Research Progress on Anti-Hyperlipidemia Peptides Derived from Foods
Abstract
1. Introduction
2. Predisposing Factors of Hyperlipidemia
2.1. Gene Expression
2.2. Eating Habits
2.3. Lifestyle Habits
3. The Anti-Hyperlipidemia Mechanism of Peptides
3.1. Inhibition of the Synthesis of Cholesterol
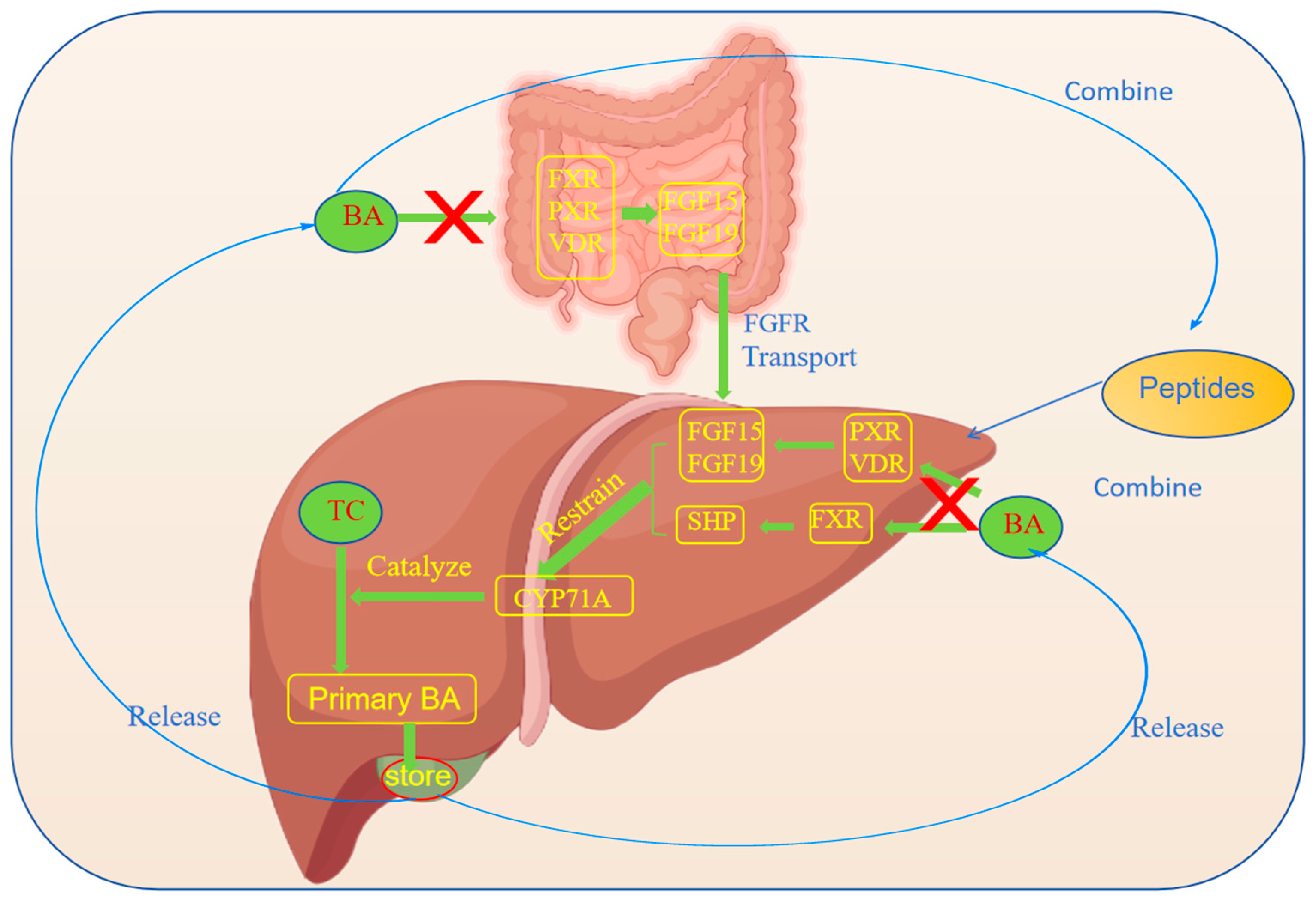
3.2. Promotion of the Excretion of Cholesterol
3.3. Regulation of the Lipoprotein Metabolism
3.4. Anti-Inflammatory and Antioxidative Effects
4. Sources of Anti-Hyperlipidemic Peptides
4.1. Marine Anti-Hyperlipidemic Peptides
4.2. Plant-Derived Anti-Hyperlipidemic Peptides
4.3. Animal-Derived Anti-Hyperlipidemic Polypeptides
5. Limitations of Peptides
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Mancini, G.B.J.; Hegele, R.A.; Leiter, L.A. Dyslipidemia. Can. J. Diabetes 2018, 42, S178–S185. [Google Scholar] [CrossRef] [PubMed]
- Berta, E.; Zsíros, N.; Bodor, M.; Balogh, I.; Lőrincz, H.; Paragh, G.; Harangi, M. Clinical Aspects of Genetic and Non-Genetic Cardiovascular Risk Factors in Familial Hypercholesterolemia. Genes 2022, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-Associated Fatty Liver Disease and Lipoprotein Metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Gau, G.T.; Wright, R.S. Pathophysiology, Diagnosis, and Management of Dyslipidemia. Curr. Probl. Cardiol. 2006, 31, 445–486. [Google Scholar] [CrossRef]
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef]
- Tokgozoglu, L.; Hekimsoy, V.; Costabile, G.; Calabrese, I.; Riccardi, G. Diet, Lifestyle, Smoking. In Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets; von Eckardstein, A., Binder, C.J., Eds.; Springer: Cham, Switzerland, 2022; ISBN 978-3-030-86075-2. [Google Scholar]
- Parhofer, K.G.; Laufs, U. Lipid Profile and Lipoprotein(a) Testing. Dtsch. Arztebl. Int. 2023, 120, 582–588. [Google Scholar] [CrossRef]
- Kushner, R.F. Weight Loss Strategies for Treatment of Obesity: Lifestyle Management and Pharmacotherapy. Progress. Cardiovasc. Dis. 2018, 61, 246–252. [Google Scholar] [CrossRef]
- Zodda, D.; Giammona, R.; Schifilliti, S. Treatment Strategy for Dyslipidemia in Cardiovascular Disease Prevention: Focus on Old and New Drugs. Pharmacy 2018, 6, 10. [Google Scholar] [CrossRef]
- Mudgil, P.; Feyisola, A.; Alyafei, A.; Yap, P.-G.; Gan, C.-Y.; Maqsood, S. Unlocking the Hypolipidemic Potential of Bioactive Peptides Derived from Probiotic Fermented Cattle, Camel, Goat, and Sheep Milk: A Comprehensive Investigation through in Vitro, in Silico, and Molecular Docking Studies. Front. Sustain. Food Syst. 2024, 8, 1443708. [Google Scholar] [CrossRef]
- Wei, L.; Wu, H.; Wang, X.; Wen, L.; Cui, B.; Cheng, Y. Comprehensive Review of Plant-Derived Anti-Hyperlipidemia Peptides: Production, Anti-Hyperlipidemia Mechanism, and Structure-Activity Relationship Study. Food Chem. 2024, 461, 140715. [Google Scholar] [CrossRef]
- Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Unveiling Familial Hypercholesterolemia—Review, Cardiovascular Complications, Lipid-Lowering Treatment and Its Efficacy. Int. J. Mol. Sci. 2024, 25, 1637. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.; Coschigano, K.T. ApoB48 as an Efficient Regulator of Intestinal Lipid Transport. Front. Physiol. 2020, 11, 796. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Poulaki, A.; Evangelopoulos, A.; Panagopoulos, F.; Stratigou, T.; Geladari, E.; Karampela, I.; Dalamaga, M. ApoB100 and Atherosclerosis: What’s New in the 21st Century? Metabolites 2024, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Shapiro, M.D. Apolipoproteins in Vascular Biology and Atherosclerotic Disease. Nat. Rev. Cardiol. 2022, 19, 168–179. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease: Pathophysiological, Genetic, and Therapeutic Insights: A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. The Complex Molecular Genetics of Familial Hypercholesterolaemia. Nat. Rev. Cardiol. 2019, 16, 9–20. [Google Scholar] [CrossRef]
