High-Fat Diet in Perinatal Period Promotes Liver Steatosis and Low Desaturation Capacity of Polyunsaturated Fatty Acids in Dams: A Link with Anxiety-Like Behavior in Rats
Abstract
1. Introduction
2. Materials and Methods
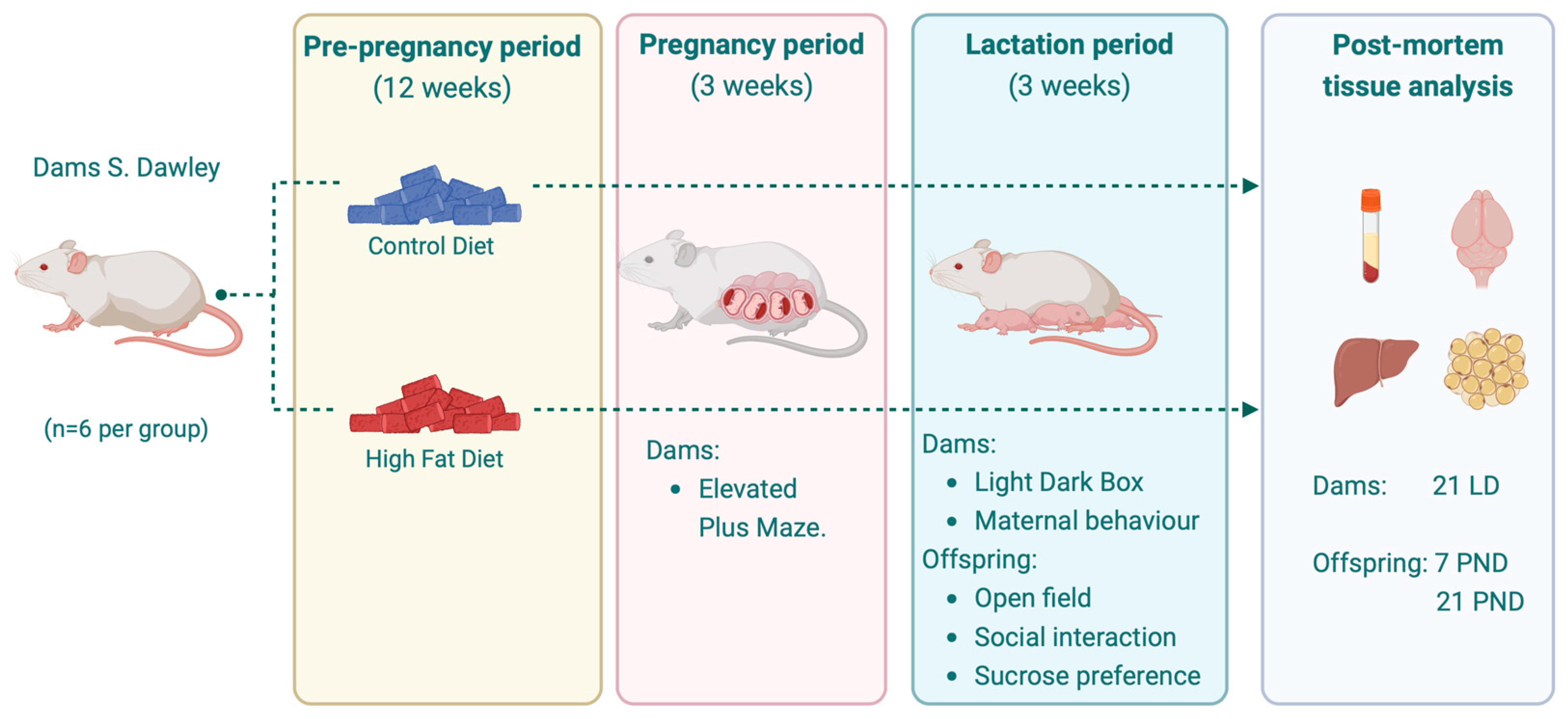
2.1. Animals and Experimental Design
2.2. Serum Parameters
2.3. Liver Parameters
2.4. Analysis of Fatty Acid by Gas Chromatography
2.5. Gene Expression Assays
2.6. Behavioral Procedures
2.6.1. Dam’s Anxiety-Like Behaviors
2.6.2. Maternal Behavior’s Assesment
2.6.3. Offspring Behavior’s Assesment
2.7. Statistical Analysis
3. Results
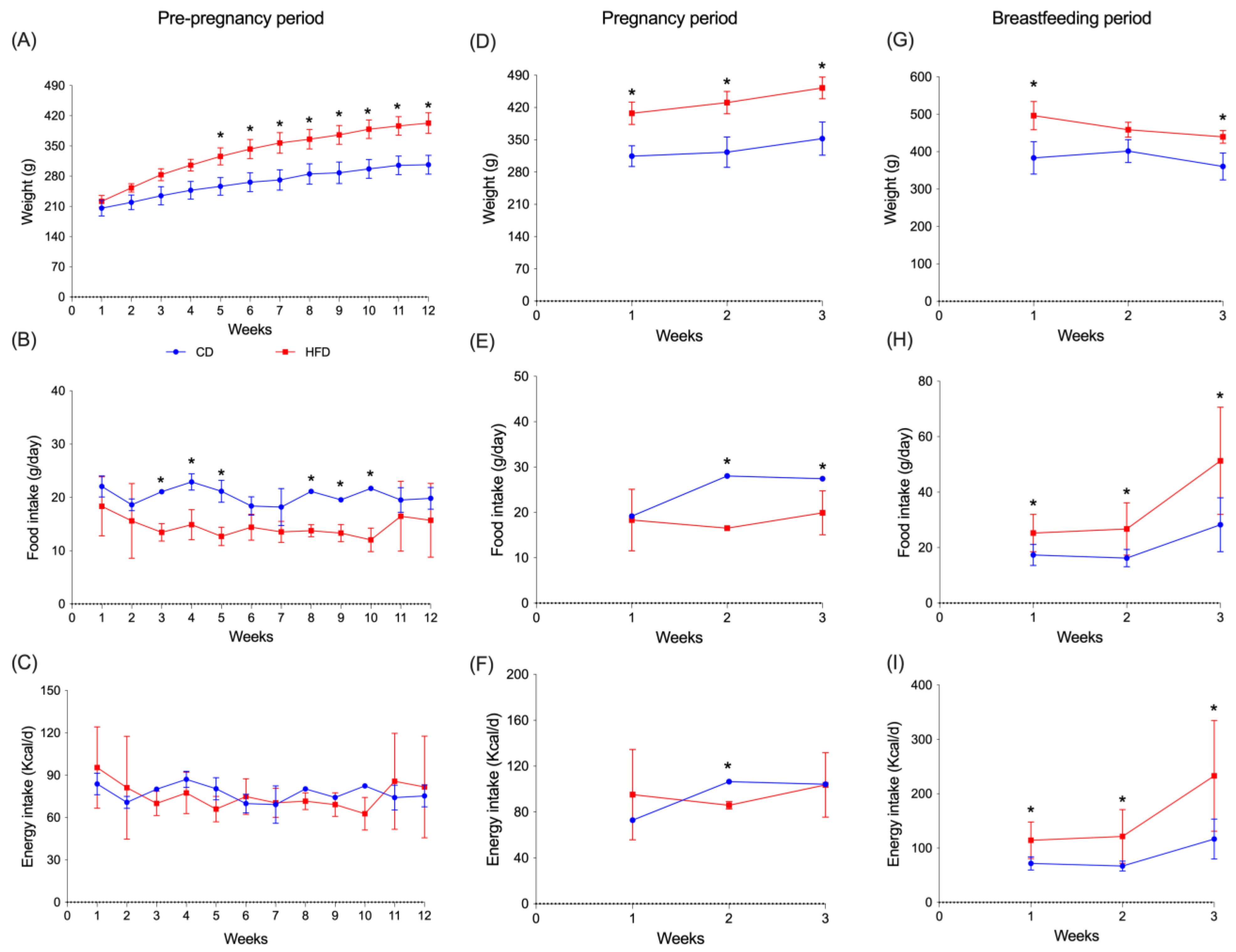
3.1. Evolution of Body Weight and Food Intake in Dam’s Rats
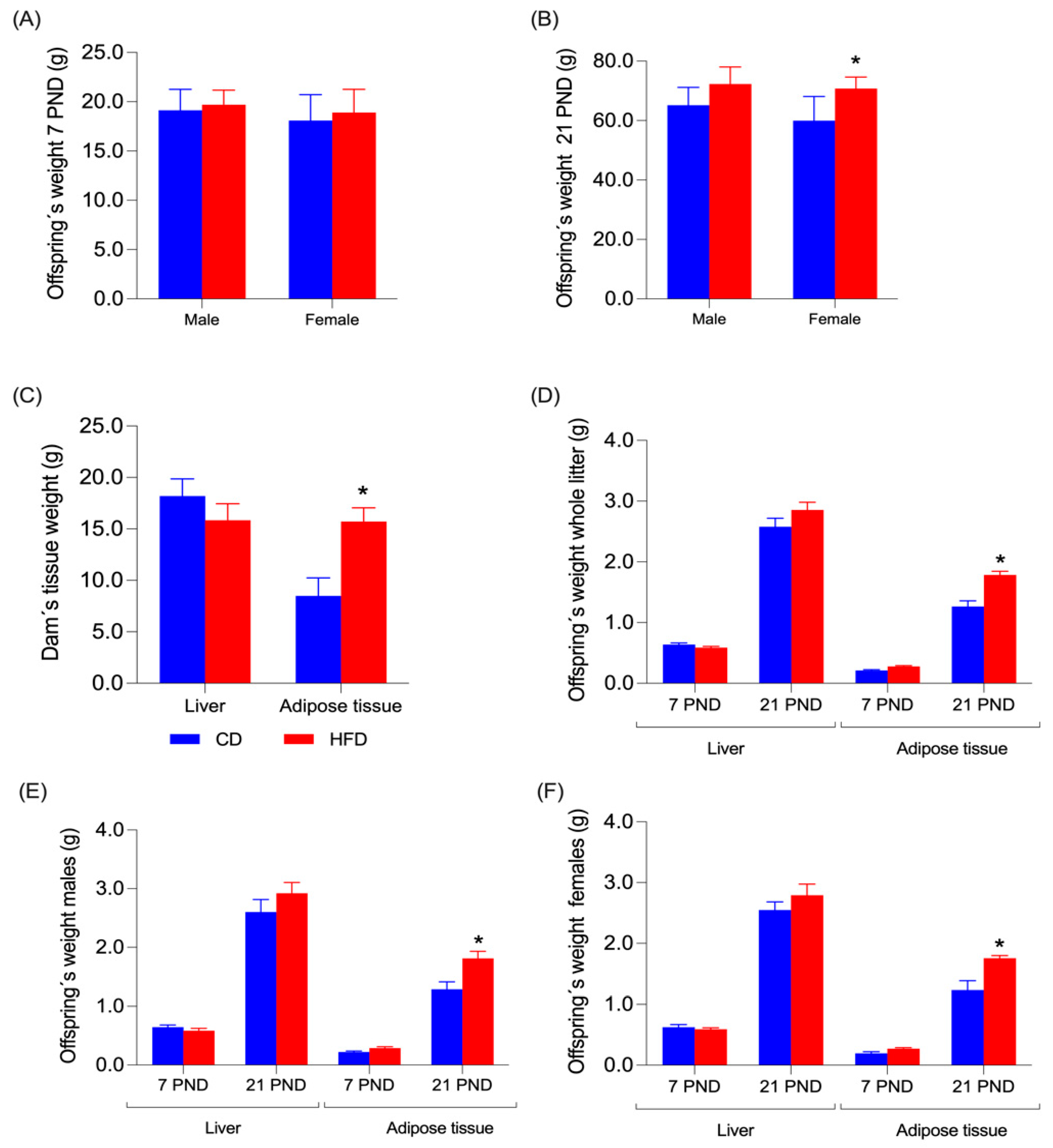
3.2. Body Weight in Offspring Rats
3.3. Liver and Adipose Tissue Weight in Dams and Offspring
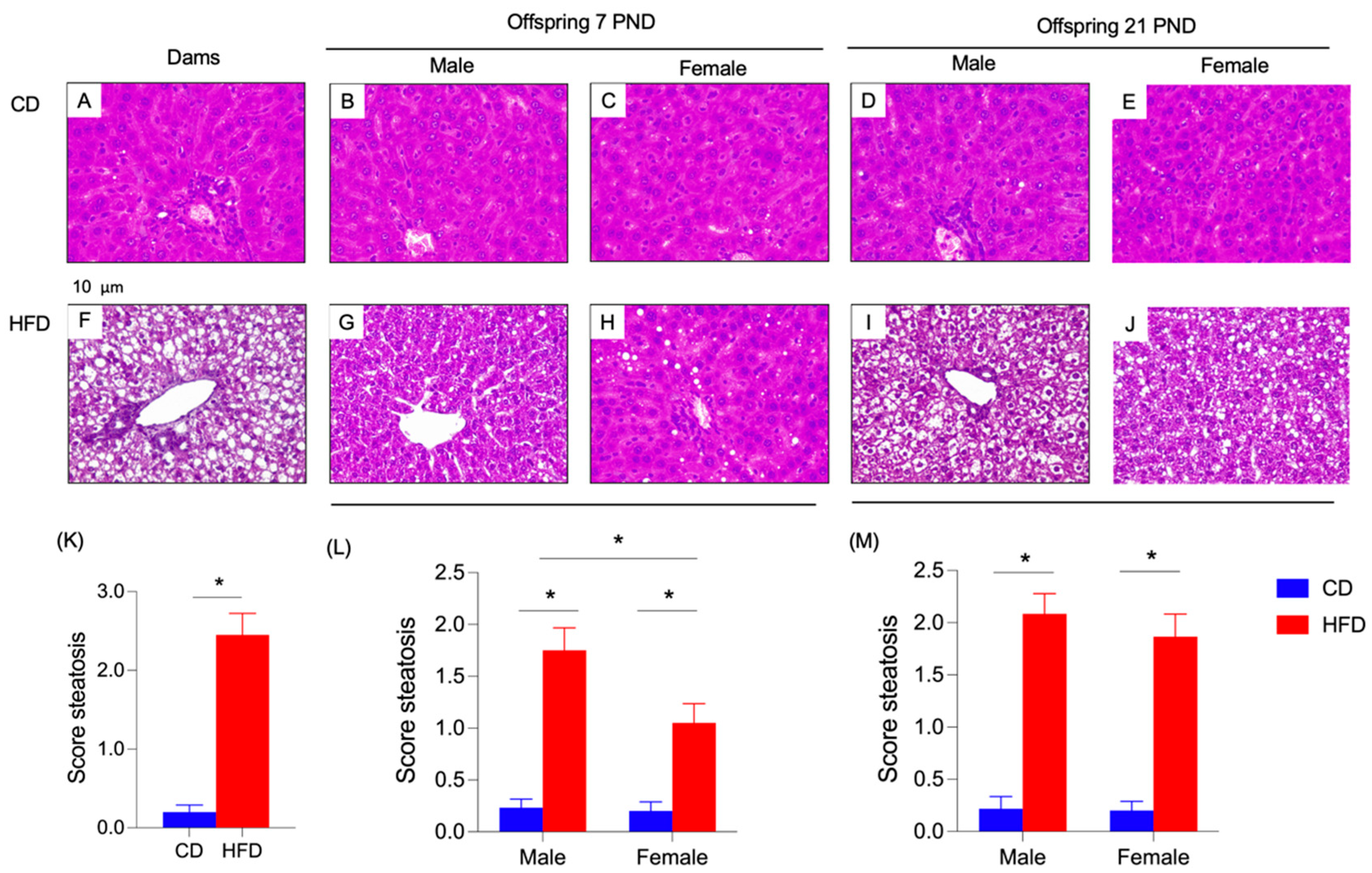
3.4. Dam and Offspring Serum and Liver Parameters
3.5. Fatty Acid Profile from Different Tissues in Dams and Offspring
3.6. Transcription, Quantitation, and Enzyme Activity in Liver from Dams and Offspring
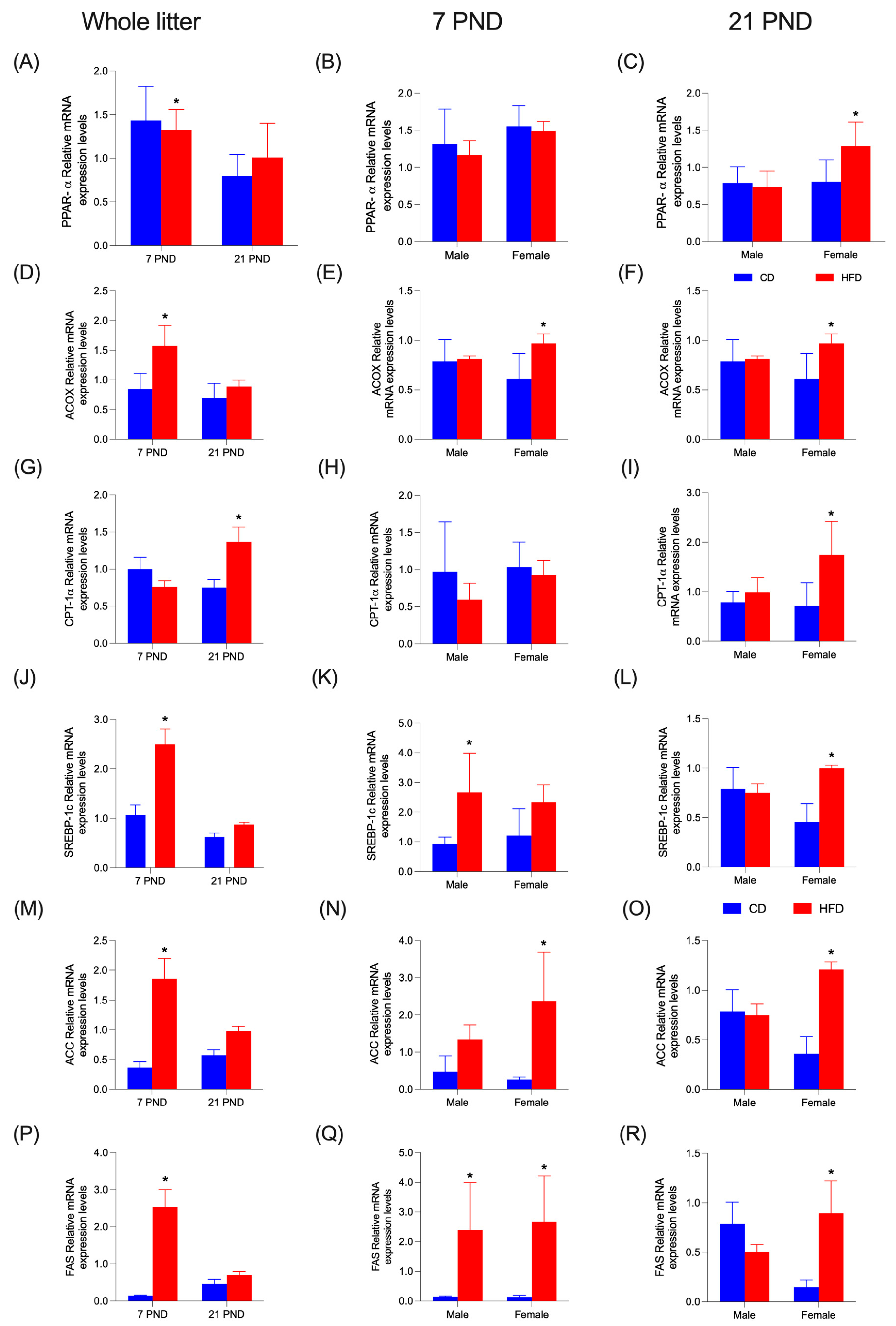
3.7. Hepatic mRNA Changes of PPAR-α and SREBP 1-c, and the Enzyme Regulated by These Transcription Factors from Offspring at 7 and 21 Days Fed with CD or HFD
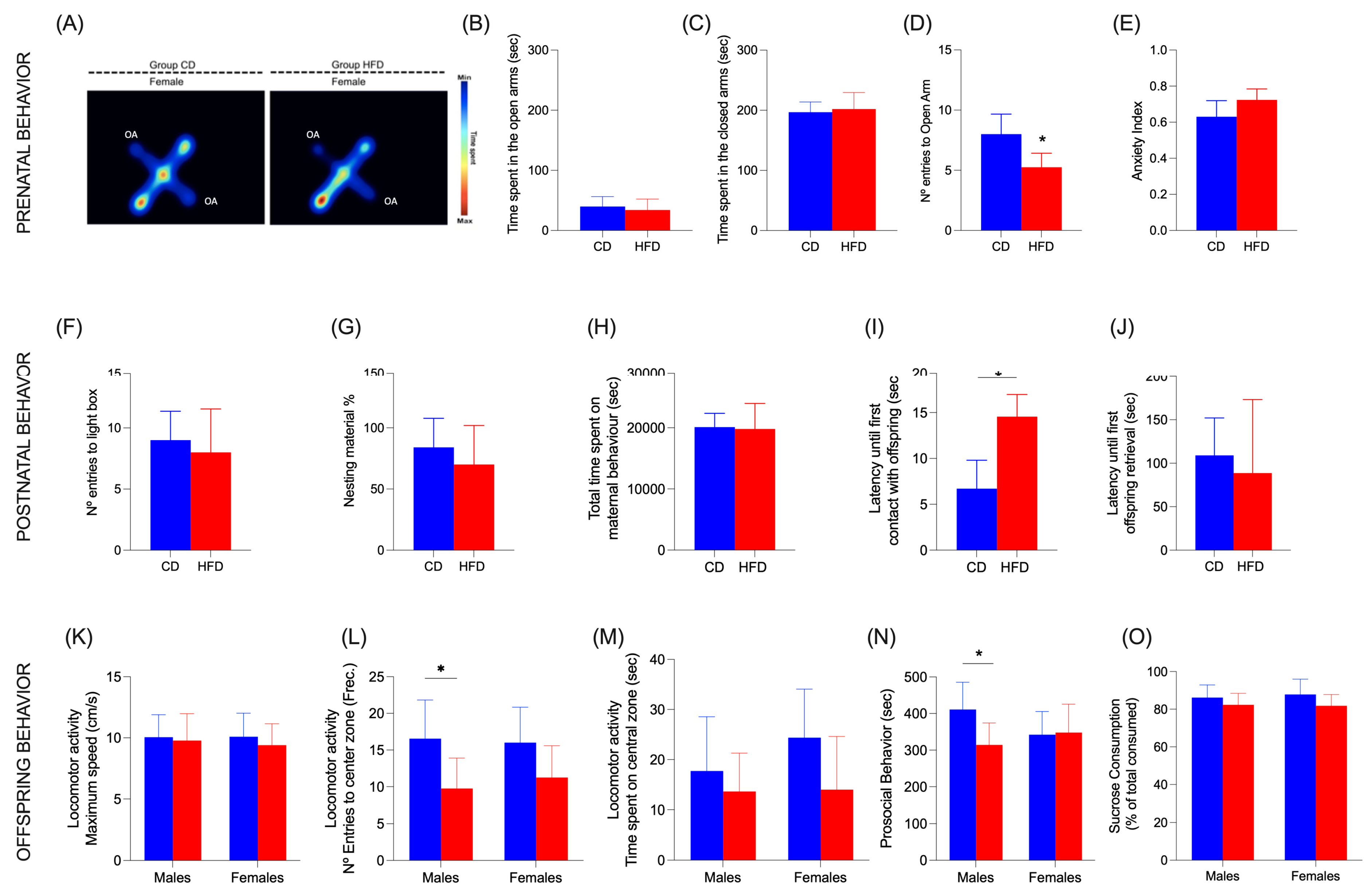
3.8. Dam Behavior
3.9. Maternal Behaviors
3.10. Offspring Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFD | High-Fat Diet |
| CD | Control Diet |
| DOHaD | Developmental Origins of Health and Disease |
| FA | Fatty Acid |
| PUFA | Polyunsaturated Fatty Acid |
| DHA | Docosahexaenoic Acid |
| ARA | Arachidonic Acid |
| ALA | Alpha-linolenic Acid |
| LA | Linoleic Acid |
| Δ5-D | Δ-5 Desaturase |
| Δ6-D | Δ-6 Ddesaturase |
| EPM | Elevated Plus Maze |
| LDB | Light Dark Box |
| PND | Post-Natal Day |
| OFT | Open Field Test |
| SPT | Sucrose Preference Test |
| HOMA | Homeostasis Model Assessment Method |
| TG | Triglycerides |
| T-Cho | Total Cholesterol |
| HDL-c | HDL-Cholesterol |
| GGT | Gamma–Glutamil-Transferase |
| GPT | Glutamat–Pyruvat-Transaminase |
| GOT | Glutamic Oxaloacetic Transaminase |
| SDA | Stearidonic Acid |
| DGLA | Dihomo-Gamma-Linolenic Acid |
| PPAR-α | Peroxisome Proliferator-Activated Receptor Alpha |
| ACOX | Acyl-CoA Oxidase |
| CPT1-α | Carnitine Palmitoyl Transferase 1α |
| SREBP-1c | Sterol Response Element Binding Protein |
| ACC | Acetyl-CoA-Carboxylase |
| FAS | FA Synthase |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase |
| SFA | Saturated Fatty Acids |
| MA | Miristic Acid |
| PA | Palmitic Acid |
| SA | Stearic Acid |
| MUFA | Monounsaturated Fatty Acid |
| POA | Palmitoleic Acid |
| OA | Oleic Acid |
| n-6 DPA | n-6 Docosapentaenoic |
| n-3 DPA | n-3 Docosapentaenoic |
| DNL | De Novo Lipogenesis |
| HPA | Hypothalamic–Pituitary–Adrenal Axis |
References
- Denizli, M.; Capitano, M.L.; Kua, K.L. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front. Cell. Infect. Microbiol. 2022, 12, 940937. [Google Scholar] [PubMed]
- Consales, A.; Morniroli, D.; Vizzari, G.; Mosca, F.; Giannì, M.L. Nutrition for Infant Feeding. Nutrients 2022, 14, 1823. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.A.; Osama, H.; Saeed, H.; Madney, Y.M.; Harb, H.S.; Abdelrahim, M.E.A. Impact of n-3 polyunsaturated fatty acid intake in pregnancy on maternal health and birth outcomes: Systematic review and meta-analysis from randomized controlled trails. Arch. Gynecol. Obstet. 2022, 307, 249–262. [Google Scholar] [PubMed]
- Álvarez, D.; Muñoz, Y.; Ortiz, M.; Maliqueo, M.; Chouinard-Watkins, R.; Valenzuela, R. Impact of Maternal Obesity on the Metabolism and Bioavailability of Polyunsaturated Fatty Acids during Pregnancy and Breastfeeding. Nutrients 2020, 13, 19. [Google Scholar] [CrossRef]
- Ortiz, M.; Sánchez, F.; Álvarez, D.; Flores, C.; Salas-Pérez, F.; Valenzuela, R.; Cantin, C.; Leiva, A.; Crisosto, N.; Maliqueo, M. Association between maternal obesity, essential fatty acids and biomarkers of fetal liver function. Prostaglandins Leukot. Essent. Fatty Acids 2023, 190, 102541. [Google Scholar]
- Gázquez, A.; Prieto-Sánchez, M.T.; Blanco-Carnero, J.E.; Ruíz-Palacios, M.; Nieto, A.; van Harskamp, D.; Oosterink, J.; Schierbeek, H.; van Goudoever, J.; Demmelmair, H.; et al. Altered materno-fetal transfer of 13C-polyunsaturated fatty acids in obese pregnant women. Clin. Nutr. 2020, 39, 1101–1107. [Google Scholar]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2017, 16, 1059–1085. [Google Scholar]
