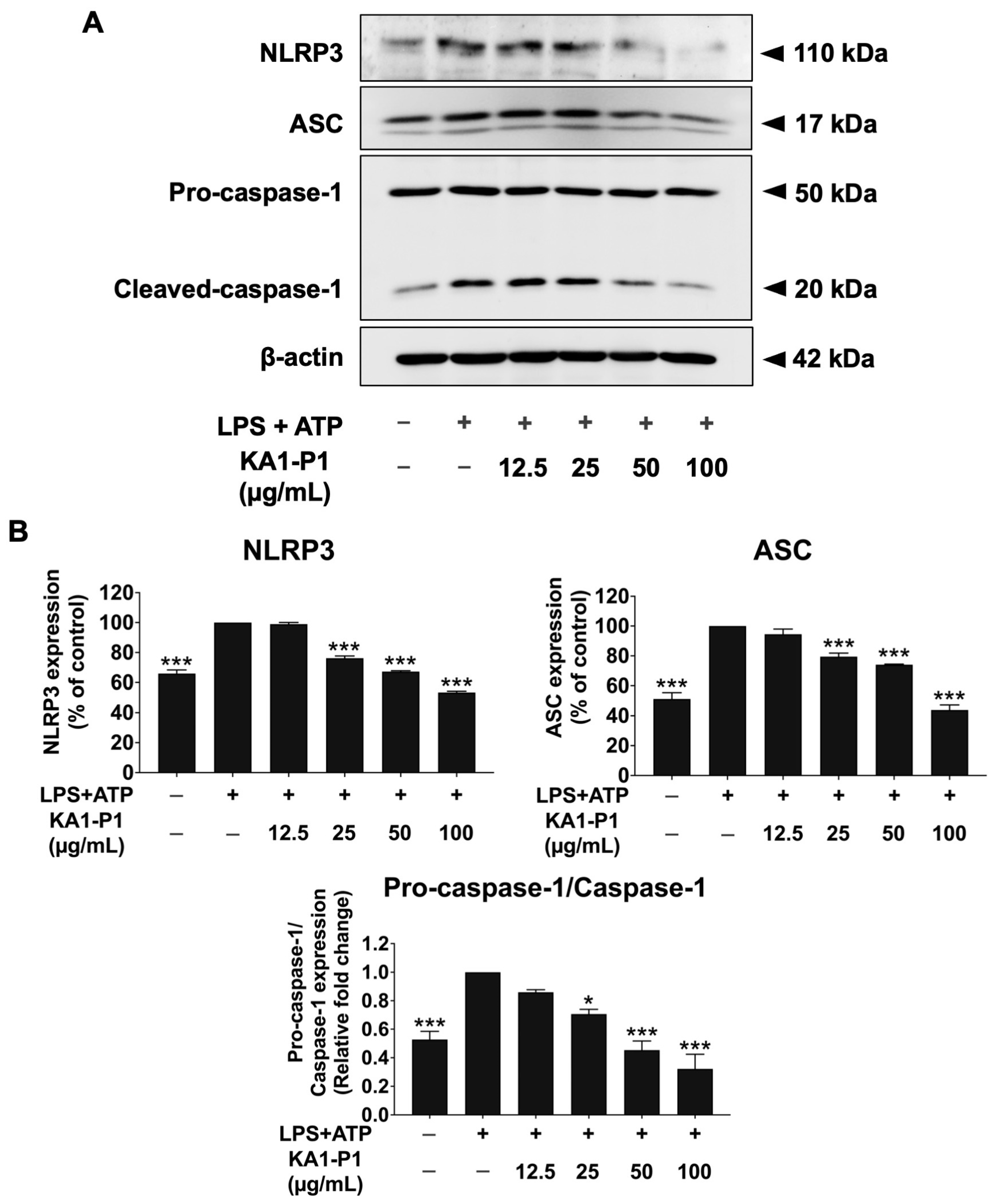
3.6. Inhibitory Effects of KA1-P1 on the NLRP3 Inflammasome Pathway in LPS + ATP-Induced A549 Cells
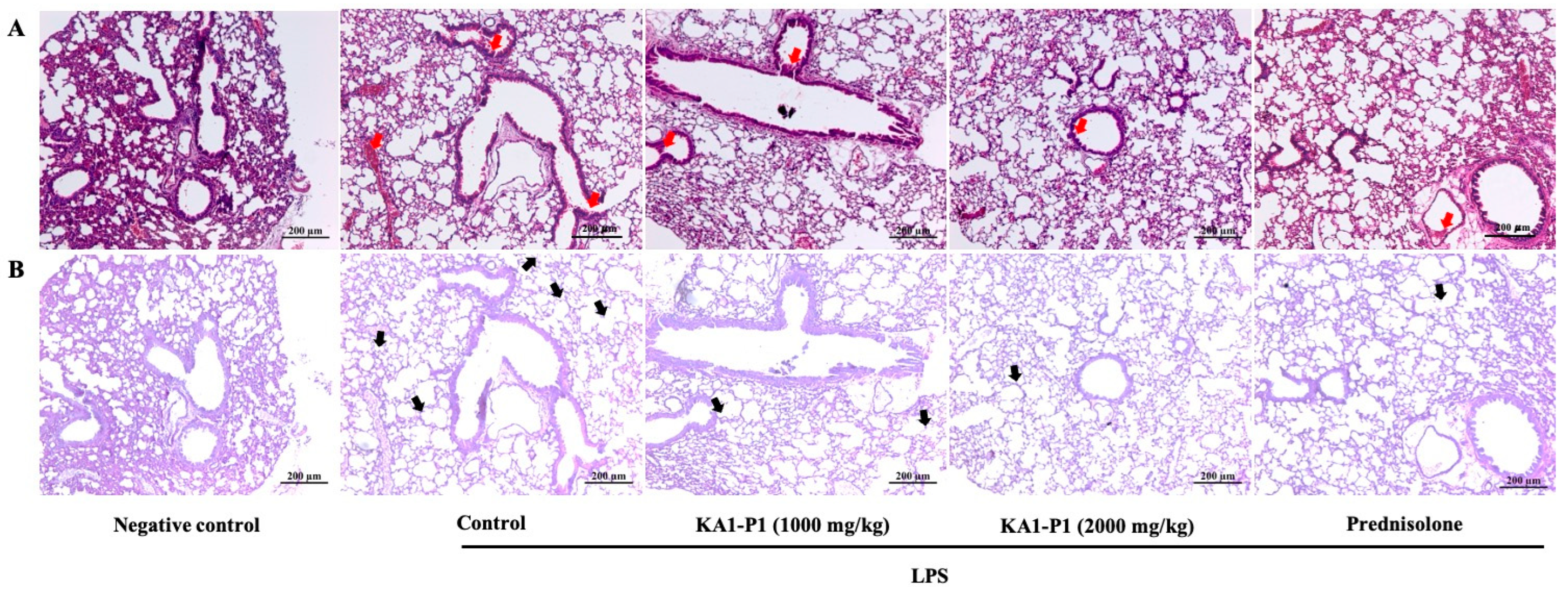
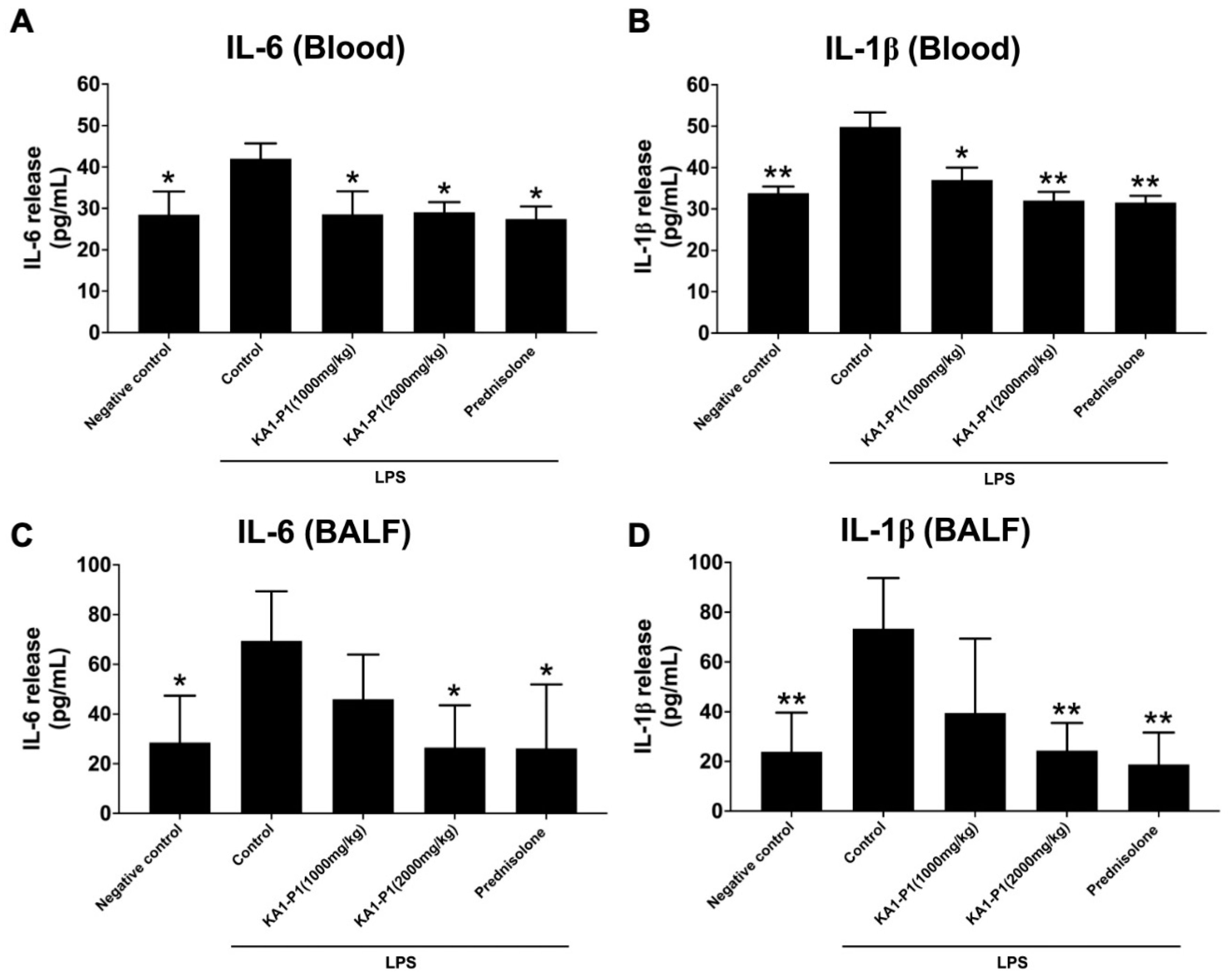
3.7. In Vivo Inhibitory Effects of KA1-P1 on Lower Respiratory Inflammation in LPS + ATP-Induced C57BL/6NJcl Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Anna, S.E.; Maniscalco, M.; Cappello, F.; Carone, M.; Motta, A.; Balbi, B.; Ricciardolo, F.L.M.; Caramori, G.; Stefano, A.D. Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease. Ann. Med. 2021, 53, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, S.; Tsubouchi, H.; Miura, A.; Matsuo, A.; Matsumoto, N.; Nakazato, M. The Impacts of Cellular Senescence in Elderly Pneumonia and in Age-Related Lung Diseases That Increase the Risk of Respiratory Infections. Int. J. Mol. Sci. 2017, 18, 503. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.P.; Orihuela, C.J.; Bogaert, D. Bacterial-Host Interactions: Physiology and Pathophysiology of Respiratory Infection. Physiol. Rev. 2018, 98, 781–811. [Google Scholar] [CrossRef]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017, 17, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.R.; Orihuela, C.J. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis. 2011, 2, 487–500. [Google Scholar]
- Xu, K.; Wei, Y.; Giunta, S.; Zhou, M.; Xia, S. Do inflammaging and coagul-aging play a role as conditions contributing to the co-occurrence of the severe hyper-inflammatory state and deadly coagulopathy during COVID-19 in older people? Exp. Gerontol. 2021, 151, 111423. [Google Scholar] [CrossRef]
- Silberberg, E.; Filep, J.G.; Ariel, A. Weathering the Storm: Harnessing the Resolution of Inflammation to Limit COVID-19 Pathogenesis. Front. Immunol. 2022, 13, 863449. [Google Scholar] [CrossRef]
- Dong, L.L.; Liu, Z.Y.; Chen, K.J.; Li, Z.Y.; Zhou, J.S.; Shen, H.H.; Chen, Z.H. The persistent inflammation in COPD: Is autoimmunity the core mechanism? Eur. Respir. Rev. 2024, 33, 230137. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef]
- Mora, A.L.; Rojas, M.; Pardo, A.; Selman, M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017, 16, 755–772. [Google Scholar] [CrossRef]