- Paixao, A.R.M.; Berry, J.D.; Neeland, I.J.; Ayers, C.R.; Rohatgi, A.; de Lemos, J.A.; Khera, A. Coronary Artery Calcification and Family History of Myocardial Infarction in the Dallas Heart Study. Jacc Cardiovasc. Imaging 2014, 7, 679–686. [Google Scholar] [CrossRef]
- Abifadel, M.; Rabès, J.-P.; Devillers, M.; Munnich, A.; Erlich, D.; Junien, C.; Varret, M.; Boileau, C. Mutations and Polymorphisms in the Proprotein Convertase Subtilisin Kexin 9 (PCSK9) Gene in Cholesterol Metabolism and Disease. Hum. Mutat. 2009, 30, 520–529. [Google Scholar] [CrossRef]
- Bao, X.; Liang, Y.; Chang, H.; Cai, T.; Feng, B.; Gordon, K.; Zhu, Y.; Shi, H.; He, Y.; Xie, L. Targeting Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): From Bench to Bedside. Signal Transduct. Target. Ther. 2024, 9, 13. [Google Scholar] [CrossRef]
- Kuzmich, N.; Andresyuk, E.; Porozov, Y.; Tarasov, V.; Samsonov, M.; Preferanskaya, N.; Veselov, V.; Alyautdin, R. PCSK9 as a Target for Development of a New Generation of Hypolipidemic Drugs. Molecules 2022, 27, 434. [Google Scholar] [CrossRef]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A Key Modulator of Cardiovascular Health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.R.; Lightstone, F.C.; Cheng, F. In Silico Insights into Protein–Protein Interaction Disruptive Mutations in the PCSK9-LDLR Complex. Int. J. Mol. Sci. 2020, 21, 1550. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Lambert, G.; Cariou, B.; Hovingh, G.K. Inhibiting PCSK9—Biology beyond LDL Control. Nat. Rev. Endocrinol. 2018, 15, 52–62. [Google Scholar] [CrossRef]
- Agarwala, A.; Petersen, K.S.; Jafari, F.; Kris-Etherton, P.M. Dietary Management of Dyslipidemia and the Impact of Dietary Patterns on Lipid Disorders. Progress. Cardiovasc. Dis. 2022, 75, 49–58. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, Z.; Liu, Y.; Zhao, Y.; Fu, J.; Zhang, Y.; Liu, Y.; Fan, Z. Triglyceride to High-Density Lipoprotein Cholesterol Ratio and Cardiovascular Events in the General Population: A Systematic Review and Meta-Analysis of Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 318–329. [Google Scholar] [CrossRef]
- Tappy, L. Metabolism of Sugars: A Window to the Regulation of Glucose and Lipid Homeostasis by Splanchnic Organs. Clin. Nutr. 2021, 40, 1691–1698. [Google Scholar] [CrossRef]
- Arnone, D.; Chabot, C.; Heba, A.-C.; Kökten, T.; Caron, B.; Hansmannel, F.; Dreumont, N.; Ananthakrishnan, A.N.; Quilliot, D.; Peyrin-Biroulet, L. Sugars and Gastrointestinal Health. Clin. Gastroenterol. Hepatol. 2022, 20, 1912–1924.e7. [Google Scholar] [CrossRef]
- Hunter, R.W.; Dhaun, N.; Bailey, M.A. The Impact of Excessive Salt Intake on Human Health. Nat. Rev. Nephrol. 2022, 18, 321–335. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Marsigliante, S. The Effects of Exercise Training on Lipid Metabolism and Coronary Heart Disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H76–H88. [Google Scholar] [CrossRef]
- Fikenzer, K.; Fikenzer, S.; Laufs, U.; Werner, C. Effects of Endurance Training on Serum Lipids. Vasc. Pharmacol. 2018, 101, 9–20. [Google Scholar] [CrossRef]
- Halverstadt, A.; Phares, D.A.; Wilund, K.R.; Goldberg, A.P.; Hagberg, J.M. Endurance Exercise Training Raises High-Density Lipoprotein Cholesterol and Lowers Small Low-Density Lipoprotein and Very Low-Density Lipoprotein Independent of Body Fat Phenotypes in Older Men and Women. Metabolism 2007, 56, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Remchak, M.-M.E.; Piersol, K.L.; Bhatti, S.; Spaeth, A.M.; Buckman, J.F.; Malin, S.K. Considerations for Maximizing the Exercise “Drug” to Combat Insulin Resistance: Role of Nutrition, Sleep, and Alcohol. Nutrients 2021, 13, 1708. [Google Scholar] [CrossRef] [PubMed]