- Larrieu, T.; Layé, S. Food for Mood: Relevance of Nutritional Omega-3 Fatty Acids for Depression and Anxiety. Front. Physiol. 2018, 9, 1047. [Google Scholar]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Layé, S. Perinatal dietary polyunsaturated fatty acids in brain development, role in neurodevelopmental disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef]
- Videla, L.A.; Hernandez-Rodas, M.C.; Metherel, A.H.; Valenzuela, R. Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: Impact on non-alcoholic fatty liver disease. Prostaglandins Leukot. Essent. Fatty Acids 2022, 181, 102441. [Google Scholar]
- Devarshi, P.P.; Grant, R.W.; Ikonte, C.J.; Hazels Mitmesser, S. Maternal Omega-3 Nutrition, Placental Transfer and Fetal Brain Development in Gestational Diabetes and Preeclampsia. Nutrients 2019, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Zehravi, M.; Maqbool, M.; Ara, I. Correlation between obesity, gestational diabetes mellitus, and pregnancy outcomes: An overview. Int. J. Adolesc. Med. Health 2021, 33, 339–345. [Google Scholar] [PubMed]
- Valenzuela, R.; Metherel, A.H.; Cisbani, G.; Smith, M.E.; Chouinard-Watkins, R.; Klievik, B.J.; Videla, L.A.; Bazinet, R.P. Protein concentrations and activities of fatty acid desaturase and elongase enzymes in liver, brain, testicle, and kidney from mice: Substrate dependency. BioFactors 2024, 50, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Tung, K.T.S.; Wong, R.S.; Mak, R.T.W. Maternal n-3 PUFA Intake During Pregnancy and Perinatal Mental Health Problems: A Systematic Review of Recent Evidence. Curr. Nutr. Rep. 2023, 12, 426–438. [Google Scholar]
- Neto, J.; Jantsch, J.; De Oliveira, S.; Braga, M.F.; Castro, L.F.D.S.; Diniz, B.F.; Moreira, J.C.F.; Giovenardi, M.; Porawski, M.; Guedes, R.P. DHA/EPA supplementation decreases anxiety-like behaviour, but it does not ameliorate metabolic profile in obese male rats. Br. J. Nutr. 2022, 128, 964–974. [Google Scholar]
- Biała, G.K.M. Amphetamine-induced anxiety-related behavior in animal models. Pharmacol. Rep. 2007, 59, 636–644. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging The Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, S.; Jarmasz, J.S.; Jin, Y.; Siddiqui, T.J.; Cattini, P.A. Effects of high fat diet-induced obesity and pregnancy on prepartum and postpartum maternal mouse behavior. Psychoneuroendocrinology 2021, 126, 105147. [Google Scholar] [CrossRef] [PubMed]
- Bosch, O.J.; Müsch, W.; Bredewold, R.; Slattery, D.A.; Neumann, I.D. Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: Implications for postpartum mood disorder. Psychoneuroendocrinology 2007, 32, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Aguggia, J.P.; Suárez, M.M.; Rivarola, M.A. Early maternal separation: Neurobehavioral consequences in mother rats. Behav. Brain Res. 2013, 248, 25–31. [Google Scholar] [CrossRef]
- Malkesman, O.; Weller, A. Two different putative genetic animal models of childhood depression—A review. Prog. Neurobiol. 2009, 88, 153–169. [Google Scholar] [CrossRef]
- Meaney, M.J.; Stewart, J. Neonatal androgens influence the social play of prepubescent rats. Horm. Behav. 1981, 15, 197–213. [Google Scholar] [CrossRef]
- Bravo-Tobar, I.D.; Fernández, P.; Sáez, J.C.; Dagnino-Subiabre, A. Long-term effects of stress resilience: Hippocampal neuroinflammation and behavioral approach in male rats. J. Neurosci. Res. 2021, 99, 2493–2510. [Google Scholar] [CrossRef]
- Cohen, H.; Matar, M.A.; Joseph, Z. Animal Models of Post-Traumatic Stress Disorder. Curr. Protoc. Neurosci. 2013, 64, 9–45. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrera, C.; Valenzuela, R.; Chamorro, R.; Bascuñán, K.; Sandoval, J.; Sabag, N.; Valenzuela, F.; Valencia, M.-P.; Puigrredon, C.; Valenzuela, A. The Impact of Maternal Diet during Pregnancy and Lactation on the Fatty Acid Composition of Erythrocytes and Breast Milk of Chilean Women. Nutrients 2018, 10, 839. [Google Scholar] [CrossRef]