- Sayan, M.; Mossman, B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. Fibre Toxicol. 2016, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Cai, W.; Zhao, Y.; Xu, H.; Tang, H.; Chen, D.; Qian, F.; Sun, L. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol. Res. 2020, 158, 104884. [Google Scholar] [CrossRef] [PubMed]
- Stout-Delgado, H.W.; Cho, S.J.; Chu, S.G.; Mitzel, D.N.; Villalba, J.; El-Chemaly, S.; Ryter, S.W.; Choi, A.M.; Rosas, I.O. Age-Dependent Susceptibility to Pulmonary Fibrosis Is Associated with NLRP3 Inflammasome Activation. Am. J. Respir. Cell Mol. Biol. 2016, 55, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Ammit, A.J. The NLRP3 inflammasome: Role in airway inflammation. Clin. Exp. Allergy 2014, 44, 160–172. [Google Scholar] [CrossRef]
- Limtrakul, P.; Semmarath, W.; Mapoung, S. Anthocyanins and Proanthocyanidins in Natural. In Phytochemicals in Human Health; IntechOpen: London, UK, 2020; Volume 3. [Google Scholar]
- Fongfon, S.; Pusadee, T.; Prom-U-Thai, C.; Rerkasem, B.; Jamjod, S. Diversity of purple rice (Oryza sativa L.) landraces in Northern Thailand. Agronomy 2021, 11, 2029. [Google Scholar] [CrossRef]
- Pintha, K.; Yodkeeree, S.; Limtrakul, P. Proanthocyanidin in red rice inhibits MDA-MB-231 breast cancer cell invasion via the expression control of invasive proteins. Biol. Pharm. Bull. 2015, 38, 571–581. [Google Scholar] [CrossRef]
- Mapoung, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Srisawad, K.; Umsumarng, S.; Phromnoi, K.; Jamjod, S.; Prom, U.T.C.; Dejkriengkraikul, P. Comparative analysis of bioactive-phytochemical characteristics, antioxidants activities, and anti-inflammatory properties of selected black rice germ and bran (Oryza sativa L.) varieties. Eur. Food Res. Technol. 2023, 249, 451–464. [Google Scholar] [CrossRef]
- Kushwaha, U.K.S.; Kushwaha, U. Black Rice; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar]
- Das, M.; Dash, U.; Mahanand, S.S.; Nayak, P.K.; Kesavan, R.K. Black rice: A comprehensive review on its bioactive compounds, potential health benefits and food applications. Food Chem. Adv. 2023, 3, 100462. [Google Scholar]
- Purnama, P.R.; Suwanchaikasem, P.; Junbuathong, S.; Chotechuen, S.; Moung-Ngam, P.; Kasettranan, W.; Paliyavuth, C.; Pongpanich, M.; Roytrakul, S.; Comai, L.; et al. Uncovering genetic determinants of antioxidant properties in Thai landrace rice through genome-wide association analysis. Sci. Rep. 2025, 15, 1443. [Google Scholar] [CrossRef]