- Pinches, I.J.L.; Pinches, Y.L.; Johnson, J.O.; Haddad, N.C.; Boueri, M.G.; Oke, L.M.; Haddad, G.E. Could “Cellular Exercise” Be the Missing Ingredient in a Healthy Life? Diets, Caloric Restriction, and Exercise-Induced Hormesis. Nutrition 2022, 99–100, 111629. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Zheng, Q.; Gao, L.; Sun, Q. Sleep Deprivation and Central Appetite Regulation. Nutrients 2022, 14, 5196. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Kohanmoo, A. Sleep Deprivation in Development of Obesity, Effects on Appetite Regulation, Energy Metabolism, and Dietary Choices. Nutr. Res. Rev. 2023, 1–21. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Astrup, A.; Hjorth, M.F.; Sjödin, A.; Pijls, L.; Markus, C.R. Does Stress Influence Sleep Patterns, Food Intake, Weight Gain, Abdominal Obesity and Weight Loss Interventions and Vice Versa? Obes. Rev. 2018, 19, 81–97. [Google Scholar] [CrossRef]
- Yu, W.; Gao, C.; Zhao, X.; Li, C.; Fan, B.; Lv, J.; Wei, M.; He, L.; Su, C.; Zhang, T. Four-Way Decomposition of Effect of Cigarette Smoking and Body Mass Index on Serum Lipid Profiles. PLoS ONE 2022, 17, e0270486. [Google Scholar] [CrossRef]
- Jin, X.; Yang, S.; Lu, J.; Wu, M. Small, Dense Low-Density Lipoprotein-Cholesterol and Atherosclerosis: Relationship and Therapeutic Strategies. Front. Cardiovasc. Med. 2021, 8, 804214. [Google Scholar] [CrossRef]
- Harris, K.K.; Zopey, M.; Friedman, T.C. Metabolic Effects of Smoking Cessation. Nat. Rev. Endocrinol. 2016, 12, 684. [Google Scholar] [CrossRef]
- Mukharjee, S.; Bank, S.; Maiti, S. Chronic Tobacco Exposure by Smoking Develops Insulin Resistance. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 869–877. [Google Scholar] [CrossRef]
- Jing, Y.-S.; Ma, Y.-F.; Pan, F.-B.; Li, M.-S.; Zheng, Y.-G.; Wu, L.-F.; Zhang, D.-S. An Insight into Antihyperlipidemic Effects of Polysaccharides from Natural Resources. Molecules 2022, 27, 1903. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Nenkov, M.; Chen, Y.; Press, A.T.; Kaemmerer, E.; Gassler, N. Fatty Acid Metabolism and Acyl-CoA Synthetases in the Liver-Gut Axis. World J. Hepatol. 2021, 13, 1512–1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Geerts, C.; Furtos, A.; Waters, P.; Cyr, D.; Wang, S.; Mitchell, G.A. The Multiple Facets of Acetyl-CoA Metabolism: Energetics, Biosynthesis, Regulation, Acylation and Inborn Errors. Mol. Genet. Metab. 2023, 138, 106966. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, K.; Mi, X.; Rajput, S.A.; Qi, D. Effects of 3-Hydroxy-3-Methylglutaryl-CoA Reductase Inhibitors on Cholesterol Metabolism in Laying Hens. Animals 2023, 13, 1868. [Google Scholar] [CrossRef]
- Li, X.; Li, M. Unlocking Cholesterol Metabolism in Metabolic-Associated Steatotic Liver Disease: Molecular Targets and Natural Product Interventions. Pharmaceuticals 2024, 17, 1073. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, P.; Bairagya, H.R.; Sharma, S.; Singh, T.P.; Tiku, P.K. Inhibition of Human 3-Hydroxy-3-Methylglutaryl CoA Reductase by Peptides Leading to Cholesterol Homeostasis through SREBP2 Pathway in HepG2 Cells. Biochim. Biophys. Acta (Bba)-Proteins Proteom. 2019, 1867, 604–615. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Yun, L.; Kwon, D.Y. Recognized Sequence and Conformation in Design of Linear Peptides as a Competitive Inhibitor for HMG-CoA Reductase. J. Mol. Recognit. 2007, 20, 197–203. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Kim, M.J.; Yun, L.; Kwon, D.Y. Binding Effect and Design of a Competitive Inhibitory Peptide for HMG-CoA Reductase through Modeling of an Active Peptide Backbone. Bioorganic Med. Chem. 2008, 16, 1309–1318. [Google Scholar] [CrossRef]
- Shi, W.; Hou, T.; Guo, D.; He, H. Evaluation of Hypolipidemic Peptide (Val-Phe-Val-Arg-Asn) Virtual Screened from Chickpea Peptides by Pharmacophore Model in High-Fat Diet-Induced Obese Rat. J. Funct. Foods 2019, 54, 136–145. [Google Scholar] [CrossRef]
- Voronova, V.; Sokolov, V.; Al-Khaifi, A.; Straniero, S.; Kumar, C.; Peskov, K.; Helmlinger, G.; Rudling, M.; Angelin, B. A Physiology-Based Model of Bile Acid Distribution and Metabolism Under Healthy and Pathologic Conditions in Human Beings. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 149–170. [Google Scholar] [CrossRef]