- Chamorro, R.; Bascuñán, K.A.; Barrera, C.; Sandoval, J.; Puigrredon, C.; Valenzuela, R. Reduced n-3 and n-6 PUFA (DHA and AA) Concentrations in Breast Milk and Erythrocytes Phospholipids during Pregnancy and Lactation in Women with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 1930. [Google Scholar] [CrossRef] [PubMed]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Domenichiello, A.F.; Kitson, A.P.; Chen, C.T.; Trépanier, M.O.; Stavro, P.M.; Bazinet, R.P. The effect of linoleic acid on the whole body synthesis rates of polyunsaturated fatty acids from α-linolenic acid and linoleic acid in free-living rats. J. Nutr. Biochem. 2016, 30, 167–176. [Google Scholar]
- Garcia-Jaramillo, M.; Spooner, M.H.; Löhr, C.V.; Wong, C.P.; Zhang, W.; Jump, D.B. Lipidomic and transcriptomic analysis of western diet-induced nonalcoholic steatohepatitis (NASH) in female Ldlr -/- mice. PLoS ONE 2019, 14, e0214387, Erratum in PLoS ONE 2019, 14, e0216535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wan, F.; Pan, F.; Mori, T.A.; O’Sullivan, T.A.; Beilin, L.J.; Oddy, W.H. Relationship between dietary intake and erythrocyte PUFA in adolescents from a Western Australian cohort. Eur. J. Clin. Nutr. 2023, 77, 283–291. [Google Scholar]
- Murru, E.; Manca, C.; Carta, G.; Banni, S. Impact of Dietary Palmitic Acid on Lipid Metabolism. Front. Nutr. 2022, 9, 861664. [Google Scholar] [CrossRef]
- Cavalcanti, C.C.L.; Manhães-de-Castro, R.; Chaves, W.F.; Cadena-Burbano, E.V.; Antonio-Santos, J.; da Silva Aragão, R. Influence of maternal high-fat diet on offspring’s locomotor activity during anxiety-related behavioral tests: A systematic review. Behav. Brain Res. 2024, 462, 114869. [Google Scholar] [CrossRef] [PubMed]
- Chaves, W.F.; Pinheiro, I.L.; da Silva, J.M.; Manhães-de-Castro, R.; da Silva Aragão, R. Repercussions of maternal exposure to high-fat diet on offspring feeding behavior and body composition: A systematic review. J. Dev. Orig. Health Dis. 2021, 12, 220–228. [Google Scholar]
- Almeida, M.M.; Dias-Rocha, C.P.; Souza, A.S.; Muros, M.F.; Mendonca, L.S.; Pazos-Moura, C.C.; Trevenzoli, I.H. Perinatal maternal high-fat diet induces early obesity and sex-specific alterations of the endocannabinoid system in white and brown adipose tissue of weanling rat offspring. Br. J. Nutr. 2017, 118, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirko, K.A.; Chai, B.; Spiegelman, D.; Campos, H.; Farvid, M.S.; Hankinson, S.E.; Willett, W.C.; Eliassen, A.H. Erythrocyte membrane fatty acids breast cancer risk: A prospective analysis in the nurses’ health study II. Int. J. Cancer 2018, 142, 1116–1129. [Google Scholar] [PubMed]
- Kitamura, Y.; Kogomori, C.; Hamano, H.; Maekawa, I.; Shimizu, T.; Shiga, S. Relationship between Changes in Fatty Acid Composition of the Erythrocyte Membranes and Fatty Acid Intake during Pregnancy in Pregnant Japanese Women. Ann. Nutr. Metab. 2017, 70, 268–276. [Google Scholar]
- Létondor, A.; Buaud, B.; Vaysse, C.; Fonseca, L.; Herrouin, C.; Servat, B.; Layé, S.; Pallet, V.; Alfos, S. Erythrocyte DHA level as a biomarker of DHA status in specific brain regions of n-3 long-chain PUFA-supplemented aged rats. Br. J. Nutr. 2014, 112, 1805–1818. [Google Scholar] [PubMed]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: Relationship to tissue development and aging. Prostaglandins Leukot. Essent. Fatty Acids 2016, 114, 28–34. [Google Scholar] [PubMed]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 23–34. [Google Scholar]
- Zhao, T.; Huang, H.; Li, J.; Shen, J.; Zhou, C.; Xiao, R.; Ma, W. Association between erythrocyte membrane fatty acids and gut bacteria in obesity-related cognitive dysfunction. AMB Express 2023, 13, 148. [Google Scholar]
- Valenzuela, R.; Das, U.N.; Videla, L.A.; Llorente, C.G. Nutrients and Diet: A Relationship between Oxidative Stress, Aging, Obesity, and Related Noncommunicable Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 7460453. [Google Scholar]
- Johnson, M.; Pace, R.D.; McElhenney, W.H. Green leafy vegetables in diets with a 25:1 omega-6/omega-3 fatty acid ratio modify the erythrocyte fatty acid profile of spontaneously hypertensive rats. Lipids Health Dis. 2018, 17, 140. [Google Scholar]
- Ren, Z.; Okyere, S.K.; Xie, L.; Wen, J.; Wang, J.; Chen, Z.; Ni, X.; Deng, J.; Hu, Y. Oral Administration of Bacillus toyonensis Strain SAU-20 Improves Insulin Resistance and Ameliorates Hepatic Steatosis in Type 2 Diabetic Mice. Front. Immunol. 2022, 13, 837237. [Google Scholar]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update. Biochem. J. 2021, 478, 3723–3739. [Google Scholar]