- Utasee, S.; Jamjod, S.; Lordkaew, S.; Prom-U-Thai, C. Improve anthocyanin and zinc concentration in purple rice by nitrogen and zinc fertilizer application. Rice Sci. 2022, 29, 435–450. [Google Scholar]
- Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway. Nutrients 2022, 14, 2738. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Rev. Int. 2021, 37, 759–811. [Google Scholar]
- Seesen, M.; Semmarath, W.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Ongprasert, K.; Chittrakul, J.; Siviroj, P.; Limtrakul Dejkriengkraikul, P. Combined Black Rice Germ, Bran Supplement and Exercise Intervention Modulate Aging Biomarkers and Improve Physical Performance and Lower-Body Muscle Strength Parameters in Aging Population. Int. J. Environ. Res. Public Health 2020, 17, 2931. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of Inflammatory Responses by Black Rice Extract in RAW 264.7 Macrophage Cells via Downregulation of NF-kB and AP-1 Signaling Pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar]
- Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J.; Limtrakul, P. Skin anti-aging assays of proanthocyanidin rich red rice extract, oryzanol and other phenolic compounds. Nat. Prod. Commun. 2018, 13, 1934578X1801300812. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Collaborators. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar]
- Krishnan, V.; Rani, R.; Pushkar, S.; Lal, S.; Srivastava, S.; Kumari, S.; Vinutha, T.; Dahuja, A.; Praveen, S.; Sachdev, A. Anthocyanin fingerprinting and dynamics in differentially pigmented exotic soybean genotypes using modified HPLC–DAD method. J. Food Meas. Charact. 2020, 14, 1966–1975. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Assessing the Activity of Natural Food Antioxidants. In Oxidation in Foods and Beverages and Antioxidant Applications; Elsevier: Amsterdam, The Netherlands, 2010; pp. 332–367. [Google Scholar]
- Koparal, A.T.; Zeytinoglu, M. Effects of carvacrol on a human non-small cell lung cancer (NSCLC) cell line, A549. Cytotechnology 2003, 43, 149–154. [Google Scholar]