- Hou, G.; Peng, W.; Wei, L.; Li, R.; Yuan, Y.; Huang, X.; Yin, Y. Lactobacillus Delbrueckii Interfere with Bile Acid Enterohepatic Circulation to Regulate Cholesterol Metabolism of Growing-Finishing Pigs via Its Bile Salt Hydrolase Activity. Front. Nutr. 2020, 7, 617676. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, K.; Li, F.; Gu, Z.; Liu, Q.; He, L.; Shao, T.; Song, Q.; Zhu, F.; Zhang, L.; et al. Probiotic Lactobacillus Rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology 2020, 71, 2050–2066. [Google Scholar] [CrossRef]
- Wang, H.H.; Portincasa, P.; de Bari, O.; Liu, K.J.; Garruti, G.; Neuschwander-Tetri, B.A.; Wang, D.Q.-H. Prevention of Cholesterol Gallstones by Inhibiting Hepatic Biosynthesis and Intestinal Absorption of Cholesterol. Eur. J. Clin. Investig. 2013, 43, 413–426. [Google Scholar] [CrossRef]
- Ding, L.; Xu, Z.-J.; Shi, H.-H.; Xue, C.-H.; Huang, Q.-R.; Yanagita, T.; Wang, Y.-M.; Zhang, T.-T. Sterol Sulfate Alleviates Atherosclerosis via Mediating Hepatic Cholesterol Metabolism in ApoE−/− Mice. Food Funct. 2021, 12, 4887–4896. [Google Scholar] [CrossRef] [PubMed]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Bile Acid Receptors and Gastrointestinal Functions. Liver Res. 2019, 3, 31–39. [Google Scholar] [CrossRef]
- Katafuchi, T.; Makishima, M. Molecular Basis of Bile Acid-FXR-FGF15/19 Signaling Axis. Int. J. Mol. Sci. 2022, 23, 6046. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Trauner, M. Role of Bile Acids and Their Receptors in Gastrointestinal and Hepatic Pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Garcia, M.; Thirouard, L.; Sedès, L.; Monrose, M.; Holota, H.; Caira, F.; Volle, D.H.; Beaudoin, C. Nuclear Receptor Metabolism of Bile Acids and Xenobiotics: A Coordinated Detoxification System with Impact on Health and Diseases. Int. J. Mol. Sci. 2018, 19, 3630. [Google Scholar] [CrossRef]
- Jia, W.; Wei, M.; Rajani, C.; Zheng, X. Targeting the Alternative Bile Acid Synthetic Pathway for Metabolic Diseases. Protein Cell 2021, 12, 411–425. [Google Scholar] [CrossRef]
- Aguchem, R.N.; Okagu, I.U.; Okorigwe, E.M.; Uzoechina, J.O.; Nnemolisa, S.C.; Ezeorba, T.P.C. Role of CETP, PCSK-9, and CYP7-Alpha in Cholesterol Metabolism: Potential Targets for Natural Products in Managing Hypercholesterolemia. Life Sci. 2024, 351, 122823. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Chiang, J.Y.L. Bile Acid Receptors and Signaling Crosstalk in the Liver, Gut and Brain. Liver Res. 2021, 5, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Chakrabarti, R. The Role of AMP Kinase in Diabetes. Indian J. Med. Res. 2007, 125, 389–398. [Google Scholar] [PubMed]
- Bellesi, F.A.; Pilosof, A.M.R. Potential Implications of Food Proteins-Bile Salts Interactions. Food Hydrocoll. 2021, 118, 106766. [Google Scholar] [CrossRef]
- Wu, S.; Bin, W.; Tu, B.; Li, X.; Wang, W.; Liao, S.; Sun, C. A Delivery System for Oral Administration of Proteins/Peptides Through Bile Acid Transport Channels. J. Pharm. Sci. 2019, 108, 2143–2152. [Google Scholar] [CrossRef]
- Han, K.; Feng, G.; Li, T.; Wan, Z.; Zhao, W.; Yang, X. Extension Region Domain of Soybean 7S Globulin Contributes to Serum Triglyceride-Lowering Effect via Modulation of Bile Acids Homeostasis. Mol. Nutr. Food Res. 2023, 67, e2200883. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional Significance of Bioactive Peptides Derived from Soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Albitar, O.; D’Souza, C.M.; Adeghate, E.A. Effects of Lipoproteins on Metabolic Health. Nutrients 2024, 16, 2156. [Google Scholar] [CrossRef]
- Stanciulescu, L.A.; Scafa-Udriste, A.; Dorobantu, M. Exploring the Association between Low-Density Lipoprotein Subfractions and Major Adverse Cardiovascular Outcomes—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 6669. [Google Scholar] [CrossRef]
- Li, J.; Bollati, C.; Bartolomei, M.; Mazzolari, A.; Arnoldi, A.; Vistoli, G.; Lammi, C. Hempseed (Cannabis Sativa) Peptide H3 (IGFLIIWV) Exerts Cholesterol-Lowering Effects in Human Hepatic Cell Line. Nutrients 2022, 14, 1804. [Google Scholar] [CrossRef]