- Lee, H.B.; Do, M.H.; Jhun, H.; Ha, S.K.; Song, H.S.; Roh, S.W.; Chung, W.-H.; Nam, Y.-D.; Park, H.-Y. Amelioration of Hepatic Steatosis in Mice through Bacteroides uniformis CBA7346-Mediated Regulation of High-Fat Diet-Induced Insulin Resistance and Lipogenesis. Nutrients 2021, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.C.; Huang, J.P.; Tsai, Y.T.; Shih, W.C.; Juan, C.C.; Hsieh, P.S.; Hung, L.-M.; Yu, C.-L. Granulocytic MDSC with Deficient CCR5 Alleviates Lipogenesis and Inflammation in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 13048. [Google Scholar] [CrossRef] [PubMed]
- Tsuduki, T.; Kitano, Y.; Honma, T.; Kijima, R.; Ikeda, I. High Dietary Fat Intake During Lactation Promotes Development of Diet-Induced Obesity in Male Offspring of Mice. J. Nutr. Sci. Vitaminol. 2013, 59, 384–392. [Google Scholar] [PubMed]
- Thompson, M.D.; Cismowski, M.J.; Trask, A.J.; Lallier, S.W.; Graf, A.E.; Rogers, L.K.; Lucchesi, P.A.; Brigstock, D.R. Enhanced Steatosis and Fibrosis in Liver of Adult Offspring Exposed to Maternal High-Fat Diet. Gene Expr. 2016, 17, 47–59. [Google Scholar] [CrossRef]
- Scheidl, T.; Wager, J.L.; Baker, L.; Brightwell, A.; Melan, K.M.; Larion, S.; Sarr, O.; Regnault, T.R.; Urbanski, S.J.; Thompson, J.A. High Maternal Adiposity During Pregnancy Programs an Imbalance in the Lipidome and Predisposes to Diet-Induced Hepatosteatosis in the Offspring. Biosci. Rep. 2023, 43, BSR20231060. [Google Scholar]
- Bautista, C.J.; Montaño, S.A.; Ramírez, V.M.; Escobedo-Morales, A.; Nathanielsz, P.W.; Bobadilla, N.A.; Zambrano, E. Changes in Milk Composition in Obese Rats Consuming a High-Fat Diet. Br. J. Nutr. 2015, 115, 538–546. [Google Scholar] [CrossRef]
- Treesukosol, Y.; Sun, B.; Moghadam, A.A.; Liang, N.C.; Tamashiro, K.L.K.; Moran, T.H. Maternal High-Fat Diet During Pregnancy and Lactation Reduces the Appetitive Behavioral Component in Female Offspring Tested in a Brief-Access Taste Procedure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R499–R509. [Google Scholar] [CrossRef][Green Version]
- Volpato, A.M.; Schultz, A.; Magalhães-da-Costa, E.; Correia, M.; Águila, M.B.; Mandarim-de-Lacerda, C.A. Maternal High-Fat Diet Programs for Metabolic Disturbances in Offspring Despite Leptin Sensitivity. Neuroendocrinology 2012, 96, 272–284. [Google Scholar] [CrossRef]
- Sheldon, R.D.; Blaize, A.N.; Fletcher, J.A.; Donkin, S.S.; Newcomer, S.C.; Rector, R.S. Gestational Exercise Protects Adult Male Offspring From High-Fat Diet-Induced Hepatic Steatosis. J. Hepatol. 2016, 64, 171–178. [Google Scholar] [CrossRef]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The impact of maternal high-fat diet on offspring neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef]
- Dagnino-Subiabre, A. Stress and Western diets increase vulnerability to neuropsychiatric disorders: A common mechanism. Nutr. Neurosci. 2021, 24, 624–634. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M. The cortisol switch between vulnerability and resilience. Mol. Psychiatry 2024, 29, 20–34. [Google Scholar] [PubMed]
- McClafferty, S.R.; Paniagua-Ugarte, C.; Hannabass, Z.M.; Jackson, P.A.; Hayes, D.M. Comparing the effects of infant maternal and sibling separation on adolescent behavior in rats (Rattus norvegicus). PLoS ONE 2024, 19, e0308958. [Google Scholar] [PubMed]


| Parameters | CD (a) | HFD (b) | p Value |
|---|---|---|---|
| Glycemia (mg/dL) | 171 ± 24 | 180 ± 74 | 0.8053 |
| Insulin (μU/mL) | 7.7 ± 0.7 | 8.8 ± 0.4 b | 0.0376 |
| HOMA-IR | 3.2 ± 0.6 | 4.4 ± 1.6 | 0.2101 |
| TG (mg/dL) | 69 ± 21 | 105 ± 55 | 0.3606 |
| T-Cho (mg/dL) | 90 ± 14 | 103 ± 34 | 0.4545 |
| HDL-c (mg/dL) | 28 ± 7.3 | 29 ± 14 | 0.8563 |
| GGT (IU/L) | 10 ± 0.2 | 10 ± 0.5 | 0.3632 |
| GPT (IU/L) | 10 ± 0.1 | 11 ± 2.4 | 0.4610 |
| GOT (IU/L) | 57 ± 13 | 75 ± 37 | 0.3257 |
| PND7 | PND21 | |||||
|---|---|---|---|---|---|---|
| Parameters | CD (a) | HFD (b) | p Value | CD (a) | HFD (b) | p Value |
| Glycemia (mg/dL) | 182 ± 78 | 201 ± 38 | 0.7343 | 243 ± 98 | 270 ± 51 | 0.5998 |
| Insulin (μU/mL) | 6.2 ± 2.6 | 7.6 ± 1.6 | 0.2816 | 6.7 ± 1.6 | 7.6 ± 1.6 | 0.3205 |
| HOMA-IR | 2.1 ± 1.4 | 3.4 ± 0.7 | 0.1146 | 3.9 ± 1.1 | 5.3 ± 1.1 | 0.0616 |
| TG (mg/dL) | 179 ± 63 | 295 ± 13 b | 0.0058 | 80 ± 45 | 178 ± 83 b | 0.0493 |