- Semmarath, W.; Srisawad, K.; Arjsri, P.; Umsumarng, S.; Yodkeeree, S.; Jamjod, S.; Prom, U.T.C.; Dejkriengkraikul, P. Protective Effects of Proanthocyanidin-Rich Fraction from Red Rice Germ and Bran on Lung Cell Inflammation via Inhibition of NF-κB/NLRP3 Inflammasome Pathway. Nutrients 2023, 15, 3793. [Google Scholar] [CrossRef]
- Håkansson, H.F.; Smailagic, A.; Brunmark, C.; Miller-Larsson, A.; Lal, H. Altered lung function relates to inflammation in an acute LPS mouse model. Pulm. Pharmacol. Ther. 2012, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Lefort, J.; Motreff, L.; Vargaftig, B.B. Airway administration of Escherichia coli endotoxin to mice induces glucocorticosteroid-resistant bronchoconstriction and vasopermeation. Am. J. Respir. Cell Mol. Biol. 2001, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Makowska, I.J.; Vickers, L.; Mancell, J.; Weary, D.M. Evaluating methods of gas euthanasia for laboratory mice. Appl. Anim. Behav. Sci. 2009, 121, 230–235. [Google Scholar]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Nwachukwu, C.F. Inflammatory reaction-A posit to the double-edged sword. Int. J. Biol. Pharm. Sci. Arch. 2021, 1, 197–209. [Google Scholar] [CrossRef]
- Deka, B.; Bhattacharjee, B.; Shakya, A.; Shivavedi, N. Antiinflammatory Therapy as a Game-Changer Toward Antiaging. In Anti-Aging Drug Discovery on the Basis of Hallmarks of Aging; Elsevier: Amsterdam, The Netherlands, 2022; pp. 325–351. [Google Scholar]
- Dias, A.L.d.S.; Pachikian, B.; Larondelle, Y.; Quetin-Leclercq, J. Recent advances on bioactivities of black rice. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 470–476. [Google Scholar]
- Sivasinprasasn, S.; Tocharus, J.; Mahatheeranont, S.; Nakrat, S.; Tocharus, C. Anthocyanin-Rich Fraction of Black Rice Bran Extract Protects Against Amyloid β-Induced Oxidative Stress, Endoplasmic Reticulum Stress, and Neuronal Apoptosis in SK-N-SH Cells. Pharmaceuticals 2024, 17, 1039. [Google Scholar] [CrossRef]
- Sutharut, J.; Sudarat, J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int. Food Res. J. 2012, 19, 215–221. [Google Scholar]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- De Nardo, D.; De Nardo, C.M.; Latz, E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am. J. Pathol. 2014, 184, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Artlett, C.M. The Role of the NLRP3 Inflammasome in Fibrosis. Open Rheumatol. J. 2012, 6, 80–86. [Google Scholar] [CrossRef]