- van Zwol, W.; van de Sluis, B.; Ginsberg, H.N.; Kuivenhoven, J.A. VLDL Biogenesis and Secretion: It Takes a Village. Circ. Res. 2024, 134, 226–244. [Google Scholar] [CrossRef]
- Wei, K.; Wei, Y.; Xu, W.; Lu, F.; Ma, H. Corn Peptides Improved Obesity-Induced Non-Alcoholic Fatty Liver Disease through Relieving Lipid Metabolism, Insulin Resistance and Oxidative Stress. Food Funct. 2022, 13, 5782–5793. [Google Scholar] [CrossRef] [PubMed]
- Dunér, P.; Mattisson, I.Y.; Fogelstrand, P.; Glise, L.; Ruiz, S.; Farina, C.; Borén, J.; Nilsson, J.; Bengtsson, E. Antibodies against apoB100 Peptide 210 Inhibit Atherosclerosis in apoE-/- Mice. Sci. Rep. 2021, 11, 9022. [Google Scholar] [CrossRef]
- Fisher, E.A. The Degradation of Apolipoprotein B100: Multiple Opportunities to Regulate VLDL Triglyceride Production by Different Proteolytic Pathways. Biochim. Biophys. Acta 2012, 1821, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-X.; He, K.-Y.; Zhang, Z.-Z.; Qu, Y.-L.; Su, X.-B.; Shi, Y.; Wang, N.; Wang, L.; Han, Z.-G. LZP Is Required for Hepatic Triacylglycerol Transportation through Maintaining Apolipoprotein B Stability. PLoS Genet. 2021, 17, e1009357. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Huang, Q.; Chen, Y.; Zeng, K.; Yang, W.; Chen, J.; Chen, J. Important Hormones Regulating Lipid Metabolism. Molecules 2022, 27, 7052. [Google Scholar] [CrossRef]
- Ran, C.; Xie, M.; Li, J.; Xie, Y.; Ding, Q.; Li, Y.; Zhou, W.; Yang, Y.; Zhang, Z.; Olsen, R.E.; et al. Dietary Nucleotides Alleviate Hepatic Lipid Deposition via Exogenous AMP-Mediated AMPK Activation in Zebrafish. J. Nutr. 2021, 151, 2986–2996. [Google Scholar] [CrossRef]
- Fan, M.; Choi, Y.-J.; Tang, Y.; Kim, J.H.; Kim, B.; Lee, B.; Bae, S.M.; Kim, E.-K. AGL9: A Novel Hepatoprotective Peptide from the Larvae of Edible Insects Alleviates Obesity-Induced Hepatic Inflammation by Regulating AMPK/Nrf2 Signaling. Foods 2021, 10, 1973. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-Features of Food-Derived Bioactive Peptides with Anti-Inflammatory Activity: A Brief Review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Petroglou, D.; Kanellos, I.; Savopoulos, C.; Kaiafa, G.; Chrysochoou, A.; Skantzis, P.; Daios, S.; Hatzitolios, A.I.; Giannoglou, G. The LDL-Receptor and Its Molecular Properties: From Theory to Novel Biochemical and Pharmacological Approaches in Reducing LDL-Cholesterol. Curr. Med. Chem. 2020, 27, 317–333. [Google Scholar] [CrossRef]
- Nagaoka, S. Structure-Function Properties of Hypolipidemic Peptides. J. Food Biochem. 2019, 43, e12539. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Abachi, S.; Pilon, G.; Marette, A.; Bazinet, L.; Beaulieu, L. Immunomodulatory Effects of Fish Peptides on Cardiometabolic Syndrome Associated Risk Factors: A Review. Food Rev. Int. 2023, 39, 3926–3969. [Google Scholar] [CrossRef]
- Priyathilaka, T.T.; Bathige, S.D.N.K.; Lee, S.; Yang, H.; Jeong, T.; Lee, S.; Lee, J. Structural and Functional Analysis of Three Iκb Kinases (IKK) in Disk Abalone (Haliotis Discus Discus): Investigating Their Role in the Innate Immune Responses. Fish. Shellfish Immunol. 2020, 103, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide Dismutase: An Updated Review on Its Health Benefits and Industrial Applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Yin, H.; Hou, T. Selenium-Containing Proteins/Peptides from Plants: A Review on the Structures and Functions. J. Agric. Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and Biological Activities of Marine-Derived Bioactive Peptides: A Review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, L.; Wang, S.; Zhao, M.; Liu, X. Anti-Diabetic and Anti-Hyperlipidemic Effects of Sea Cucumber (Cucumaria Frondosa) Gonad Hydrolysates in Type II Diabetic Rats. Food Sci. Human. Wellness 2022, 11, 1614–1622. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ryu, B.; Kim, S.-K. Purification of Novel Anti-Inflammatory Peptides from Enzymatic Hydrolysate of the Edible Microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Shih, M.F.; Chen, L.C.; Cherng, J.Y. Chlorella 11-Peptide Inhibits the Production of Macrophage-Induced Adhesion Molecules and Reduces Endothelin-1 Expression and Endothelial Permeability. Mar. Drugs 2013, 11, 3861–3874. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a Seaweed Derived Platelet Activating Factor Acetylhydrolase (PAF-AH) Inhibitory Hydrolysate, Synthesis of Inhibitory Peptides and Assessment of Their Toxicity Using the Zebrafish Larvae Assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef]