| T-Cho (mg/dL) | 154 ± 19 | 145 ± 6.1 | 0.2916 | 116 ± 16 | 122 ± 11 | 0.5137 |
| HDL-c (mg/dL) | 58 ± 5.0 | 57 ± 4.0 | 0.6248 | 44 ± 8.3 | 48 ± 8.1 | 0.4645 |
| GPT (IU/L) | 10 ± 0.5 | 13 ± 3.0 | 0.0639 | 11 ± 1.2 | 17 ± 5.3 b | 0.0288 |
| GGT (IU/L) | 10 ± 0.5 | 11 ± 1.1 b | 0.0422 | 10 ± 0.2 | 10 ± 0.4 | 0.3632 |
| GOT (IU/L) | 60 ± 13 | 102 ± 45 | 0.0518 | 81 ± 19 | 82 ± 9.2 | 0.9306 |
| Fatty Acids (%mmol) | Liver | Adipose Tissue | Erythrocytes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | |
| SFA | |||||||||
| C14:0 | 1.5 ± 0.2 | 1.5 ± 0.4 | 0.958 | 2.7 ± 0.4 | 5.3 ± 0.5 a | <0.001 | 2.6 ± 0.4 a | 1.8 ± 0.3 | 0.004 |
| C16:0 | 31.9 ± 3.2 a | 23.7 ± 2.2 | <0.001 | 29.3 ± 4.0 a | 14.8 ± 1.7 | <0.001 | 29.8 ± 1.6 a | 26.8 ± 1.3 | 0.004 |
| C18:0 | 7.1 ± 1.5 | 15.3 ± 6.4 a | 0.012 | 3.7 ± 0.4 | 4.2 ± 2.3 | 0.634 | 18.9 ± 1.9 | 22.1 ± 1.2 a | 0.005 |
| MUFA | |||||||||
| C 16:1 | 4.2 ± 1.1 a | 0.8 ± 0.4 | <0.001 | 3.5 ± 1.4 a | 1.0 ± 0.4 | 0.002 | 0.7 ± 0.2 a | 0.5 ± 0.1 | 0.037 |
| C18:1n9c | 46.6 ± 3.5 a | 30.3 ± 7.4 | <0.001 | 51.1 ± 2.6 | 47.2 ± 3.4 | 0.052 | 12.6 ± 0.9 a | 10.3 ± 1.1 | 0.002 |
| PUFA | |||||||||
| C18:2n-6c (LA) | 4.9 ± 2.0 | 20.0 ± 3.7 a | <0.001 | 8.4 ± 1.6 | 24.5 ± 2.0 a | <0.001 | 6.0 ± 0.8 | 10.7 ± 0.4 a | <0.001 |
| C18:3n-3 (ALA) | 0.4 ± 0.1 | 0.8 ±0.1 a | <0.001 | 0.1 ± 0.1 | 0.6 ± 0.1 a | <0.001 | 0.6 ± 0.1 a | 0.4 ± 0.1 | 0.001 |
| C20:4n-6 (AA) | 0.3 ± 0.2 | 1.0 ± 0.4 a | 0.007 | 0.4 ± 0.2 | 1.0 ± 0.2 a | <0.001 | 22.2 ± 1.0 | 21.1 ± 1.4 | 0.169 |
| C22:5n-6 (DPA) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.522 | 0.1 ± 0.1 | 0.03 ± 0.02 | 0.161 | 0.6 ± 0.2 a | 0.3 ± 0.1 | 0.025 |
| C22:5n-3 (DPA) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.575 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.335 | 1.6 ± 1.1 | 1.9 ± 1.0 | 0.622 |
| C20:5n-3 (EPA) | 0.2 ± 0.1 | 0.7 ± 0.2 a | <0.001 | 0.1 ± 0.03 | 0.2 ± 0.04 a | <0.001 | 1.3 ± 0.2 | 1.5 ± 0.2 | 0.209 |
| C22:6n-3 (DHA) | 2.6 ± 0.2 | 5.4 ± 0.6 a | <0.001 | 0.2 ± 0.03 | 0.2 ± 0.04 a | 0.025 | 3.1 ± 0.5 | 4.2 ± 0.7 a | 0.008 |
| ΣSFA | 40.4 ± 2.3 | 40.5 ± 7.4 | 0.981 | 35.9 ± 4.0 | 24.4 ± 3.9 a | <0.001 | 51.3 ± 0.9 | 50.7 ± 2.3 | 0.537 |
| ΣMUFA | 50.7 ± 3.1 a | 31.1 ± 7.4 | <0.001 | 54.7 ± 3.0 a | 48.6 ± 2.9 | 0.005 | 13.3 ± 1.0 a | 10.8 ± 1.2 | 0.002 |
| ΣPUFA | 8.8 ± 2.2 | 28.4 ± 3.2 a | <0.001 | 9.4 ± 1.4 | 27.0 ± 1.4 a | <0.001 | 35.4 ± 1.0 | 40.2 ± 1.7 a | <0.001 |
| Σn-6 PUFA | 5.4 ± 2.0 | 21.3 ± 3.4 a | <0.001 | 9.0 ± 1.4 | 25.8 ± 1.7 a | <0.001 | 28.8 ± 1.0 | 32.2 ± 1.6 a | 0.001 |
| Σn-3 PUFA | 3.4 ± 0.2 | 7.1 ± 0.7 a | <0.001 | 0.5 ± 0.2 | 1.2 ± 0.4 a | 0.001 | 6.6 ± 0.8 | 8.0 ± 1.3 a | 0.044 |
| Ratio n-6:n-3 | 1.6 ± 0.5 | 3.0 ± 0.7 a | 0.002 | 24.6 ± 15.1 | 23.2 ± 8.1 | 0.848 | 4.4 ± 0.6 | 4.1 ± 0.8 | 0.515 |
| Fatty Acids (%mmol) | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PND7 | PND21 | PND7 | PND21 | |||||||||
| CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | |
| SFA | ||||||||||||
| C14:0 | 2.6 ± 0.8 | 3.0 ± 0.3 | 0.295 | 3.3 ± 0.4 | 2.6 ± 0.2 | 0.089 | 4.4 ± 0.3 a | 1.7 ± 0.1 | <0.001 | 3.6 ± 0.5 a | 2.5 ± 0.3 | <0.001 |
| C16:0 | 33.9 ± 2.3 a | 21.2 ± 1.5 | <0.001 | 25.9 ± 2.8 a | 18.3 ± 1.3 | <0.001 | 32.1 ± 2.4 a | 24.2 ± 1.9 | <0.001 | 28.3 ± 1.9 a | 20.0 ± 1.7 | <0.001 |
| C18:0 | 10.3 ± 1.1 | 11.8 ± 1.6 | 0.181 | 14.05 ± 1.07 | 17.3 ± 1.7 a | 0.001 | 9.9 ± 0.8 | 14.7 ± 1.7 a | <0.001 | 12.1 ± 1.5 | 14.8 ± 1.0 a | 0.004 |
| MUFA | ||||||||||||
| C16:1 | 0.9 ± 0.3 a | 0.4 ± 0.04 | <0.012 | 1.4 ± 0.4 a | 0.3 ± 0.03 | <0.001 | 1.4 ± 0.1 a | 0.4 ± 0.1 | <0.001 | 1.8 ± 0.3 a | 0.5 ± 0.1 | <0.001 |
| C18:1n9c | 28.3 ± 3.0 | 27.4 ± 2.2 | 0.741 | 27.1 ± 2.6 a | 22.4 ± 1.6 | 0.006 | 30.6 ± 3.9 | 29.2 ± 3.8 | 0.704 | 29.1 ± 2.6 | 25.4 ± 2.2 | 0.123 |
| PUFA | ||||||||||||
| C18:2n-6c (LA) | 13.2 ± 2.4 | 22.5 ± 1.6 a | <0.001 | 12.4 ± 1.3 | 27.7 ± 2.2 a | <0.001 | 13.7 ± 2.9 | 17.6 ± 1.6 a | 0.005 | 13.5 ± 1.1 | 26.6 ± 1.9 a | <0.001 |
| C18:3n-3 (ALA) | 1.1 ± 0.1 | 2.3 ± 0.2 a | <0.001 | 1.9 ± 0.1 a | 3.3 ± 0.3 | <0.001 | 1.2 ± 0.3 | 3.6 ± 0.3 a | <0.001 | 1.4 ± 0.4 | 3.2 ± 0.3 a | <0.001 |
| C20:4n-6 (AA) | 1.0 ± 0.1 | 1.3 ± 0.1 a | 0.001 | 0.7 ± 0.1 | 1.0 ± 0.07 a | <0.001 | 1.1 ± 0.2 | 0.9 ± 0.2 | 0.145 | 0.9 ± 0.1 | 0.8 ± 0.08 | 0.486 |
| C22:5n-6 (DPA) | 1.2 ± 0.4 a | 0.6 ± 0.1 | 0.014 | 1.0 ± 0.5 a | 0.3 ± 0.05 | 0.004 | 0.8 ± 0.1 a | 0.5 ± 0.1 | <0.001 | 0.3 ± 0.1 | 0.2 ± 0.02 | 0.113 |