- Artlett, C.M. The Mechanism and Regulation of the NLRP3 Inflammasome during Fibrosis. Biomolecules 2022, 12, 634. [Google Scholar] [CrossRef]
- Patel, S. Danger-Associated Molecular Patterns (DAMPs): The Derivatives and Triggers of Inflammation. Curr. Allergy Asthma Rep. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Groslambert, M.; Py, B.F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 2018, 11, 359–374. [Google Scholar] [CrossRef]
- Jin, C.; Flavell, R.A. Molecular mechanism of NLRP3 inflammasome activation. J. Clin. Immunol. 2010, 30, 628–631. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Thepthanee, C.; Liu, C.C.; Yu, H.S.; Huang, H.S.; Yen, C.H.; Li, Y.H.; Lee, M.R.; Liaw, E.T. Evaluation of Phytochemical Contents and In Vitro Antioxidant, Anti-Inflammatory, and Anticancer Activities of Black Rice Leaf (Oryza sativa L.) Extract and Its Fractions. Foods 2021, 10, 2987. [Google Scholar] [CrossRef]
- Ravi Kumar, S.; Paudel, S.; Ghimire, L.; Bergeron, S.; Cai, S.; Zemans, R.L.; Downey, G.P.; Jeyaseelan, S. Emerging Roles of Inflammasomes in Acute Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, N.; Cho, Y.; Lockey, R.F.; Kolliputi, N. The role of the NLRP3 inflammasome in pulmonary diseases. Ther. Adv. Respir. Dis. 2015, 9, 188–197. [Google Scholar]
- Gonçalves, M.T.; Mitchell, T.J.; Lord, J.M. Immune ageing and susceptibility to Streptococcus pneumoniae. Biogerontology 2016, 17, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Qi, X.; Cai, Q.; Niu, L.; Huang, X.; Zhang, D.; Ling, J.; Wu, Y.; Chen, Y.; Yang, P.; et al. The role of NLRP3 inflammasome in aging and age-related diseases. Immun. Ageing 2024, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Sebag, S.C.; Koval, O.M.; Paschke, J.D.; Winters, C.J.; Jaffer, O.A.; Dworski, R.; Sutterwala, F.S.; Anderson, M.E.; Grumbach, I.M. Mitochondrial CaMKII inhibition in airway epithelium protects against allergic asthma. JCI Insight 2017, 2, e88297. [Google Scholar] [CrossRef]
- Hsieh, P.-C.; Peng, C.-K.; Liu, G.-T.; Kuo, C.-Y.; Tzeng, I.-S.; Wang, M.-C.; Lan, C.-C.; Huang, K.-L. Aqueous extract of descuraniae semen attenuates lipopolysaccharide-induced inflammation and apoptosis by regulating the proteasomal degradation and IRE1α-dependent unfolded protein response in A549 cells. Front. Immunol. 2022, 13, 916102. [Google Scholar]