- Pei, Y.; Cai, S.; Ryu, B.; Zhou, C.; Hong, P.; Qian, Z.-J. An ACE Inhibitory Peptide from Isochrysis Zhanjiangensis Exhibits Antihypertensive Effect via Anti-Inflammation and Anti-Apoptosis in HUVEC and Hypertensive Rats. J. Funct. Foods 2022, 92, 105061. [Google Scholar] [CrossRef]
- Wang, K.; Han, L.; Tan, Y.; Hong, H.; Fan, H.; Luo, Y. Novel Hypocholesterolemic Peptides Derived from Silver Carp Muscle: The Modulatory Effects on Enterohepatic Cholesterol Metabolism In Vitro and In Vivo. J. Agric. Food Chem. 2023, 71, 5565–5575. [Google Scholar] [CrossRef] [PubMed]
- Nasri, R.; Abdelhedi, O.; Jemil, I.; Amor, I.B.; Elfeki, A.; Gargouri, J.; Boualga, A.; Karra-Châabouni, M.; Nasri, M. Preventive Effect of Goby Fish Protein Hydrolysates on Hyperlipidemia and Cardiovascular Disease in Wistar Rats Fed a High-Fat/Fructose Diet. Rsc Adv. 2018, 8, 9383–9393. [Google Scholar] [CrossRef]
- Lassoued, I.; Mezghani, M.; Jridi, M.; Rahmouni, F.; Jamoussi, K.; Rebai, T.; El Feki, A.; Nasri, M.; Barkia, A. Protective Effects of Thornback Ray Muscle Protein Hydrolysate against Dyslipidemia, Oxidative Stress and Reduced Fertility Induced by High Cholesterol Diet in Adult Male Rats. Rsc Adv. 2018, 8, 22303–22312. [Google Scholar] [CrossRef]
- Xu, H.; Mu, Y.; Zhang, Y.; Li, J.; Liang, M.; Zheng, K.; Wei, Y. Graded Levels of Fish Protein Hydrolysate in High Plant Diets for Turbot (Scophthalmus Maximus): Effects on Growth Performance and Lipid Accumulation. Aquaculture 2016, 454, 140–147. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Purification and Identification of Antioxidant Peptides from Fermented Fish Sauce (Budu). J. Aquat. Food Product. Technol. 2019, 28, 14–24. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive Peptides Derived from Marine Sources: Biological and Functional Properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Chen, H.; Dong, X.; Zhou, M.; Xu, Z.; Xiang, X. Modulation of Lipid Metabolism by Oyster Peptides in Mice with High-Fat Diet-Induced Obesity. Food Biosci. 2024, 58, 103529. [Google Scholar] [CrossRef]
- Tastesen, H.S.; Keenan, A.H.; Madsen, L.; Kristiansen, K.; Liaset, B. Scallop Protein with Endogenous High Taurine and Glycine Content Prevents High-Fat, High-Sucrose-Induced Obesity and Improves Plasma Lipid Profile in Male C57BL/6J Mice. Amino Acids 2014, 46, 1659–1671. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A. IAVPGEVA, IAVPTGVA, and LPYP, Three Peptides from Soy Glycinin, Modulate Cholesterol Metabolism in HepG2 Cells through the Activation of the LDLR-SREBP2 Pathway. J. Funct. Foods 2015, 14, 469–478. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Investigations on the Hypocholesterolaemic Activity of LILPKHSDAD and LTFPGSAED, Two Peptides from Lupin β-Conglutin: Focus on LDLR and PCSK9 Pathways. J. Funct. Foods 2017, 32, 1–8. [Google Scholar] [CrossRef]
- Fisayo Ajayi, F.; Mudgil, P.; Gan, C.-Y.; Maqsood, S. Identification and Characterization of Cholesterol Esterase and Lipase Inhibitory Peptides from Amaranth Protein Hydrolysates. Food Chem. X 2021, 12, 100165. [Google Scholar] [CrossRef]
- Marques, M.R.; Fontanari, G.G.; Pimenta, D.C.; Soares-Freitas, R.M.; Areas, J.A.G. Proteolytic Hydrolysis of Cowpea Proteins Is Able to Release Peptides with Hypocholesterolemic Activit. Food Res. Int. 2015, 77, 43–48. [Google Scholar] [CrossRef]