| C22:5n-3 (DPA) | 1.4 ± 0.3 | 1.2 ± 0.2 | 0.084 | 1.0 ± 0.1 | 1.0 ± 0.07 | 0.825 | 1.0 ± 0.1 a | 0.6 ± 0.07 | <0.001 | 1.2 ± 0.2 a | 0.7 ± 0.07 | <0.001 |
| C20:5n-3 (EPA) | 1.1 ± 0.1 a | 0.5 ± 0.1 | <0.001 | 0.7 ± 0.1 a | 0.3 ± 0.06 | <0.001 | 1.2 ± 0.1 a | 0.4 ± 0.05 | <0.001 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.320 |
| C22:6n-3 (DHA) | 4.2 ± 0.8 a | 9.0 ± 1.4 | <0.001 | 9.9 ± 1.4 a | 6.7 ± 0.5 | <0.001 | 3.8 ± 0.8 | 7.4 ± 1.7 a | <0.001 | 6.9 ± 0.8 | 5.9 ± 0.4 | 0.253 |
| ΣSFA | 46.9 ± 3.1 a | 36.1 ± 3.0 | <0.001 | 43.3 ± 4.2 a | 38.3 ± 3.0 | 0.038 | 46.4 ± 3.2 a | 40.7 ± 3.7 | 0.013 | 44.1 ± 3.2 a | 37.4 ± 2.8 | 0.003 |
| ΣMUFA | 29.3 ± 2.8 a | 27.8 ± 2.2 | 0.502 | 28.6 ± 2.6 a | 22.8 ± 1.6 | <0.001 | 32.0 ± 4.0 | 29.6 ± 3.7 | 0.396 | 30.9 ± 2.9 a | 26.0 ± 2.3 | 0.037 |
| ΣPUFA | 23.7 ± 2.2 | 37.6 ± 2.7 a | <0.001 | 28.0 ± 3.2 | 40.5 ± 2.9 a | <0.001 | 23.1 ± 2.0 | 31.2 ± 3.9 a | <0.001 | 24.8 ± 1.6 | 38.2 ± 2.7 a | <0.001 |
| Σn-6 PUFA | 15.6 ± 2.5 | 24.5 ± 1.8 a | <0.001 | 14.2 ± 1.6 | 29.0 ± 2.2 a | <0.001 | 15.7 ± 2.8 | 19.1 ± 1.9 a | 0.021 | 14.8 ± 1.3 | 27.6 ± 2.0 a | <0.001 |
| Σn-3 PUFA | 8.1 ± 1.2 | 13.1 ± 1.5 a | <0.001 | 13.7 ± 1.7 a | 11.4 ± 0.9 | 0.020 | 7.3 ± 1.4 | 12.1 ± 2.0 a | <0.001 | 10.0 ± 1.0 | 10.5 ± 1.0 | 0.816 |
| Ratio n-6:n-3 | 1.9 ± 0.6 | 1.8 ± 0.2 | 0.854 | 1.0 ± 0.08 | 2.5 ± 0.1 a | <0.001 | 2.2 ± 0.8 a | 1.6 ± 0.1 | 0.035 | 1.4 ± 0.2 | 2.6 ± 0.2 a | <0.001 |
| Fatty Acids (%mmol) | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PND7 | PND21 | PND7 | PND21 | |||||||||
| CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | |
| SFA | ||||||||||||
| C14:0 | 13.5 ± 2.1 a | 5.5 ± 0.4 | <0.001 | 10.7 ± 1.0 a | 7.0 ± 0.8 | <0.001 | 13.0 ± 0.9 a | 7.4 ± 0.7 | <0.001 | 9.7 ± 0.9 a | 2.0 ± 0.1 | <0.001 |
| C15:0 | 0.2 ± 0.04 | 1.1 ± 0.5 a | 0.010 | 1.4 ± 0.9 | 0.9 ± 0.3 | 0.322 | 0.2 ± 0.1 | 1.0 ± 0.3 | 0.072 | 10.3 ± 1.0 a | 0.8 ± 0.6 | <0.001 |
| C16:0 | 25.2 ± 6.6 a | 14.2 ± 1.1 | 0.001 | 26.8 ± 6.1 a | 18.6 ± 1.5 | 0.011 | 28.8 ± 4.6 a | 16.2 ± 1.2 | <0.001 | 25.1 ± 2.2 a | 18.5 ± 3.8 | <0.001 |
| C16:1 | 4.3 ± 0.6 a | 0.5 ± 0.14 | <0.001 | 5.3 ± 1.1 ª | 1.8 ± 0.4 | <0.001 | 3.9 ± 0.3 a | 0.4 ± 0.04 | <0.001 | 3.7 ± 0.3 a | 0.4 ± 0.1 | 0.017 |
| C18:0 | 4.1 ± 0.3 | 5.6 ± 0.4 | 0.070 | 4.7 ± 1.0 | 19.9 ± 1.9 a | <0.001 | 5.2 ± 0.7 | 9.4 ± 2.4 a | 0.017 | 3.5 ± 0.3 | 10.3 ± 4.3 a | <0.001 |
| MUFA | ||||||||||||
| C16:1 | 4.3 ± 0.6 a | 0.5 ± 0.14 | <0.001 | 5.3 ± 1.1 ª | 1.8 ± 0.4 | <0.001 | 3.9 ± 0.3 a | 0.4 ± 0.04 | <0.001 | 3.7 ± 0.3 a | 0.4 ± 0.1 | 0.017 |
| C18:1n9c | 42.4 ± 5.9 | 42.8 ± 3.5 | 0.986 | 38.5 ± 3.3 a | 26.3 ± 3.4 | <0.001 | 39.2 ± 2.8 | 41.0 ± 3.4 | 0.457 | 37.9 ± 2.8 | 43.5 ± 6.3 | 0.057 |
| PUFA | ||||||||||||
| C18:2n-6c (LA) | 6.3 ± 0.5 | 23.6 ± 1.9 a | <0.001 | 10.2 ± 2.4 | 25.7 ± 1.9 a | <0.001 | 6.9 ± 0.5 | 19.3 ± 3.2 a | <0.001 | 7.4 ± 0.9 | 24.6 ± 5.6 a | <0.001 |
| C18:3n-3 (ALA) | 0.6 ± 0.04 a | 0.5 ± 0.05 | <0.001 | 0.1 ± 0.03 | 0.2 ± 0.01 | 0.055 | 0.5 ± 0.04 a | 0.3 ± 0.1 | 0.006 | 0.1 ± 0.01 | 0.2 ± 0.1 | 0.098 |
| C20:1n9 | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.986 | 0.2 ± 0.1 | 0.02 ± 0.01 | 0.191 | 1.0 ± 0.4 a | 0.1 ± 0.03 | <0.001 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.931 |
| C20:2 | 0.4 ± 0.1 | 0.6 ± 0.2 a | 0.029 | 0.3 ± 0.07 | 0.3 ± 0.2 | 0.962 | 1.0 ± 0.4 a | 0.1 ± 0.03 | <0.001 | 0.2 ± 0.04 | 0.1 ± 0.1 | 0.865 |
| C20:4n-6 (AA) | 1.5 ± 0.2 | 1.4 ± 0.3 | 0.838 | 0.8 ± 0.2 | 0.6 ± 0.05 | 0.438 | 1.7 ± 0.1 | 1.5 ± 0.1 | 0.172 | 0.6 ± 0.1 | 0.5 ± 0.2 | 0.246 |
| C22:5n-6 (DPA n-6) | 0.1 ± 0.04 | 0.3 ± 0.1 a | <0.001 | 0.05 ± 0.03 | 0.04 ± 0.01 | 0.980 | 0.1 ± 0.1 a | 0.05 ± 0.01 | <0.001 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.977 |
| C22:5n-3 (DPA n-3) | 0.2 ± 0.1 | 0.4 ± 0.2 a | 0.017 | 0.1 ± 0.06 | 0.1 ± 0.004 | 0.647 | 0.2 ± 0.1 a | 0.1 ± 0.1 | <0.001 | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.265 |
| C20:5n-3 (EPA) | 0.3 ± 0.1 | 0.7 ± 0.1 a | <0.001 | 0.3 ± 0.1 a | 0.1 ± 0.01 | 0.003 | 0.3 ± 0.1 | 0.9 ± 0.1 a | <0.001 | 0.3 ± 0.1 | 0.2 ± 0.05 | 0.012 |
| C22:6n-3 (DHA) | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.059 | 0.6 ± 0.2 a | 0.2 ± 0.01 | <0.001 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.777 | 0.7 ± 0.2 a | 0.2 ± 0.03 | <0.001 |