- Gugliandolo, E.; Fusco, R.; Ginestra, G.; D’Amico, R.; Bisignano, C.; Mandalari, G.; Cuzzocrea, S.; Di Paola, R. Involvement of TLR4 and PPAR-α Receptors in Host Response and NLRP3 Inflammasome Activation, Against Pulmonary Infection with Pseudomonas Aeruginosa. Shock 2019, 51, 221–227. [Google Scholar] [CrossRef]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef]
- Rodriguez, I.; Carnevale, K.J. Systematic Review: JAK-STAT Regulation and Its Impact on Inflammation Response in ARDS from COVID-19. Immuno 2024, 4, 147–158. [Google Scholar] [CrossRef]
- Zahid, A.; Li, B.; Kombe, A.J.K.; Jin, T.; Tao, J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2538. [Google Scholar] [CrossRef]
- Bagherniya, M.; Khedmatgozar, H.; Fakheran, O.; Xu, S.; Johnston, T.P.; Sahebkar, A. Medicinal plants and bioactive natural products as inhibitors of NLRP3 inflammasome. Phytother. Res. 2021, 35, 4804–4833. [Google Scholar] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Meng, F.; Köhler, K.; Bülow, J.M.; Wagner, A.; Neunaber, C.; Bundkirchen, K.; Relja, B. Age-related exacerbation of lung damage after trauma is associated with increased expression of inflammasome components. Front. Immunol. 2023, 14, 1253637. [Google Scholar] [CrossRef]
- Yuan, Z.; Yu, D.; Gou, T.; Tang, G.; Guo, C.; Shi, J. Research progress of NLRP3 inflammasome and its inhibitors with aging diseases. Eur. J. Pharmacol. 2023, 957, 175931. [Google Scholar] [CrossRef]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2008, 2, 1–11. [Google Scholar]
- Morazzoni, P.; Bombardelli, E. Vaccinium myrtillus L. Fitoterapia 1996, 67, 3–29. [Google Scholar]
- Cladis, D.P.; Weaver, C.M.; Ferruzzi, M.G. (Poly)phenol toxicity in vivo following oral administration: A targeted narrative review of (poly)phenols from green tea, grape, and anthocyanin-rich extracts. Phytother. Res. 2022, 36, 323–335. [Google Scholar] [CrossRef]
- Additives, E.P.o.F.; Food, N.S.a.t. Scientific Opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145. [Google Scholar]
- Zhou, Y.; Wang, S.; Wan, T.; Huang, Y.; Pang, N.; Jiang, X.; Gu, Y.; Zhang, Z.; Luo, J.; Yang, L. Cyanidin-3-O-β-glucoside inactivates NLRP3 inflammasome and alleviates alcoholic steatohepatitis via SirT1/NF-κB signaling pathway. Free Radic. Biol. Med. 2020, 160, 334–341. [Google Scholar]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef]