- Wang, N.; Tong, Z.; Wang, D.; Zhang, Y.; Liu, T. Effects of Hericium Erinaceus Polypeptide on Lowering Blood Lipids of Mice with Hyperlipidemia Induced by a High-Fat Diet. J. Future Foods 2022, 2, 346–357. [Google Scholar] [CrossRef]
- Xue, Z.; Hou, X.; Yu, W.; Wen, H.; Zhang, Q.; Li, D.; Kou, X. Lipid Metabolism Potential and Mechanism of CPe-III from Chickpea (Cicer arietinum L.). Food Res. Int. 2018, 104, 126–133. [Google Scholar] [CrossRef]
- Yang, F.; Huang, J.; He, H.; Ju, X.; Ji, Y.; Deng, F.; Wang, Z.; He, R. Study on the Hypolipidemic Activity of Rapeseed Pro-tein-Derived Peptides. Food Chem. 2023, 423, 136315. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Mudgil, P.; Jobe, A.; Antony, P.; Vijayan, R.; Gan, C.-Y.; Maqsood, S. Novel Plant-Protein (Quinoa) Derived Bioactive Peptides with Potential Anti-Hypercholesterolemic Activities: Identification, Characterization and Molecular Docking of Bioactive Peptides. Foods 2023, 12, 1327. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Zhong, D.-Y.; Wang, G.-L.; Zhang, R.-G.; Zhang, Y.-L. Effect of Walnut Meal Peptides on Hyperlipidemia and Hepatic Lipid Metabolism in Rats Fed a High-Fat Diet. Nutrients 2021, 13, 1410. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Song, X.; Yang, M.; Xu, Y.; Zeng, X.; Wu, Z.; Pan, D.; Luo, H.; Lv, L.; et al. The Cholesterol-Lowering Effects and Mechanisms of Novel Milk Casein-Derived Peptides in Hyperlipidemia and Hypercholesterol Mice. Food Biosci. 2024, 61, 104730. [Google Scholar] [CrossRef]
- Zhang, Y.; Kouguchi, T.; Shimizu, K.; Sato, M.; Takahata, Y.; Morimatsu, F. Chicken Collagen Hydrolysate Reduces Proinflammatory Cytokine Production in C57BL/6.KOR-ApoEshl Mice. J. Nutr. Sci. Vitaminol. 2010, 56, 208–210. [Google Scholar] [CrossRef]
- Shimizu, M.; Tanabe, S.; Morimatsu, F.; Nagao, K.; Yanagita, T.; Kato, N.; Nishimura, T. Consumption of Pork-Liver Protein Hydrolysate Reduces Body Fat in Otsuka Long-Evans Tokushima Fatty Rats by Suppressing Hepatic Lipogenesis. Biosci. Biotechnol. Biochem. 2006, 70, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Al Musa, R.; Hussen Al-Garory, N. Biological Activities of Fermented Camel Milk Peptides: A Review. Al-Qadisiyah J. Agric. Sci. QJAS 2024, 14, 64–71. [Google Scholar] [CrossRef]
- Yang, K.-T.; Lin, C.; Liu, C.-W.; Chen, Y.-C. Effects of Chicken-Liver Hydrolysates on Lipid Metabolism in a High-Fat Diet. Food Chem. 2014, 160, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Z.; Xing, L.; Zhou, L.; Zhang, W. Bovine Bone Gelatin-Derived Peptides: Food Processing Characteristics and Evaluation of Antihypertensive and Antihyperlipidemic Activities. J. Agric. Food Chem. 2022, 70, 9877–9887. [Google Scholar] [CrossRef]
- Al-Shamsi, K.A.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel Milk Protein Hydrolysates with Improved Technofunctional Properties and Enhanced Antioxidant Potential in in Vitro and in Food Model Systems. J. Dairy. Sci. 2018, 101, 47–60. [Google Scholar] [CrossRef]
- Prete, V.; Abate, A.C.; Di Pietro, P.; De Lucia, M.; Vecchione, C.; Carrizzo, A. Beneficial Effects of Spirulina Supplementation in the Management of Cardiovascular Diseases. Nutrients 2024, 16, 642. [Google Scholar] [CrossRef]
- Wergedahl, H.; Liaset, B.; Gudbrandsen, O.A.; Lied, E.; Espe, M.; Muna, Z.; Mørk, S.; Berge, R.K. Fish Protein Hydrolysate Reduces Plasma Total Cholesterol, Increases the Proportion of HDL Cholesterol, and Lowers Acyl-CoA:Cholesterol Acyl-transferase Activity in Liver of Zucker Rats. J. Nutr. 2004, 134, 1320–1327. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed Peptides Exert Hypocholesterolemic Effects with a Statin-Like Mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Li, K.; Zhang, J.; Wei, B.; Wang, H. Absorption of Food-Derived Peptides: Mechanisms, Influencing Factors, and Enhancement Strategies. Food Res. Int. 2024, 197, 115190. [Google Scholar] [CrossRef]



| Polypeptide Source | Polypeptide Sequence | Anti-Hyperlipidemic Mechanism | Objects | Reference | Time | |
|---|---|---|---|---|---|---|
| Ocean source | SCGH | Polypeptide mixtures | Eliminate BA | Rats | [88] | 2022 |