| ΣSFA | 43.0 ± 5.7 a | 26.4 ± 2.2 | <0.001 | 43.6 ± 4.2 | 46.4 ± 3.3 | 0.425 | 47.2 ± 4.8 a | 34.0 ± 3.5 | 0.001 | 48.6 ± 3.9 a | 31.6 ± 8.7 | <0.001 |
| ΣMUFA | 47.1 ± 6.1 a | 43.7 ± 3.8 | 0.330 | 44.0 ± 3.3 a | 28.1 ± 3.1 | <0.001 | 43.1 ± 3.2 | 41.5 ± 3.5 | 0.764 | 41.8 ± 3.2 | 44.1 ± 6.3 | 0.590 |
| ΣPUFA | 9.9 ± 0.7 | 28.2 ± 2.4 a | <0.001 | 12.4 ± 2.6 | 27.1 ± 1.9 a | <0.001 | 11.4 ± 0.8 | 22.8 ± 3.6 a | <0.001 | 9.6 ± 0.9 | 26.0 ± 5.5 a | <0.001 |
| Σn-6 PUFA | 8.3 ± 0.6 | 26.0 ± 2.1 a | <0.001 | 11.3 ± 2.7 | 26.7 ± 1.9 a | <0.001 | 9.8 ± 0.8 | 20.9 ± 3.4 a | <0.001 | 8.3 ± 1.0 | 25.3 ± 5.5 a | <0.001 |
| Σn-3 PUFA | 1.6 ± 0.4 | 2.2 ± 0.4 a | 0.004 | 1.1 ± 0.3 a | 0.5 ± 0.03 | 0.006 | 1.6 ± 0.3 | 1.9 ± 0.2 | 0.080 | 1.3 ± 0.2 a | 0.7 ± 0.1 | <0.001 |
| Ratio n-6:n-3 | 5.5 ± 1.6 | 11.8 ± 1.7 a | <0.001 | 11.1 ± 4.1 | 58.1 ± 0.8 a | <0.001 | 6.2 ± 1.5 | 11.1 ± 0.8 | 0.224 | 6.6 ± 1.6 | 36.5 ± 10.0 a | <0.001 |
| Fatty Acids (%mmol) | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PND7 | PND21 | PND7 | PND21 | |||||||||
| CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | |
| SFA | ||||||||||||
| C14:0 | 3.3 ± 1.1 | 3.0 ± 0.8 | 0.811 | 4.2 ± 1.0 a | 1.8 ± 0.4 | <0.001 | 2.9 ± 0.8 | 3.9 ± 1.3 | 0.100 | 2.4 ± 0.6 | 1.9 ± 0.7 | 0.576 |
| C16:0 | 34.2 ± 4.6 | 30.3 ± 2.5 | 0.127 | 35.4 ± 4.0 a | 29.3 ± 2.1 | 0.011 | 37.7 ± 2.8 | 30.5 a ± 2.6 | <0.001 | 35.2 ± 3.1 a | 28.4 ± 2.1 | <0.001 |
| C18:0 | 11.8 ± 1.6 | 14.9 ± 2.0 a | 0.006 | 12.6 ± 1.1 | 19.6 ± 1.5 a | <0.001 | 11.9 ± 0.9 | 13.9 ± 1.3 a | 0.045 | 16.7 ± 1.2 | 20.3 ± 2.1 a | <0.001 |
| MUFA | ||||||||||||
| C16:1 | 1.5 ± 0.3 a | 0.6 ± 0.1 | <0.001 | 1.5 ± 0.5 a | 0.3 ± 0.02 | <0.001 | 1.0 ± 0.1 | 0.9 ± 0.2 | 0.334 | 1.1 ± 0.3 a | 0.5 ± 0.1 | <0.001 |
| C18:1n9c | 25.6 ± 2.9 a | 19.4 ± 2.1 | <0.001 | 22.6 ± 2.9 a | 14.5 ± 1.5 | <0.001 | 20.7 ± 1.5 | 20.0 ± 1.7 | 0.660 | 18.0 ± 1.8 a | 15.5 ± 1.2 | 0.021 |
| PUFA | ||||||||||||
| C18:2n-6c (LA) | 9.9 ± 1.6 | 15.2 ± 2.0 a | <0.001 | 10.8 ± 1.3 | 14.5 ± 1.0 a | 0.001 | 8.1 ± 1.8 | 16.4 ± 1.4 a | <0.001 | 12.3 ± 1.8 | 13.2 ± 1.4 | 0.558 |
| C18:3n-3 (ALA) | 0.9 ± 0.2 | 1.2 ± 0.2 a | 0.001 | 1.3 ± 0.1 | 1.7 ± 0.2 a | <0.001 | 0.7 ± 0.05 | 0.7 ± 0.1 | 0.796 | 0.9 ± 0.2 | 1.4 ± 0.3 a | <0.001 |
| C20:4n-6 (AA) | 7.9 ± 0.8 | 12.7 ± 2.8 a | <0.001 | 6.5 ± 1.8 | 15.0 ± 1.6 a | <0.001 | 10.3 ± 0.9 | 11.1 ± 1.1 | 0.263 | 9.4 ± 0.6 | 12.5 ± 1.0 a | <0.001 |
| C22:5n-6 (DPA) | 0.7 ± 0.4 a | 0.3 ± 0.04 | 0.009 | 0.6 ± 0.3 a | 0.2 ± 0.02 | 0.037 | 0.4 ± 0.1 a | 0.3 ± 0.1 | 0.021 | 0.5 ± 0.1 | 0.5 ± 0.04 | 0.687 |
| C22:5n-3 (DPA) | 1.1 ± 0.4 | 0.8 ± 0.1 | 0.128 | 0.9 ± 0.4 | 0.8 ± 0.1 | 0.843 | 0.9 ± 0.1 a | 0.6 ± 0.1 | 0.003 | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.945 |
| C20:5n-3 (EPA) | 1.1 ± 0.4 | 1.3 ± 0.2 | 0.303 | 0.6 ± 0.1 | 1.5 ± 0.2 a | <0.001 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.856 | 1.0 ± 0.1 | 1.5 ± 0.2 a | <0.001 |
| C22:6n-3 (DHA) | 2.0 ± 0.2 | 2.0 ± 0.5 | 0.999 | 1.4 ± 0.6 | 2.3 ± 0.2 a | 0.002 | 2.7 ± 0.2 a | 2.2 ± 0.2 | <0.001 | 1.7 ± 0.1 | 1.8 ± 0.2 | 0.350 |
| ΣSFA | 49.4 ± 4.9 | 48.3 ± 4.1 | 0.893 | 52.3 ± 5.1 | 50.7 ± 3.6 | 0.798 | 52.5 ± 4.0 | 48.3 ± 3.6 | 0.171 | 54.3 ± 4.7 | 50.6 ± 3.8 | 0.232 |
| ΣMUFA | 27.1 ± 2.8 a | 20.0 ± 2.2 | <0.001 | 24.1 ± 3.4 a | 14.8 ± 1.5 | <0.001 | 21.8 ± 1.6 | 20.9 ± 1.6 | 0.591 | 19.0 ± 2.0 a | 15.9 ± 1.2 | 0.007 |
| ΣPUFA | 23.5 ± 2.8 | 33.4 ± 2.9 a | <0.001 | 22.0 ± 4.5 | 36.2 ± 3.0 a | <0.001 | 24.1 ± 2.1 | 32.5 ± 2.9 a | <0.001 | 26.6 ± 2.5 | 31.8 ± 3.0 a | 0.005 |
| Σn-6 PUFA | 18.5 ± 1.9 | 28.1 ± 2.2 a | <0.001 | 17.9 ± 3.3 | 29.8 ± 2.4 a | <0.001 | 18.8 ± 1.8 | 27.8 ± 2.6 a | <0.001 | 22.2 ± 2.1 | 26.2 ± 2.4 a | 0.010 |
| Σn-3 PUFA | 5.0 ± 0.9 | 5.3 ± 0.9 | 0.840 | 4.1 ± 1.2 | 6.4 ± 0.7 a | <0.001 | 5.3 ± 0.4 a | 4.7 ± 0.3 | 0.038 | 4.5 ± 0.4 | 5.6 ± 0.6 a | <0.001 |
| Ratio n-6:n-3 | 3.7 ± 0.4 | 5.4 ± 0.8 a | <0.001 | 4.5 ± 0.6 | 4.7 ± 0.2 | 0.803 | 3.5 ± 0.2 | 5.9 ± 0.2 a | <0.001 | 5.0 ± 0.1 a | 4.7 ± 0.1 | 0.034 |
| Fatty Acids (%mmol) | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PND7 | PND21 | PND7 | PND21 | |||||||||
| CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | CD (n = 6) | HFD (n = 6) | ANOVA p-Value | |
| SFA | ||||||||||||
| C14:0 | 6.0 ± 1.3 a | 3.3 ± 0.3 | <0.001 | 2.9 ± 0.7 | 3.1 ± 0.3 | 0.849 | 3.6 ± 0.3 | 3.6 ± 0.4 | 0.881 | 3.2 ± 0.3 | 2.9 ± 0.2 | 0.171 |