- Kay, C.D. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr. Res. Rev. 2006, 19, 137–146. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Cooke, D.; Brown, K.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother. Pharmacol. 2009, 64, 1261–1268. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Aqil, F.; Vadhanam, M.V.; Jeyabalan, J.; Cai, J.; Singh, I.P.; Gupta, R.C. Detection of anthocyanins/anthocyanidins in animal tissues. J. Agric. Food Chem. 2014, 62, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Cheng, Q.; Wang, Y.; Mu, H.; Zhang, Y. COPD and Gut-Lung Axis: How Microbiota and Host Inflammasome Influence COPD and Related Therapeutics. Front. Microbiol. 2022, 13, 868086. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Wang, H.; Lv, C.; Wang, S.; Ying, H.; Weng, Y.; Yu, W. NLRP3 Inflammasome Involves in the Acute Exacerbation of Patients with Chronic Obstructive Pulmonary Disease. Inflammation 2018, 41, 1321–1333. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Job, E.R.; Saelens, X.; Roose, K. Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration. J. Vis. Exp. 2017, 123, 55398. [Google Scholar] [CrossRef]
- Brass, D.M.; Hollingsworth, J.W.; Cinque, M.; Li, Z.; Potts, E.; Toloza, E.; Foster, W.M.; Schwartz, D.A. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, K.; Wrenger, S.; Liu, B.; Schaudien, D.; Hesse, C.; Gomez-Mariano, G.; Perez-Luz, S.; Sewald, K.; DeLuca, D.; Wurm, M.J.; et al. Mice inflammatory responses to inhaled aerosolized LPS: Effects of various forms of human alpha1-antitrypsin. J. Leukoc. Biol. 2023, 113, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.J.; Nanchal, R. Cotton dust lung diseases. Curr. Opin. Pulm. Med. 2007, 13, 137–141. [Google Scholar] [CrossRef]
- Brass, D.M.; Savov, J.D.; Gavett, S.H.; Haykal-Coates, N.; Schwartz, D.A. Subchronic endotoxin inhalation causes persistent airway disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 285, L755–L761. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequences |
|---|---|
| NLRP3 | Forward, 5′-AAC ATG CCC AAG GAG GAA GA-3′ |
| Reverse, 5′-GGC TGT TCA CCA ATC CAT GA-3′ | |
| IL-1β | Forward, 5′-TGC TCA AGT GTC TGA AGC AG-3′ |