| Spirulina | LDAVNR/MMLDF, C-phycocyanin | Prevent vascular disease | Cells | [89] | 2013 | |
| Chlorella | 11-Peptides | Prevent chronic inflammation | Cells | [90] | 2013 | |
| Palmaria palmata | PAF-AH inhibitory peptide | Prevent atherosclerosis | Cells | [91] | 2013 | |
| Microalgae | Polypeptide mixtures | Reduce OX-LDL apoptosis | Rats | [92] | 2022 | |
| Chub | Polypeptide mixtures | Inhibit TC absorption Promote LDL uptake | Mice | [93] | 2023 | |
| Goby fish | Polypeptide mixtures | Reduce blood fat | Rats | [94] | 2018 | |
| Thornback | Polypeptide mixtures | Reduce blood fat | Rats | [95] | 2018 | |
| Alaska cod | Polypeptide mixtures | Promote fecal BA excretion and expression of IBAT mRNA levels | Pigs, Rats, Mice | [96] | 2016 | |
| Salmon | Polypeptide mixtures | Promote fecal BA excretion and expression of IBAT mRNA levels | Pigs, Rats, Mice | [96] | 2016 | |
| Fermented chicken tail fish | Polypeptide mixtures | Antioxidant | [97] | 2018 | ||
| Ocean bass | Polypeptide mixtures | Reduce blood fat | [98] | 2013 | ||
| Oyster | Polypeptide mixtures | Inhibit PL activity | Mice | [99] | 2024 | |
| Pectinid | Polypeptide mixtures | Promote fecal BA excretion | Rats | [100] | 2014 | |
| Plant origin | Soybean | IAVPGEVA, IAVPTGVA, LPYP, FPFPRPPHQ, FMYL, MMLM, YSPHs, SFFFPFELPRE | Inhibit the activity of HMGCoAR, inhibit CEase | Cells | [101] | 2015 |
| Lupine | LILPKHSDAD, LTFPGSAED | Inhibitor of the HMGCoAR, lower PCSK9 | Cells | [102] | 2017 | |
| Amaranth | FPFVPAPT, MPFLPR, FPFVGP, FPFPPTLGY, FGAPR, FPFVPAPT | CEase inhibitor, PL inhibitor | Pigs | [103] | 2021 | |
| Cowpea | GCTLN | Inhibit the activity of HMGCoAR | [104] | 2015 | ||
| Hempseed | Polypeptide mixtures | Inhibit the catalytic activity of HMGCoAR | Cells | [102] | 2017 | |
| Hericium erinaceus | Polypeptide mixtures | Improve the antioxidant capacity | Mice | [105] | 2022 | |
| Chickpea | RQSHFANAQP | Block TC transport | Mice | [106] | 2018 | |
| Rapeseed | EFLELL | Regulate the LDLR-PCSK9 signaling pathway | Cells | [107] | 2023 | |
| Quinoa | QHPHGLGALCAAPPST, HVQGHPALPGVPAHW, ASNLDNPSPEGTVM, FSAGGL, PQHPHGLGALCAAPPST, KIVLDSDDPLFGGF, MFVPVPH, HVQGHPALPGVPAHW | CEase inhibitor, PL inhibitor | [108] | 2023 | ||
| Walnut | Polypeptide mixtures | Increase ApoB levels | Rats | [109] | 2021 | |
| Animal origin | Milk | LQPE, VLPVPQ, VAPFPE | Inhibit HNF4α Increase intestinal TC efflux | Mice | [110] | 2024 |
| Chicken | Polypeptide mixtures | Inhibit the expression of inflammatory cytokines | Mice | [111] | 2010 | |
| Pork liver | Polypeptide mixtures | Inhibit fat biosynthesis | Rats | [112] | 2006 | |
| Camel fermented milk | Polypeptide mixtures | Decompose bile salts | [113] | 2024 | ||
| Chicken liver | Polypeptide mixtures | Inhibit LP activity and BA binding activity | Hamsters | [114] | 2014 | |
| Ox bone | Polypeptide mixtures | Inhibit ACE/AngII/AT1R Activate AngII/AT2R | Rats | [115] | 2022 | |
| Honey bee venom | Mellitin, apamin, secapin, tertiapin, ado lapin, MCD peptide | Increase insulin secretion in pancreatic β -cells | [116] | 2018 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Qiao, K.; Zhang, L.; Liang, L.; Chen, S.; Chen, L.; Zhang, Y. Research Progress on Anti-Hyperlipidemia Peptides Derived from Foods. Nutrients 2025, 17, 1181. https://doi.org/10.3390/nu17071181
Zhao M, Qiao K, Zhang L, Liang L, Chen S, Chen L, Zhang Y. Research Progress on Anti-Hyperlipidemia Peptides Derived from Foods. Nutrients. 2025; 17(7):1181. https://doi.org/10.3390/nu17071181
Chicago/Turabian StyleZhao, Mingxia, Kaina Qiao, Lili Zhang, Li Liang, Shuxing Chen, Lishui Chen, and Yuyu Zhang. 2025. "Research Progress on Anti-Hyperlipidemia Peptides Derived from Foods" Nutrients 17, no. 7: 1181. https://doi.org/10.3390/nu17071181
APA StyleZhao, M., Qiao, K., Zhang, L., Liang, L., Chen, S., Chen, L., & Zhang, Y. (2025). Research Progress on Anti-Hyperlipidemia Peptides Derived from Foods. Nutrients, 17(7), 1181. https://doi.org/10.3390/nu17071181