| C16:0 | 34.3 ± 2.3 | 34.8 ± 2.2 | 0.910 | 29.6 ± 1.9 | 29.8 ± 2.1 | 0.987 | 35.9 ± 2.7 | 34.1 ± 2.4 | 0.360 | 28.9 ± 1.9 | 29.2 ± 1.9 | 0.979 |
| C18:0 | 16.0 ± 1.1 | 17.2 ± 1.1 | 0.296 | 21.1 ± 1.5 | 21.9 ± 1.7 | 0.493 | 17.0 ± 1.3 | 16.2 ± 1.1 | 0.524 | 20.8 ± 1.4 | 20.9 ± 1.3 | 0.992 |
| MUFA | ||||||||||||
| C16:1 | 2.9 ± 0.4 a | 2.2 ± 0.3 | <0.001 | 1.4 ± 0.3 a | 0.7 ± 0.1 | <0.001 | 2.5 ± 0.2 | 5.1 ± 2.6 a | 0.004 | 0.8 ± 0.1 | 1.1 ± 0.5 | 0.890 |
| C18:1n9c | 12.4 ± 0.9 | 12.5 ± 0.8 | 0.986 | 14.9 ± 1.0 | 16.4 ± 1.2 a | 0.032 | 13.1 ± 1.0 | 12.6 ± 0.8 | 0.728 | 16.8 ± 1.1 | 15.9 ± 1.2 | 0.278 |
| PUFA | ||||||||||||
| C18:2n-6c (LA) | 2.3 ± 0.3 | 4.5 ± 0.7 a | <0.001 | 2.1 ± 0.2 | 1.9 ± 0.2 | 0.677 | 1.6 ± 0.1 | 4.7 ± 0.5 a | <0.001 | 1.5 ± 0.2 | 1.8 ± 0.2 | 0.147 |
| C18:3n-3 (ALA) | 0.3 ± 0.02 | 0.4 ± 0.1 a | 0.002 | 0.4 ± 0.1 | 0.8 ± 0.1 a | <0.001 | 0.3 ± 0.02 | 0.3 ± 0.1 | 0.471 | 0.6 ± 0.1 | 0.6 ± 0.04 | 0.916 |
| C20:4n-6 (AA) | 12.7 ± 1.0 | 12.5 ± 0.9 | 0.927 | 12.5 ± 0.9 | 12.1 ± 1.1 | 0.687 | 14.0 ± 1.0 a | 11.6 ± 1.2 | <0.001 | 12.6 ± 0.8 | 12.6 ± 0.8 | 0.994 |
| C22:5n-6 (DPA) | 2.2 ± 0.2 a | 1.6 ± 0.2 | <0.001 | 1.7 ± 0.2 a | 1.3 ± 0.2 | 0.002 | 2.4 ± 0.2 a | 1.2 ± 0.2 | <0.001 | 1.6 ± 0.1 a | 1.2 ± 0.1 | 0.001 |
| C22:5n-3 (DPA) | 2.7 ± 0.3 | 2.8 ± 0.5 | 0.934 | 2.9 ± 0.2 | 2.9 ± 0.2 | 0.999 | 2.9 ± 0.2 a | 2.5 ± 0.2 | 0.010 | 3.0 ± 0.2 | 3.1 ± 0.3 | 0.389 |
| C20:5n-3 (EPA) | 0.3 ± 0.1 | 0.2 ± 0.02 | 0.129 | 0.2 ± 0.03 a | 0.2 ± 0.01 | 0.004 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.305 | 0.2 ± 0.01 | 0.2 ± 0.03 | 0.459 |
| C22:6n-3 (DHA) | 7.8 ± 0.6 | 8.0 ± 0.7 | 0.801 | 10.2 ± 0.7 | 10.6 ± 0.8 | 0.495 | 8.0 ± 0.6 | 7.7 ± 0.8 | 0.720 | 10.0 ± 0.7 | 10.5 ± 0.7 | 0.451 |
| ΣSFA | 56.4 ± 3.6 | 55.3 ± 3.5 | 0.853 | 53.6 ± 3.5 | 54.9 ± 4.0 | 0.801 | 56.5 ± 4.2 | 53.9 ± 3.6 | 0.421 | 52.9 ± 3.4 | 52.9 ± 3.4 | >0.999 |
| ΣMUFA | 15.3 ± 1.1 | 14.7 ± 1.0 | 0.515 | 16.3 ± 1.1 | 17.1 ± 1.2 | 0.428 | 15.5 ± 1.2 | 17.8 ± 3.0 | 0.084 | 17.6 ± 1.2 | 17.0 ± 1.2 | 0.835 |
| ΣPUFA | 28.3 ± 1.8 | 30.0 ± 1.9 | 0.285 | 30.1 ± 2.0 | 29.7 ± 2.4 | 0.941 | 29.4 ± 2.2 | 28.3 ± 2.3 | 0.603 | 29.5 ± 1.9 | 30.1 ± 1.9 | 0.875 |
| Σn-6 PUFA | 17.2 ± 1.1 | 18.6 ± 1.2 | 0.157 | 16.3 ± 1.1 | 15.3 ± 1.5 | 0.284 | 18.0 ± 1.3 | 17.6 ± 1.4 | 0.802 | 15.7 ± 1.0 | 15.6 ± 1.0 | 0.986 |
| Σn-3 PUFA | 11.0 ± 0.7 | 11.5 ± 0.9 | 0.662 | 13.8 ± 1.0 | 14.5 ± 1.0 | 0.365 | 11.4 ± 0.8 | 10.7 ± 1.0 | 0.375 | 13.8 ± 0.9 | 14.4 ± 0.9 | 0.406 |
| Ratio n-6:n-3 | 1.6 ± 0.01 | 1.6 ± 0.1 | 0.324 | 1.2 ± 0.01 a | 1.1 ± 0.1 | 0.007 | 1.6 ± 0.0 | 1.6 ± 0.1 a | 0.005 | 1.1 ± 0.02 a | 1.1 ± 0.01 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercado-López, L.; Muñoz, Y.; Farias, C.; Beyer, M.P.; Carrasco-Gutiérrez, R.; Caicedo-Paz, A.V.; Dagnino-Subiabre, A.; Espinosa, A.; Valenzuela, R. High-Fat Diet in Perinatal Period Promotes Liver Steatosis and Low Desaturation Capacity of Polyunsaturated Fatty Acids in Dams: A Link with Anxiety-Like Behavior in Rats. Nutrients 2025, 17, 1180. https://doi.org/10.3390/nu17071180
Mercado-López L, Muñoz Y, Farias C, Beyer MP, Carrasco-Gutiérrez R, Caicedo-Paz AV, Dagnino-Subiabre A, Espinosa A, Valenzuela R. High-Fat Diet in Perinatal Period Promotes Liver Steatosis and Low Desaturation Capacity of Polyunsaturated Fatty Acids in Dams: A Link with Anxiety-Like Behavior in Rats. Nutrients. 2025; 17(7):1180. https://doi.org/10.3390/nu17071180
Chicago/Turabian StyleMercado-López, Lorena, Yasna Muñoz, Camila Farias, María Paz Beyer, Robinson Carrasco-Gutiérrez, Angie Vanessa Caicedo-Paz, Alexies Dagnino-Subiabre, Alejandra Espinosa, and Rodrigo Valenzuela. 2025. "High-Fat Diet in Perinatal Period Promotes Liver Steatosis and Low Desaturation Capacity of Polyunsaturated Fatty Acids in Dams: A Link with Anxiety-Like Behavior in Rats" Nutrients 17, no. 7: 1180. https://doi.org/10.3390/nu17071180
APA StyleMercado-López, L., Muñoz, Y., Farias, C., Beyer, M. P., Carrasco-Gutiérrez, R., Caicedo-Paz, A. V., Dagnino-Subiabre, A., Espinosa, A., & Valenzuela, R. (2025). High-Fat Diet in Perinatal Period Promotes Liver Steatosis and Low Desaturation Capacity of Polyunsaturated Fatty Acids in Dams: A Link with Anxiety-Like Behavior in Rats. Nutrients, 17(7), 1180. https://doi.org/10.3390/nu17071180