| Reverse, 5′-TGG TGG TCG GAG ATT CGT AG-3′ | |
| IL-18 | Forward, 5′-TCG GGA AGA GGA AAG GAA CC-3′ |
| Reverse, 5′-TTC TAC TGG TTC AGC AGC CA-3′ | |
| IL-6 | Forward: 5′-ATG AAC TCC TTC ACA AGC-3′ |
| Reverse: 5′-GTT TTC TGC CAG TGC CTC TTT G-3′ | |
| GAPDH | Forward, 5′-TCA ACA GCG ACA CCC AC-3′ |
| Reverse, 5′-GGG TCT CTC TCT TCC TCT TGT G-3′ |
| Phytochemicals | KA1-P1 | KA1-P2 |
|---|---|---|
| Total phenolic contents (mg GAE/g extract) | 168.82 ± 7.65 ** | 73.62 ± 0.69 |
| Total flavonoid contents (mg CE/g extract) | 97.45 ± 6.96 ** | 39.29 ± 4.11 |
| Total anthocyanins (mg/g extract) | 74.63 ± 1.66 ** | 33.26 ± 1.11 |
| Cyanidin-3-glucoside (C3G) (mg/g extract) | 45.58 ± 0.48 | ND |
| Peonidin-3-glucoside (P3G) (mg/g extract) | 6.92 ± 0.29 | ND |
| Extracts | IC50 (μg/mL) | |
|---|---|---|
| DPPH Assay | ABTS Assay | |
| KA1-P1 | 47.82 ± 4.65 * | 17.35 ± 0.66 * |
| KA1-P2 | 215.10 ± 28.23 | 60.05 ± 0.42 |
| Vitamin E | 24.74 ± 0.60 | - |
| Trolox | - | 2.45 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Intanil, I.; Jamjod, S.; Prom-u-thai, C.; Dejkriengkraikul, P. Anthocyanin-Rich Fraction from Kum Akha Black Rice Attenuates NLRP3 Inflammasome-Driven Lung Inflammation In Vitro and In Vivo. Nutrients 2025, 17, 1186. https://doi.org/10.3390/nu17071186
Umsumarng S, Semmarath W, Arjsri P, Srisawad K, Intanil I, Jamjod S, Prom-u-thai C, Dejkriengkraikul P. Anthocyanin-Rich Fraction from Kum Akha Black Rice Attenuates NLRP3 Inflammasome-Driven Lung Inflammation In Vitro and In Vivo. Nutrients. 2025; 17(7):1186. https://doi.org/10.3390/nu17071186
Chicago/Turabian StyleUmsumarng, Sonthaya, Warathit Semmarath, Punnida Arjsri, Kamonwan Srisawad, Intranee Intanil, Sansanee Jamjod, Chanakan Prom-u-thai, and Pornngarm Dejkriengkraikul. 2025. "Anthocyanin-Rich Fraction from Kum Akha Black Rice Attenuates NLRP3 Inflammasome-Driven Lung Inflammation In Vitro and In Vivo" Nutrients 17, no. 7: 1186. https://doi.org/10.3390/nu17071186
APA StyleUmsumarng, S., Semmarath, W., Arjsri, P., Srisawad, K., Intanil, I., Jamjod, S., Prom-u-thai, C., & Dejkriengkraikul, P. (2025). Anthocyanin-Rich Fraction from Kum Akha Black Rice Attenuates NLRP3 Inflammasome-Driven Lung Inflammation In Vitro and In Vivo. Nutrients, 17(7), 1186. https://doi.org/10.3390/nu17071186










